EXENDIN-4, AN ANALOGUE OF GLUCAGON-LIKE PEPTIDE-1, ATTENUATES HYPERALGESIA THROUGH SEROTONERGIC PATHWAYS IN RATS WITH NEONATAL COLONIC SENSITIVITY
2Department of Gastroenterology, the Affiliated Hospital of Taishan Medical University, Taian, China
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the common functional gastrointestinal disorders, with a prevalence of 10–15% worldwide (1). IBS is primarily characterized by chronic abdominal pain or discomfort, and altered bowel habits in the absence of evidence of organic diseases. The mechanisms underlying this condition are not well understood. Accumulating evidence demonstrates that it is related to visceral hypersensitivity, abnormal gastrointestinal motility, inflammation, and/or infection of the gut (2). Among these, visceral hypersensitivity is recognized as a common biological feature of IBS, which manifests as pain associated with bowel disturbances (3).
Currently, various approaches have been proposed to treat IBS, but there is no satisfactory management available for IBS. Recently, Hellstrom et al. demonstrated that glucagon-like peptide-1 (GLP-1) analogue ROSE-010 provided effective pain relief in IBS patients (4). However, the mechanism remains largely unknown. GLP-1 is a gut-derived hormone released from intestinal L-cells following a meal (5). GLP-1 is considered to inhibit glucagon release, as well as potentiate glucose-dependent insulin release (6). Moreover, GLP-1 is associated with other potentially beneficial effects related to gastric emptying, small bowel motility and wall tone in healthy subjects and rodents (7-10). GLP-1 acts on the GLP-1 receptor (GLP-1R), a specific trans-membrane G-protein receptor which has been found in nodose neurons and the gastrointestinal tract (11, 12). In vivo, GLP-1 has a short plasma half-life ranging from 1 to 2 min because of its’ rapid proteolytic degradation, particularly by the serine protease dipeptidyl peptidase-IV (DPP-IV). The proteolytic degradation of GLP-1 presents a considerable barrier to its therapeutic use, although recently, a range of analogues of GLP-1 such as exenatide (exendin-4) have been developed.
The gastrointestinal mucosa receives a variety of stimuli that can impart various kinds of signal to the central nervous system and lead to sensory deregulation. Our previous study showed that GLP-1R was primarily expressed in the colon mucosal layer of Sprague-Dawley rats (13). Our findings highlighted that the GLP-1R co-localized with 5-HT in colon mucosa. Based on this specific distribution of GLP-1R in rats’ colon mucosa and GLP-1 analogue relieving pain in IBS patients, we hypothesized that GLP-1 analogue might be involved in ameliorating visceral hypersensitivity.
It has been ascertained that serotonin (5-hydrodytryptamine, 5-HT), released by enterochromaffin (EC) cells, plays a critical role in the regulation of gastrointestinal motility, secretion, and sensation (14, 15). Alterations in 5-HT biosynthesis, release, or uptake may contribute to abnormal gastrointestinal function. Here, we investigate how the GLP-1 analogue exendin-4 effect on visceral hypersensitivity and the association of exendin-4 with serotonergic pathway.
MATEALS AND METHODS
Animals
Male Sprague-Dawley rats obtained from Beijing Vital River Laboratories Animal Technology Co., Ltd were used in this study. All of the procedures used in this study were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University and were in accordance with the guidelines of the International Association for the Study of Pain.
Rats were obtained as litters of 4-day-old pups and divided into two groups: the colonic sensitized group with acetic acid infusion (n=40), and the control group (n=16). 10-day-old rat pups received an intra-colonic infusion of a 0.2 ml acetic acid (0.5% in saline salt solution) into the colon (roughly, 2 cm from the anus), while controls received an equal volume of saline, as described previously (16, 17). The experiments were performed until the animals were 8–12 weeks of age.
