THE CURRENT VIEW ON BIOLOGICAL POTENCY
OF CATIONICALLY
MODIFIED CHITOSAN
2Department of Physical Chemistry and Electrochemistry, Jagiellonian University, Cracow, Poland
INTRODUCTION
Cationic polymers, both of natural and synthetic origin, have been intensively studied due to their immense therapeutical potential including delivery of gene which is probably their best known biomedical application (1). Synthetic polycations are synthesized often with desired molecular weight, they can be easily modified and do not show batch-to-batch variation, a typical disadvantage of natural polymers. The synthetic polycations, which are the most intensively studied in biomedical applications, especially in drug delivery, tissue engineering and gene delivery, are polyethyleneimines (PEIs) (2) poly(L-lysine) (PLL) (3), poly(amino-co--esters) (PAEs) (4, 5) and poly(dimethylaminoethylmethacrylates) (PDMAEMAs) (6, 7). However, they are often toxic, show poor hemocompatibility, and low biodegradability (8, 9).
On the other hand, the cationic polymers of natural origin are actually limited to proteins with chitosan being a unique polymer as the only natural cationic polysaccharide. Chitosan is a random copolymer of N-acetyl-D-glucosamine and d-glucosamine (10). In nature it occurs in small amounts in the cell walls of several genera of mushrooms e.g., Mucor, Rhizopus, and Absidia (11, 12). However, on a synthetic scale it is obtained by deacetylation of chitin, the main component of the crustacean shells (13, 14) being regarding the validity of the importance second (after cellulose) substance in the biosphere. The process of chitin deacetylation may be carried out to different degree leading to chitosans of various degrees of deacetylation (DD). DD and the molecular weight are two important characteristics of chitosan that determine its physicochemical properties, solubility in particular. Its structure, physicochemical properties and biological functions are determined by the presence of three types of functional reactive groups, i.e., hydroxyl, amino, and acetamido groups. Chitosan is soluble only in acidic solutions. Its solubility is higher for chitosans with higher DDs and lower molecular weight (15). Chitosan has found many commercial applications in cosmetics (16), food industry (17), agriculture (18), environmental protection (19), wastewater management (20), and chemical industry (13).
UNMODIFIED CHITOSANS
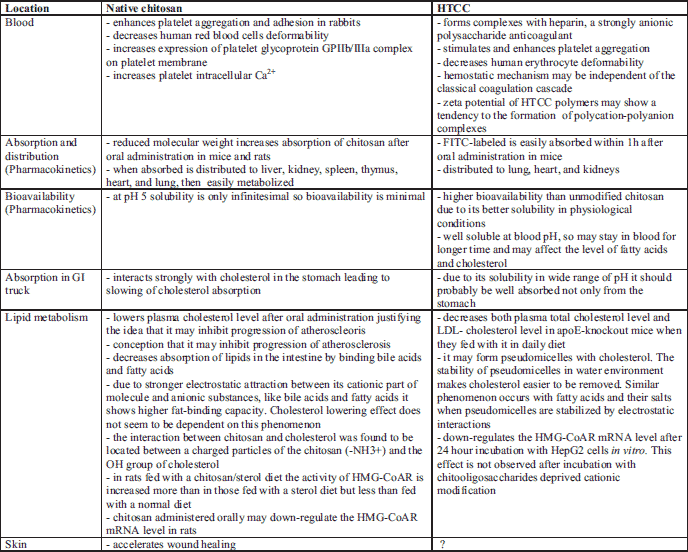
Chitosan has many beneficial biomedical properties such as biocompatibility, biodegradability, and nontoxicity. Biological activity of chitosan is closely related to its solubility and thus to both molecular weight and DD. Decreasing molecular weight increases absorption of chitosan after oral administration in mice (21) and rats (22). When absorbed, low-molecular-weight chitosan was found to be distributed to various of tested organs, i.e., liver, kidney, spleen, thymus, heart, and lung, and easily metabolized.