Measurement of visceral sensitivity
Visceral sensitivity was measured by grading the response to colorectal distention (CRD) as previously described (16, 17). In brief, 8-week old rats underwent anesthesia with pentobarbital sodium 50 mg/kg intraperitoneally for 15 min, one pair of electrodes were implanted into the lateral abdominal wall of the rats, and exteriorized at the back of the neck for recording EMG.. The rats were allowed to recover from the surgery for a week. Rats were fasted overnight (with free access to water) before the experiment. On the day of the experiment, rats were sedated with 1–1.5% isoflurane and a 5 cm flexible balloon (made from the finger of a latex glove) attached to Tygon tubing was inserted 8 cm into the rectum and descending colon through the anus, then fixed in suitable place. The rat was placed in small Lucite cubicles (20×8×8 cm) (Medical Instrument Co., Ltd. Ningbo Madain, Zhejiang, China) and allowed to adapt for 30 minutes. CRD was performed by inflating the balloon to constant pressure measured using a sphygmomanometer connected to a pressure transducer. The balloon was inflated to 20, 40, 60 and 80 mmHg for a 20-second stimulation period followed by a 2-min rest (16, 17). Behavioral responses to CRD were assessed by visual observation of the AWR by blinded observer. The assignment of an AWR score is as follows: 1 = normal behavior without response; 2 = contraction of abdominal muscles; 3 = lifting of abdominal wall; 4 = body arching and lifting of pelvic structures (16). At the same time, the EMG was continuously recorded during CRD at different pressures. The EMG measurement was performed as described previously (16), 20-second distention was followed by 2-minute rest during CRD at pressures of 20, 40, 60, and 80 mmHg. The EMG signal was amplified (×1000) using an AC/DC differential amplifier (3000; A-M Systems Inc., Sequim, WA, USA) with a band-pass filter (low frequency 30 Hz, high frequency 3 kHz). The area under the curve for the EMG signal (during each 20 seconds of distention, plus 10-second post-distention period, for a total of 30 seconds) was simultaneously recorded on a PowerLab data acquisition system (8SP; AD Instruments, Sydney, Australia) and stored on a hard disk until analysis. The net value for each distention was calculated by subtracting the baseline value derived from the average area under the curve (30-second interval) for the 2-minute pre-distention period as previously established (16).
Evaluation of colon inflammation/damage
Colonic sensitized rats and matched controls were randomly selected. Colonic tissue specimens were collected for H&E-staining. An expert pathologist scored the inflammatory grade on H&E stained sections, as previously described (18).
Detection of glucagon-like peptide-1 level in plasma
Rats fasting for 12 hours were sedated with ether, and then approximately 1 mL blood sample from the orbital canthus vein plexus was collected. Blood samples were quickly mixed with a DPP-4 inhibitor (Millipore, Massachusetts, USA). Following centrifugation at 4000 g for 10 min at 4°C, the supernatants were stored at –20°C until analysis. Plasma GLP-1 levels were measured by means of a standardized ELISA kit assay (Linco). The concentration of active GLP-1 was proportional to the fluorescence generated by umbelliferone, which is produced by alkaline phosphatase-catalyzed hydrolysis of methyl umbelliferyl phosphate (conjugated with anti-GLP-1 monoclonal antibodies). Samples (100 µl/each sample) were added to each assay well. This ELISA featured a working range between 2 and 100 pM.
Analysis of the effect of exendin-4 on visceral sensitivity
Colonic sensitized rats were randomly divided into four groups (each group n=8) as follows: vehicle group (saline), different doses of exendin-4 group (exendin-4 at a dose of 1.0, 5.0 and 10 µg kg–1), were intraperitoneally injected once a day for 7 days respectively (19). The EMG was recorded and the AWR scores were assessed one week after seven-day intraperitoneal injection of exendin-4. After completing visceral sensitivity assessment, rats were deeply anesthetized with ketamine. Blood samples were collected and segments of distal colon (roughly, 5–6 cm from anus) were dissected and stored at –80°C until used.