Chitosan decreases absorption of lipids in the intestine by binding bile acids and fatty acids (23). Therefore, the polysaccharide is a well-known dietary weight-reducing supplement. Oral chitosan lowers plasma cholesterol (24, 25) without adverse effects. The mechanism of hypocholesterolemic action of chitosan is still unclear. However, it has been suggested that chitosan acts by inhibiting the absorption of cholesterol and bile acids (23). Due to stronger electrostatic attraction between this cationic polysaccharide and anionic substances like bile acids and fatty acids, chitosan with higher DD (about 90%) and molecular weight shows higher fat-binding capacity (12). On the other hand, its cholesterol lowering effect does not show a clear dependence on the above features (12). Its hypocholesterolemic potency seems to be related to inhibition of progression of atherosclerosis (26).
One of the most important properties of chitosan is its inherent antimicrobial effect, that was first recognized more than 40 years ago (27), against many Gram-positive and Gram-negative bacteria, yeasts, and fungi (28, 29).
Furthermore, chitosan is one of the most intensively studied and safe component of drug delivery systems (30) prepared in different forms, e.g. as a hydrogel (31), nanoparticles (32-35), microparticles (28), and films (36); and administered by various routes, including topical (33) e.g. general mucosal (37), nasal (31), intraocular (38) and oral. Chitosan-based oral drug delivery systems may be quite advanced and include oral insulin administration (32), colon drug delivery (39), gastroretentive floating drug systems (40), or gene delivery (41, 42).
Other pharmacological properties of chitosan are: antitumor (43), immuno-enhancing (44, 45), wound healing (46), and antidiabetic (47, 48). In biotechnology chitosan is employed in tissue engineering (49, 50) and as a cell culture support (51, 52). Chitosan reveals immuno-stimulating effect because of its impact on the body’s immunological response to inflammation (45, 53). This polysaccharide increases production of interleukin 1 (IL-1), platelet-delivered growth factor, transforming growth factor β1 and phagocytosis of macrophages. Chitosan enhances also phagocytosis of polymorphonuclear leukocytes (PMN) and production of leukotriene B4 and osteopontin. These effects are crucial for healing of large open wounds as found in animals. Chitosan polymers induce fibroblasts to release interleukin-8 (IL-8) involved in proliferation and migration of vascular endothelial cells and fibroblasts (45, 53). Chitosan when delivered intranasally enhances systemic and local immune responses to tetanus toxoid, influenza pertussis and diphtheria vaccines (45, 54-57). Similar effects were observed after oral gene-delivery with chitosan-DNA nanoparticles in animal studies (45, 58, 59). Another studies showed that chitosan might limit an immunosuppression (45, 59). It was observed that after chitosan subcutaneous implantation in dogs the number of white blood cells was increased (45, 60).
CATIONIC CHITOSAN DERIVATIVES
The poor solubility of chitosan in water significantly constrains its possible applications. These limitations can be at least partially overcome by its cationic modification with ammonium groups which are ionized irrespective of the pH value.
Biological activity of the cationically modified chitosan overlaps significantly with that of the native chitosan and includes antibacterial (60) and antifungal (61) properties, enhancing adsorption of hydrophilic drugs through mucous membranes (62-64) improving proliferation of human periodontal ligament cells (HPDLC) (65) and inhibition the proliferation of cancer cells (66).
However, some properties are unique to cationically-modified chitosan. Studies on one of these type of polymers, including our recently performed studies, are as follows:
Antiheparin properties of cationically-modified chitosan
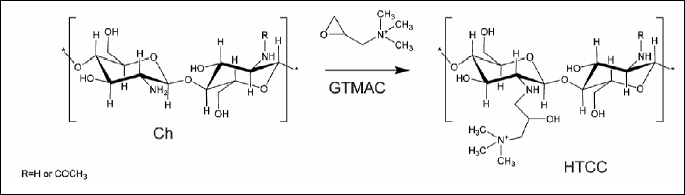
As previously described by us cationic modification (quaternization) of chitosan was conducted by covalent attachment of glycidyltrimethylammonium chloride (GTMAC) (67).
Two polymers of N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride with degree of substitution (DS) 63.6% and 90.5%, referred to as HTCC1 and HTCC2 respectively, were obtained. The modified chitosans showed very good solubility in water at physiological pH. In PBS buffer at pH=7.4 the zeta potential of HTCC1 and HTCC2 was found to be 13.28±1.21 and 24.2±0.83 respectively, indicating that these polymers may have a tendency to the formation of polycation-polyanion complexes. Indeed, it was shown that HTCC polymers form complexes with heparin - a strongly anionic polysaccharide anticoagulant - accompanied by the inhibition of anticoagulative activity of both unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) (61). Moreover, heparin activity could not only be blocked by HTCC but also removed from the solution in vitro in the form of microspheres crosslinked with genipin, a naturally-occurring compound obtained from Gardenia fruit (68).
Absorption and distribution of HTCC in mice after oral administration
HTCC absorption we have studied in C57BL/6j mice after oral administration of this polymer previously labeled with fluorescein isothiocyanate (FITC) (unpublished data). High level of FITC fluorescence that has been found in serum 1 hour after oral administration indicates that HTCC is easily absorbed from GI tract. HTCC was distributed mainly to lung and heart and particularly high HTCC concentration was found in kidney. Twenty four hours after oral administration HTCC was completely eliminated both from serum and the studied organs. However, there are studies indicating that some of unmodified high-molecular weight chitosan samples (HCS, Mw=760,000 Da) would gradually precipitate at the lower intestine and would be hardly absorbed if their molecules were not degraded into soluble ones (21). In mice plasma level of HCS half an hour after oral administration was very low and this level was negligible later (1 hour time point or even at 4 hour time point after administration). In kidney the highest concentration of HCS was found after 2 hours and then at 4th hour was significantly decreased. A large quantity of HSC was observed in feces when HSC was used as dietary fiber supplements (21).
HTCC stimulates and enhances platelet aggregation
Chitosan with moderately high molecular weight (Mw=50,000 Da) and high degree of deacetylation (DD>90%) enhances platelet aggregation and adhesion in rabbits, increases expression of platelet glycoprotein GPIIb/IIIa complex on platelet membrane and significantly increases platelet intracellular Ca2+ (69). We have found that HTCC with DS of 63.6% essentially stimulates human platelet aggregation in vitro and this phenomenon is dose-dependent (unpublished data). This phenomenon occurs in the absence of collagen and it is in contrast to platelet aggregation accompanying the bleeding from wounded vessels. The above results suggest that cationically modified chitosan may be an effective hemostatic agent but its hemostatic mechanism seems to be independent on the classical coagulation cascade (70). It also is well established that chitosan deprived cationic modification accelerate wound healing (71, 72). Unmodified chitosan inhibits the diffuse capillary bleeding in brain tissue (73) and the lingual bleeding in heparinized rabbits (74).
The effect on red blood cell deformability
It is well established that atherosclerosis and hyperlipidemia are related to microcirculatory disturbances and they are accompanied by decrease of red blood cell (RBC) deformability. However, in rabbits with hypercholesterolemia induced by the hen’s egg enriched diet the deformability of RBC was found to be increased (75). It was speculated that this could be a result of the changes in the mean corpuscular volume, viscosity of erythrocyte cytoplasm, and oxidative/antioxidative balance. We have found that HTCC with DS of 63.6% causes significant decrease of RBC deformability in human blood in vitro (unpublished data). Significant decrease of human RBC deformability was also found to be induced by chitooligosaccharide with Mw =50,000 Da, however its potency was much lower than HTCC.