Measurement of 5-HT levels in colon tissue and plasma
A standardized enzyme immunoassay kit (Millipore, Massachusetts, USA) was used to detect 5-HT levels in colon tissues and plasma. The blood samples were collected from fasting rats as preciously described, following centrifugation at 4000 g for 10 min at 4°C, the supernatants were stored at –80°C until analysis. For the preparation of colon tissues, briefly, a segment of distal colon (0.1 g) was homogenized in a 0.2 M perchloric acid solution, and subsequently centrifuged at 10,000×g for 5 min. The supernatant was neutralized with an equal volume of borate buffer (1M, pH = 9.25) and centrifuged at 10,000×g for 1 min. After acylation using N-hydroxysuccinimide ester-succinyl glycinamide, the samples were incubated in antibody-coated wells in the presence of acetylcholinesterase-acylated-serotonin conjugate. After washing the wells to remove non-bound components, the acetylcholinesterase activity was measured as previously described by Chauveau et al. (20). A chromogenic substrate was added to the wells and the absorbance was measured at 405 nm. 5-HT levels were expressed as ng/100 mg wet weight of tissue.
Western blot for serotonin transporter and tryptophan hydroxylase-1
Colonic tissue samples (100 mg) were homogenized in a buffer containing 25 mmol/L Tris-HCl (pH=7.5), 5 mmol/L EDTA, 5 mmol/L EGTA, 0.5 mmol/L PMSF, 25 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mmol/L sodium vandate. The crude protein homogenates were immersed in sample buffer and boiled for 5 min. Samples were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (10 mg/lane), and then transferred to a polyvinylidene difluoride membrane for immunoblotting. The membranes were incubated in blocking buffer (5% non-fat dry milk in Tween/TRIS-buffered salt solution, TTBS) containing rabbit anti-SERT monoclonal antibody (1:500, Millipore, Massachusetts, USA, AB9726) or rabbit anti-TPH-1 monoclonal antibody (1:500, Abcam Ltd. Chicago, USA, AB52954) overnight at 4°C. After multiple washes, the membranes were incubated for 1.5 hours at room temperature with secondary antibodies (dilution: 1:4000) linked to horseradish peroxidase (HRP). The immunopositive proteins on the membrane were detected by enhanced chemiluminescence detection kit (ECL, Millipore, Bedford, USA). Target bands and the band for GAPDH were quantified by Tianneng GIS gel image processing system for optical density analysis.
Reverse transcription-polymerase chain reaction for serotonin transporter and tryptophan hydroxylase-1 mRNA
Total RNA from frozen rat colon samples were extracted using a TRIzol RNA extract reagent (Invitrogen, Carlsbad, CA). RNA (1 µg) was subjected to first-strand cDNA synthesis using oligo(dT) 18 and ThermoScript Reverse transcriptase (Invitrogen) in a 10 µl total reaction volume. Amplification of cDNA (1 µl) was carried out using GoTaq Green Master mix (Promega, Madison, WI) with SERT or TPH-1-specific sense and anti-sense primers. PCR reaction of SERT mRNA was performed as follows: denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 52°C for 30 s and extension at 72°C for 30 s (the final extension for 10 min at 72°C). PCR reaction of TPH-1 mRNA was performed as follows: denaturation at 95°C for 7 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 40 s and extension at 72°C for 40 s (the final extension for 10 min at 72°C). The primers (Invitrogen) used were the following:
SERT: (forward) 5’-AGGTGGCCAAAGACGCAGGC-3’,
(reverse) 5’-GCTGGCCCCGTGGCATACTC-3’;
TPH-1: (forward) 5’-TCCGAACTCGACGCGGACCA-3’,
(reverse) 5’-CTCCCTGCAGGCGTGGGTTG-3’;
β-actin: (forward) 5‘-TGGAGAAGAGCTATGAGCTGCCTG-3’,
(reverse) 5‘-GTGCCACCAGACAGCACTGTGTTG-3’.
The amplified PCR products were subjected to electrophoresis using a 1.5% agarose gel and stained with ethidium bromide. All amplifications were executed at least three times.
Statistical analysis
Data were expressed as mean ±S.D. Analyses were performed using SPSS 17.0 software (SPSS Science, Chicago, IL). Friedman analysis of variance (ANOVA) was used to determine whether AWR behavioral grades changed with different distention pressure in each experimental group. Median scores of AWR at each distention pressure were compared between different treatment groups by a Mann-Whitney rank sum test. EMG data were analyzed by two-way repeated measures ANOVA with distention pressure as the repeated measure. Other data were evaluated using one-way ANOVA followed by Bonferroni post-test comparisons. P<0.05 was considered statistically significant.
RESULTS
Acetic acid treatment of colon of neonatal rats produces visceral hypersensitivity
To analyze whether a mild chemical irritation of colon of neonatal rats with 0.5% acetic acid would produce visceral hypersensitivity in adults, rats aged 8 weeks were tested for sensitivity to CRD. Compared with controls, neonatal acetic acid-treated rats exhibited higher median AWR scores at all distention pressures (Fig. 1A). These increases in neonatal acetic acid-treated rats were significantly higher at 40 mmHg (2.75±0.52 vs. 1.33±0.51, P=0.007) and 60 mmHg (3.25±0.52 vs. 2.33±0.53, P=0.014). EMG activity, measured in response to graded CRD, was significantly higher in neonatal acetic acid-treated rats compared to controls at 40 mmHg (245.15±43.87 vs. 125.15±37.48, P<0.0001), 60 mmHg (383.00±38.79 vs. 177.80±29.77, P=0.0014) and 80 mmHg (461.50±40.33 vs. 289.80±35.87, P=0.0004) (Fig. 1B, 1C). These data suggest that acetic acid treatment of the colon in neonatal rats produces a persistent visceral hypersensitivity.
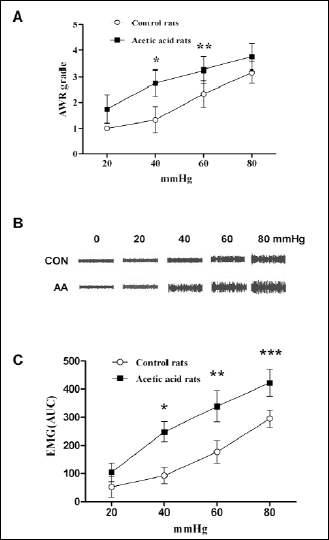 |
Fig. 1. Effect of neonatal acetic acid treatment on visceral sensitivity to CRD at 8 weeks of age. (A): AWR scores in response to CRD at different pressures; neonatal saline treated, (n= 10), neonatal acetic acid treated, (n= 10), *P=0.007, **P=0.014. (B): Examples of EMG activities recorded from control (CON) and neonatal acetic acid-sensitized (AA) treated, response to 0, 20, 40 and 80 mmHg distention pressures. (C): Representative EMG traces in the external oblique muscle in response to grading CRD. EMG is expressed in units of V/s; each point represents the mean of 10 rats from 3 independent experiments. Neonatal saline treated, (n=10); neonatal acetic acid treated, (n=10). *P<0.001, **P=0.0014, ***P=0.0004. Analysis performed using two way ANOVA with repetitive measures, F=32.697, P<0.001. |
No evidence of inflammation or structural abnormalities was found in either controls or acetic acid-treated rats (Fig. 2).
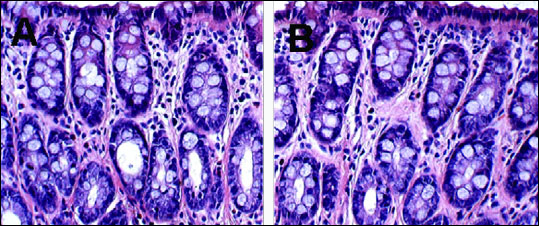 |
Fig. 2. Evaluating of colons from neonatal acetic acid-treated rat. H&E-stained sections were scored for evaluating inflammation. (A): neonatal saline treatment (B): neonatal acetic acid treatment (×400 magnification). |
The plasma levels of glucagon-like peptide-1 in rats with visceral hypersensitivity
To determine whether the levels of GLP-1 in plasma were different between colonic sensitized rats and control rats, we measured the plasma levels of GLP-1 in two groups. We found that the plasma levels of GLP-1 in colonic sensitized rats were significantly decreased compared with the controls (4.453±0.520 vs. 6.657±1.062 pmol/L, P=0.0012, Fig. 3).