The effect on lipid metabolism and hypercholesterolemia in the apolipoprotein E-deficient mouse model of atherosclerosis
To study the effect of HTCC on lipid and cholesterol metabolism we have used the apolipoprotein E-knockout mice model of atherosclerosis (76, 77). The apoE-knockout homozygous mutant mice have total plasma cholesterol level five times higher than that of normal animals while triglyceride levels are 68% higher than in normal litter mates. The high density lipoprotein (HDL) cholesterol levels reach only 45% of the normal level. Gene-targeted apoE-knockout mouse model proved to be very useful in the development of the inflammatory theory of atherosclerosis (78). In addition, process of atherogenesis seems to be affected by a gender of ApoE(–/–) mice. This effect is connected with markedly elevated production of thromboxane A2 (TXA2) and reduced prostacyclin (prostaglandin I2, PGI2) production in female ApoE(–/–) mice (79). To restrain of atherosclerosis progress some compounds were studied in mice model successfully, e.g., antileukotriene drugs (80), AVE 0991 - angiotensin(1-7) receptor agonist (81) and doxycycline (82). Chitooligosaccharides have angiotensin-I-converting enzyme (ACE) inhibitory activity (13, 83).
In apoE-deficient mice fed for 20 weeks on a diet containing 5% w/w of chitosan (DD=78%) blood cholesterol level was significantly decreased to 64% of that in the control animals (i.e. on a diet without chitosan). Examination of the area of aortic plaque showed a potent inhibition of atherogenesis in the whole aorta (about 42%) as well as in the aortic arch (50%) as compared to control mice (26). Moreover, we found that apoE-knockout mice fed for 16 weeks with a diet containing 200 mg of HTCC (with DS of 63.6%) per 1 kg of body weight, had total plasma cholesterol and LDL level lower by about 15% and 32%, respectively, as compared to the control (unpublished data). Fig. 2 contains an explanation of the possible mechanism of binding of cholesterol to chitosan according to molecular simulation of complexes of chitosan-cholesterol obtained with the Gaussian 98 software (84).
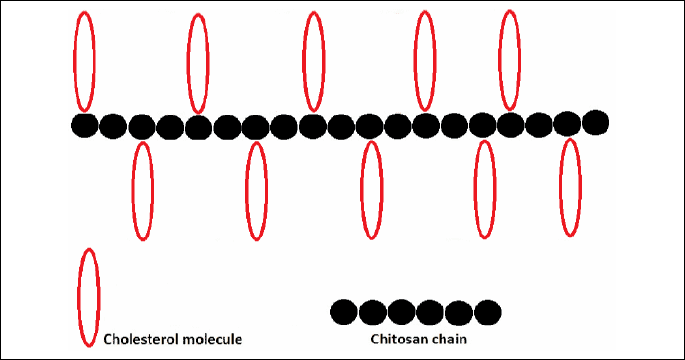
It is supposed that cationically modified chitosan (HTCC) may form pseudomicelles with cholesterol. In these structures cholesterol is inside pseudomicelle and more hydrophobic fragments of chitosan (main hydrocarbon chain) are immersed in hydrophobic centrum of cholesterol. Hydrophilic fragments of chitosan molecule through its electrostatic charges become oriented towards peripheries of pseudomicelles and this way the structures turn to more stabilized form. Stability of these microstructures in water environment possibly makes removal of cholesterol from the body much easier than in the form of agglutinated drops. Similar effect occurs in the case of fatty acids and their salts where pseudomicelles are additionally stabilized by electrostatic interactions between carboxyl groups of acids and cationic hydrophilic domains in chitosan molecule. In addition, it seems that these pseudomicellar structures may be stabilized by hydrogen bonds between number of OH groups present in chitosan and nucleophile atoms in cholesterol structure.
The effect of chitosan on the activity of 3-hydroxy-3-methylglutaryl coenzyme-A reductase (HMG-CoAR) - a hepatic enzyme involved in metabolism of lipoproteins, has previously been studied in rats (85). In rats fed a chitosan-sterol diet (DD=92%) the activity of HMG-CoAR increased more than in those fed with a sterol diet but less than in rats fed with a normal diet. Further studies with rats fed with chitosan using semi-quantitative RT-PCR showed that chitosan could downregulate the HMG-CoAR mRNA level (13). We found that also HTCC significantly down-regulates the HMG-CoAR mRNA level after 24 hour incubation with HepG2 cells in vitro (real-time PCR results). This effect was not observed after incubation with chitooligosaccharide (Mw=5,000 Da) deprived cationic modification (unpublished data).