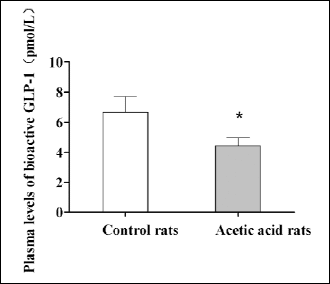 |
Fig. 3. The plasma level of bioactive GLP-1 in rats with visceral hypersensitivity. The plasma level of bioactive GLP-1 was detected by ELISA method. Control rats, n=10; acetic acid treated rats, n=10; *P=0.0012, t-test. Bioactive GLP-1: GLP-1(7–36) amide. |
Exendin-4 decreased visceral sensitivity in rats with visceral hypersensitivity
To determine the effect of exendin-4 on visceral hypersensitivity, we observed AWR scores and EMG responses to CRD at different pressures in colonic sensitized rats treated with various doses of exendin-4. As shown in Fig. 4A, AWR scores during CRD at 40, 60 mmHg significantly decreased with exendin-4 at dose of 5 µg/kg compared with vehicle group (1.56±0.53 vs. 2.33±0.50, P=0.013; 2.23±0.45 vs. 3.0±0.5, P=0.008; at 40, 60 mmHg respectively). Similar findings were observed with exendin-4 at dose of 10 µg/kg. The AWR scores during CRD at 20, 40, 60, 80 mmHg were also significantly reduced with exendin-4 at 10 µg/kg compared with the vehicle group (0.667±0.50 vs. 1.889±0.782, P=0.004; 1.33±0.5 vs. 2.33±0.50, P=0.001; 2.34±0.51 vs. 3.0±0.5 P=0.031; 2.57±0.53 vs. 3.78±0.44 P<0.001; respectively, Fig. 4A). Similar to the findings from AWR assessment, exendin-4 at a dose of 5 mg/kg significantly reduced the EMG responses to CRD at 40, 60, 80 mmHg distention pressures (compared with vehicle group, P=0.004 at 40 mmHg, P<0.001 at 60 and 80 mmHg, Fig. 4B and 4C). Similar findings were observed with exendin-4 at a dose of 10 µg/kg. Exendin-4 at 10 µg/kg significantly reduced visceral hypersensitivity with decreases in the AUC of EMG during CRD at 40, 60, 80 mmHg compared with vehicle group (P=0.004 at 40 mmHg, P<0.001 at 60 and 80 mmHg, Fig. 4B and 4C).
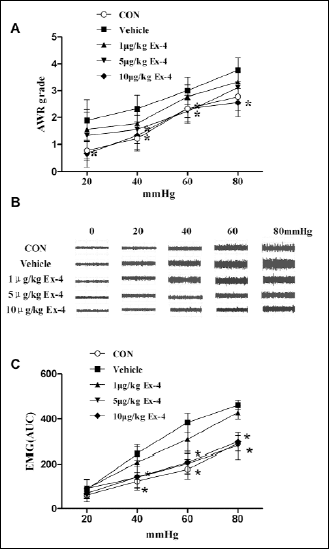 |
Fig. 4. Effect of exendin-4 on visceral sensitivity to CRD after consecutive 7 days treatment. (A): Exendin-4 at all doses reduced AWR responses to CRD at different pressures (compared with vehicle group, for 5 µg kg–1: at 40 mmHg P=0.013, at 60 mmHg P=0.008; for 10 µg kg–1: at 20 mmHg P=0.004, at 40 mmHg P=0.001, at 60 mmHg P=0.031, at 80 mmHg P<0.001). (B): Representative EMG traces with vehicle or exendin-4 at all doses in an acetic acid-treated rat and controls. (C): In acetic acid-treated rats, exendin-4 at all doses reduced EMG responses to CRD at different pressures (compared with vehicle group, at the dose of 5.0 µg/kg: at 40 mmHg P=0.004, at 60 and 80 mmHg P<0.001; at the dose of 10 µg/kg: at 40 mmHg P=0.004, at 60 and 80 mmHg P<0.001). Analysis performed using two-way repeated measures ANOVA, F=12.150, P<0.001. |
Effect of exendin-4 on 5-HT level in rats with visceral hypersensitivity
To determine the effect of exendin-4 on 5-HT levels in colonic sensitized rats, the 5-HT concentrations in colon tissue and plasma were measured. 5-HT concentrations in colon and plasma from colonic sensitized rats were increased compared to controls (P=0.005 in colon, P=0.047 in plasma). The 5-HT colon concentration was 2.894±0.232, 2.343±0.447, 2.175±0.360 ng/100 mg, respectively, with exendin-4 at doses of 1.0 µg/kg, 5.0 µg/kg, or 10.0 µg/kg, which significantly decreased compared with vehicle group (3.607±0.628 ng/100mg, P=0.025 at dose of 1.0 µg/kg, P<0.001 at dose of 5.0 µg/kg and 10.0 µg/kg, Fig. 5A). 5-HT plasma levels were also significantly decreased in colonic sensitized rats treated with exendin-4 at a dose of 5 µg/kg, 10 µg/kg respectively, compared with colonic sensitized rats without exendin-4 (4.366±0.535, 4.168±0.436 ng/ml vs. 5.325±0.784 ng/ml, P=0.007 at 5 µg/kg, P=0.001 at 10 µg/kg, Fig. 5B). But 5-HT plasma level was no significantly difference between the colonic sensitized rats with exendin-4 at 1.0 µg/kg and vehicle group.
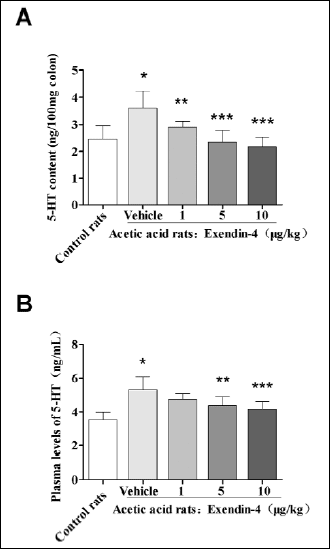 |
Fig. 5. The 5-HT concentration in colon and plasma in acetic acid treated rats and controls. (A): The 5-HT concentration in colon was significantly increased in acetic acid treated rats (vehicle vs. control, *P=0.005).After treatment with exendin-4 at all doses, 5-HT contents in colon were significantly decreased (compared with vehicle group, **P=0.025, ***P<0.001; n=10/group, ANOVA). (B): The 5-HT plasma concentration was significantly increased in acetic acid treated rats (compared with control, *P=0.047); 5-HT plasma concentrations in acetic acid treated rats were significantly decreased after treatment with exendin-4 at all doses (compared with vehicle group, **P=0.007, ***P=0.001; n=10/group ANOVA). |
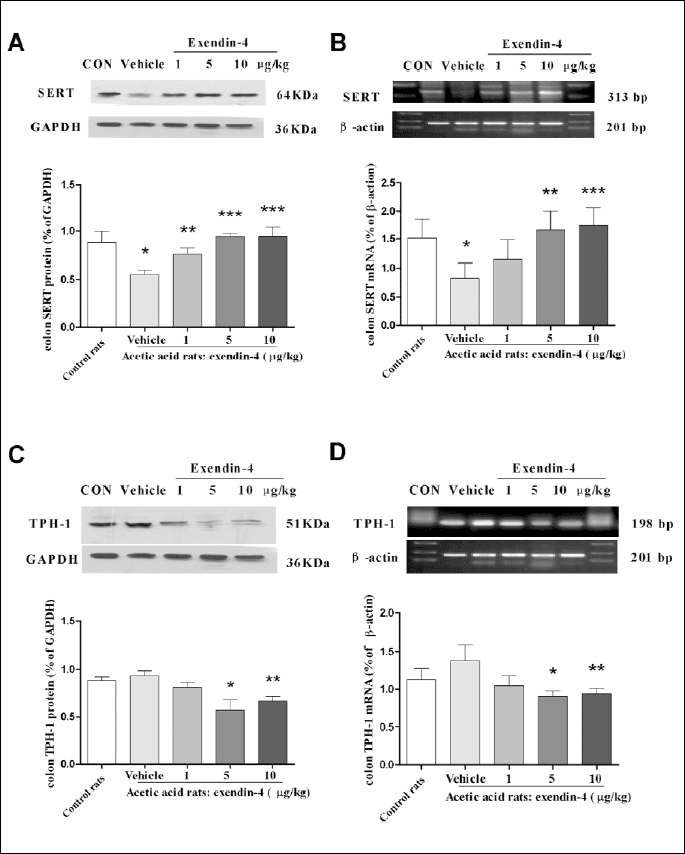
The effect of exendin-4 on the expressions of tryptophan hydroxylase-1 and for serotonin transporter in colonic sensitized rats
To observe TPH-1 and SERT expressions in colonic sensitized rats, we measured mRNA and protein levels by RT-PCR and western blot, respectively. The levels of SERT protein expression were significantly decreased in colonic sensitized rats compared to controls (P<0.001, Fig. 6A), similar to the findings of SERT mRNA expression (P=0.049, Fig. 6B). However, the SERT protein expression in colonic sensitized rats treated with exendin-4 (1 mg/kg, 5 µg/kg, 10 µg/kg) was significantly increased compared with vehicle control (without exendin-4 treatment) (P=0.025 at 1 µg/kg, P<0.001 at 5 µg/kg and 10 µg/kg, Fig. 6A). At the mRNA level the SERT