Is cationically modified chitosan (HTCC) more efficient in regulating lipid metabolism than native chitosan?
It is believed that HTCC has higher bioavailability than unmodified chitosan and this due to its better solubility in physiological conditions. Native chitosan at pH 5 is soluble only infinitesimally so its bioavailability is minimal. On the other hand, at pH in the stomach unmodified chitosan becomes soluble and in such conditions it may easily be absorbed to blood. But pH of blood is 7.4, so concentration of the chitosan in blood is expected to be rather low and then chitosan may be subject to rapid uptake by organs. Furthermore, beneficial function of chitosan is binding of fatty acids and cholesterol in blood. Unmodified chitosan interacts with cholesterol in the stomach so effectively that practically it doesn’t let cholesterol to be absorbed from digestive tract. In contrast to unmodified chitosan, HTCC, due to its solubility at wide range of pH, has a big chance to be absorbed not only from the stomach. On the other hand, HTCC is well soluble at pH of blood, so it may stay in blood for longer time and therefore to influence the blood level of fatty acids and cholesterol very effectively. To sum up, HTCC having the characteristics of a chitosan it is just a more effective (Table 1).
In summary, HTCC is a well-absorbed cationic chitosan derivative that displays important biological activities including recently found effect on lipid metabolism. Some of its activities, e.g. binding of heparin or cholesterol lowering effect, appear to be quite unique. Although HTCC in principle mimics the actions of unmodified chitosan but it is doing this with clearly better effectiveness and this may prove useful from the therapeutic point of view.
Acknowledgements: Authors JS and RK acknowledge the financial support from the project Interdisciplinary PhD Studies “Molecular sciences for medicine” (co-financed by the European Social Fund within the Human Capital Operational Programme). Co-authors MN and KS gratefully acknowledge financial support of the Foundation for Polish Science TEAM Programme (PolyMed, TEAM/2008-2/6) co-financed by the European Regional Development Fund.
Conflict of interests: None declared.
REFERENCES
- Samal SK, Dash M, Van Vlierberghe S, et al. Cationic polymers and their therapeutic potential. Chem Soc Rev 2012; 41: 7147-7194.
- Lai WF. in vivo nucleic acid delivery with PEI and its derivatives: current status and perspectives. Expert Rev Med Devices 2011; 8: 173-185.
- Hamano Y. Occurrence, biosynthesis, biodegradation, and industrial and medical applications of a naturally occurring e-poly-L-lysine. Biosci Biotechnol Biochem 2011; 75: 1226-1233.
- Song W, Tang Z, Li M, et al. Tunable pH-sensitive poly(β-amino ester)s synthesized from primary amines and diacrylates for intracellular drug delivery. Macromol Biosci 2012; 12: 1375-1383.
- Shen Y, Tang H, Zhan Y, Van Kirk EA, Murdoch WJ. Degradable poly(β-amino ester) nanoparticles for cancer cytoplasmic drug delivery. Nanomedicine 2009; 5: 192-201.
- Agarwal S, Zhang Y, Maji S, Greiner A. PDMAEMA based gene delivery materials. Materials Today 2012; 15: 388-393.
- Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev 2009; 109: 259-302.
- Itaka K, Ishii T, Hasegawa Y, Kataoka K. Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials 2010; 31: 3707-3714.
- Moreau E, Domurado M, Chapon P, Vert M, Domurado D. Biocompatibility of polycations: in vitro agglutination and lysis of red blood cells and in vivo toxicity. J Drug Target 2002; 10: 161-173.
- Riva R, Ragelle H, Des Rieux A, Duhem N, Jerome C, Preat V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv Polymer Sci 2011; 244: 19-44.
- Ruiz-Herrera J. Fungal Cell Walls: Structure, Synthesis, and Assembly. Boca Raton, CRC Press, 1992.
- Khor E. Chitin: Fulfilling a Biomaterials Promise. Oxford, Elsevier Science and Technology, 2001.
- Xia W, Liu P, Zhang J, Chen J. Biological activities of chitosan and chitooligosaccharides. J Food Hydrocoll 2011; 25: 170-179.
- Knorr D. Use of chitinous polymers in food - a challenge for food research and development. Food Technol 1984; 38: 85-97.
- Kim S, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr Polym 2005; 62: 357-368.
- Majeti NV, Kumar R. A review of chitin and chitosan. React Funct Polym 2000; 46: 1-27.
- Shahidi F, Synowiecki J. Isolation and characterization of nutrients and value-added product from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agric Food Chem 1991; 39: 1527-1532.
- Yamada A, Shibbuya N, Komada O, Akatsuka T. Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci Biotechnol Biochem 1993; 57: 405-409.
- Peniche-Covas C, Alwarez LW, Arguelles-Monal W. The adsorption of mercuric ions by chitosan. J Appl Polym Sci 1987; 46: 1147-1150.
- Jeuniaux C. Chitosan as a tool for the purification of waters. In: Chitin in Nature and Technology. RA Muzzarelli, C Jeuniaux, GW Gooday (eds.). New York, Plenum Press 1986, 551-570.
- Zeng L, Qin C, Wang W, Chi W, Li W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr Polym 2008; 71: 435-340.
- Chae SY, Jang M, Nah J. Influence of molecular weight on oral absorption of water soluble chitosans. J Control Release 2005; 102: 383-394.
- Ventura P. Lipid lowering activity of chitosan, a new dietary integrator. Chitin Enzymol 1996; 2: 55-62.
- Bokura H, Kobayashi S. Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 2003; 57: 721-725.
- Maezaki Y, Tsuji K, Nakagawa Y, et al. Hypocholesterolemic effect of chitosan in adult males. Biosci Biotechnol Biochem 1993; 57: 1439-1444.
- Ormrod DJ, Holmes CC, Miller TE. Dietary chitosan inhibits hypercholesterolaemia and atherogenesis in the apolipoprotein E-deficient mouse model of atherosclerosis. Atherosclerosis 1998; 138: 329-334.
- Allan CR, Hadwiger LA. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp Mycol 1979; 3: 285-287.
- Vinsova J, Vavrikova E. Recent advances in drugs and prodrugs design of chitosan. Curr Pharm Des 2008; 14: 1311–1326.
- Qin CQ, Li HR, Xiao Q, Liu Y, Zhu JC, Du YM. Water-solubility of chitosan and its antimicrobial activity. Carbohydr Polym 2006; 63: 367-374.
- Bernkop-Schnurch A, Dunnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm 2012; 81: 463-9.
- Sumit S, Shikha L, Murthy RS. Potential of chitosan for nose to brain drug delivery. Int J Pharm Sci Rev Res 2012; 16: 47-55.
- Mukhopadhyay P, Mishra R, Rana D, Kundu PP. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: a review. Prog Polym Sci 2012; 37: 1457-1475.
- Sezer AD, Cevher E. Topical drug delivery using chitosan nano- and microparticles. Expert Opin Drug Deliv 2012; 9: 1129-1146.
- Garcia-Fuentes M, Alonso MJ. Chitosan-based drug nanocarriers: where do we stand? J Control Release 2012; 161: 496-504.
- Grenha A. Chitosan nanoparticles: a survey of preparation methods. J Drug Target 2012; 20: 291-300.
- Mengatto LN, Helbling IM, Luna JA. Recent advances in chitosan films for controlled release of drugs. Recent Pat Drug Deliv Formul 2012; 6: 156-170.
- Sandri G, Rossi S, Bonferoni MC, Ferrari F, Mori M, Caramella C. The role of chitosan as a mucoadhesive agent in mucosal drug delivery. J Drug Deliv Sci Technol 2012; 22: 275-284.
- Basaran E, Yazan Y. Ocular application of chitosan. Expert Opin Drug Deliv 2012; 9: 701-712.
- Gulbake A, Jain SK. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin Drug Deliv 2012; 9: 713-729.
- Pahwa R, Saini N, Kumar V, Kohli K. Chitosan-based gastroretentive floating drug delivery technology: an updated review. Expert Opin Drug Deliv 2012; 9: 525-539.
- Ramesan RM, Sharma CP. Modification of chitosan nanoparticles for improved gene delivery. Nanomedicine (Lond) 2012; 7: 5-8.
- Al-Qadi S, Grenha A, Remunan-Lopez C. Chitosan and its derivatives as nanocarriers for siRNA delivery. J Drug Deliv Sci Technol 2012; 22: 29-42.
- Jeon YJ, Kim SK. Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reaction system. J Microbiol Biotechnol 2002; 12: 503-507.
- Peluso G, Petillo O, Ranieri M, et al. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994; 15: 1215-1220.
- Senel S, McClure SJ. Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev 2004; 56: 1467-1480.
- Rodriguez-Merchan EC. Local fibrin glue and chitosan-based dressings in haemophilia surgery. Blood Coagul Fibrinolysis 2012; 23: 473-476.
- Liu SH, He SP, Chiang MT. Effects of long-term feeding of chitosan on postprandial lipid responses and lipid metabolism in a high-sucrose-diet-impaired glucose-tolerant rat model. J Agric Food Chem 2012; 60: 4306-4313.
- Hayashi K, Ito M. Antidiabetic action of low molecular weight chitosan in genetically obese diabetic KK-A y mice. Biol Pharm Bull 2002; 25: 188-192.
- Xu C, Lei C, Meng L, Wang C, Song Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J Biomed Mater Res B Appl Biomater 2012; 100: 1435-1443.
- Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym 2012; 89: 723-739.
- Gao W, Lai JC, Leung SW. Functional enhancement of chitosan and nanoparticles in cell culture, tissue engineering, and pharmaceutical applications. Front Physiol 2012; 3: 321.
- Grolik M, Szczubialka K, Wowra B, et al. Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering. J Mater Sci Mater Med 2012; 23: 1991-2000.
- Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev 2001; 52: 105-115.
- Bacon A, Makin J, Chatfield N. A novel mucosal influenza vaccine. Res Immunol 1998; 149: 98.
- McNeela EA, O’Connor D, Jabbal-Gill I, et al. A mucosal vaccine against diptheria: formulation of cross reacting material (CRM 197) of diptheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vacccine 2001; 19: 1198-1199.
- Le Buanec H, Vetu C, Lachgar A, et al. Induction in mice of anti-Tat mucosal immunity by the intranasal and oral routes. Biomed Pharmacother 2001; 55: 316-320.
- Westerink MA, Smithson SL, Srivastava N, Blonder J, Coeshott C, Rosenthal GJ. ProJuvant (Pluronic F127/chitosan) enhances the immune response to intranasally administered tetanus toxoid. Vaccine 2002; 20: 711-723.
- Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine peanut allergy model. Nat Med 1999; 5: 387-391.
- Kosaka T, Kaneko Y, Nakada Y, Matsuura M, Tanaka S. Effect of chitosan implantation on activation of canine macrophages and polymorphonuclear cells after surgical stress. J Vet Med Sci 1996; 58: 963-967.
- Lim SH, Hudson SM. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res 2004; 339: 313-319.
- Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, Li P. The influence of the cationic of quaternized chitosan on antifungal activity. Int J Food Microbiol 2007; 118: 214-217.
- Sandri G, Rossi S, Bonferoni MC, et al. Buccal penetration enhancement properties of N-trimethyl chitosan: influence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm 2005; 297: 146-155.
- Kotze AF, Luessen HL, de Leeuw BJ, Boer BG, Verhoef JC, Junginger HE. N-trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: in vitro evaluation in intestinal epithelial cells (Caco-2). Pharm Res 1997; 14: 1197-1202.
- Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials 2007; 28: 2220-2232.
- Ji QX, Zhong DY, Lu R, Zhang WQ, Deng J, Chen XG. in vitro evaluation of the biomedical properties of chitosan and quaternized chitosan for dental applications. Carbohydr Res 2009; 344: 1297-1302.
- Toshkova R, Manolova N, Gardeva E, et al. Antitumor activity of quaternized chitosan-based electrospun implants against graffi myeloid tumor. Int J Pharm 2010; 400: 221-233.
- Kaminski K, Szczubialka K, Zazakowny K, Lach R, Nowakowska M. Chitosan derivatives as novel potential heparin reversal agents. J Med Chem 2010; 53: 4141-4147.
- Kaminski K, Zazakowny K, Szczubialka K, Nowakowska M. pH-sensitive genipin-cross-linked chitosan microspheres for heparin removal. Biomacromolecules 2008; 9: 3127-3132.
- Chou TC, Fu E, Wu CJ, Yeh JH. Chitosan enhances platelet adhesion and aggregation. Biochem Biophys Res Commun 2003; 302: 480-483.
- Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res 1997; 34: 21-28.
- Okamoto Y, Shibazaki K, Minami S, Matsuhashi A, Tanioka S, Shigemasa Y. Evaluation of chitin and chitosan on open wound healing in dogs. J Vet Med Sci 1995; 57: 851-854.
- Ueno H, Yamada H, Tanaka I, et al. Accelerating effects of chitosan for healing and early phase of experimental open wound in dogs. Biomaterials 1999; 20: 1407-1414.
- Brandenberg G, Leibrock LG, Shuman R, Malette WG, Quigley H. Chitosan: a new topical hemostatic agent for diffuse capillary bleeding in brain tissue. Neurosurgery 1984; 15: 9-13.
- Klokkevold PR, Fukayama H, Sung EC, Bertolami CN. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J Oral Maxillofac Surg 1999; 57: 49-52.
- Chmiel B, Grabowska-Bochenek R, Piskorska D, Cierpka L. Diet-induced hypercholesterolaemia causes increase of red blood cell deformability in rabbits. Chirurg Pol 2002; 4: 107-112.
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992; 258: 468-471.
- Plump AS, Smith JD, Hayek T, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992; 71: 343-353.
- Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol 2004; 55: 503-517.
- Smith DD, Tan X, Tawfik O, Milne G, Stechschulte DJ, Dileepan KN. Increased aortic atherosclerotic in female apolipoprotein E-null mice is associated with elevated thromboxane A2 and decreased prostacyclin production. J Physiol Pharmacol 2010; 61: 309-316.
- Jawien J, Korbut R. The current view on the role of leukotrienes in atherogenesis. J Physiol Pharmacol 2010; 61: 647-650.
- Toton-Zuranska J, Gajda M, Pyka-Fosciak G, et al. AVE 0991 - angiotensin-(1-7) receptor agonist, inhibits atherogenesis in apoE-knockout mice. J Physiol Pharmacol 2010; 61: 181-183.
- Pawlowska M, Gajda M, Pyka-Fosciak G, et al. The effect of doxycycline on atherogenesis in ApoE-knockout mice. J Physiol Pharmacol 2011; 62: 247-250.
- Ngo DN, Qian ZJ, Je JY, Kim MM, Kim SK. Aminoethyl chitooligosaccharides inhibit the activity of angiotensin converting enzyme. Process Biochem 2008; 43: 119-123.
- Parra-Barraza H, Burboa MG, Sanchez-Vazquez M, Juarez J, Goycoolea FM, Valdez MA. Chitosan-cholesterol and chitosan-stearic acid interactions at the air-water interface. Biomacromolecules 2005; 6: 2416-2426.
- Xu G, Huang X, Qiu L, Wu J, Hu Y. Mechanism study of chitosan on lipid metabolism in hyperlipidemic rats. Asia Pac J Clin Nutr 2007; 16 (Suppl 1): 313-317.
A c c e p t e d : April 2, 2014