expression in colonic sensitized rats treated with exendin-4 (5 µg/kg or 10 µg/kg) was also significantly increased compared with vehicle control (P=0.0293 at 5 µg/kg, P=0.0194 at 10 µg/kg, Fig. 6B).The levels of TPH-1 mRNA and protein were not significantly different between colonic sensitized rats and control rats (Fig. 6C and 6D). However, the levels of TPH-1 protein expression in colonic sensitized rats treated with exendin-4 at a dose of 5 µg/kg or 10 µg/kg were significantly decreased compared to colonic sensitized rats which were not exposed to exendin-4 (P=0.001 at 5 µg/kg, P=0.009 at 10 µg/kg, Fig. 6C), similar to the findings of TPH-1 mRNA expression (P=0.015 at 5 µg/kg, P=0.025 at 10 µg/kg Fig. 6D).
DISCUSSION
Gut hormones such as motilin and ghrelin are involved in the formation of MMCs, while others (gastrin, cholecystokinin, serotonin) are involved in the generation of spikes upon the slow waves in the small intestine and colon. Additionally, melatonin secreted by neuroendocrine cells of the gut mucosa plays an important role in the internal biological clock, related to food intake and the myoelectric rhythm (21).
GLP-1 is also a gut hormone, which secreted by intestinal L-cells located in the mucosa of duodenum, small intestine and colon (5), following cleavage from a pro-glucagon precursor molecule by post-translational processing. It has been ascertained that GLP-1 could inhibit gastrointestinal motility. Furthermore, a randomized, double-blinded, prospective clinical trial was carried out by Hellstrom et al. (4) to investigate the effect of ROSE-010 in 166 IBS patients suffering from IBS-related pain. This study showed that the GLP-1 analogue ROSE-010 was twice as effective as placebo in terms of total pain relief response in IBS patients affected by pain. IBS-associated pain is usually accompanied by abnormal bowel function, which is believed to result in part from disordered smooth muscle activity, coupled with sensory deregulation, leading to visceral hypersensitivity (22).
The present study demonstrates that GLP-1 analogue exendin-4 produced an analgesic effect on chronic visceral hypersensitivity induced by neonatal acetic acid treatment. Our findings showed that exendin-4 could reverse the magnified visceromotor responses to CRD in colonic sensitized rats. Moreover, the elevated 5-HT levels in colon and plasma in colonic sensitized rats decreased after treatment with exendin-4. These effects may be mediated in part by activation of 5-HT pathways in the colon. These findings agree with previous reports (4), and provide additional support to the potential application of GLP-1 for the treatment of visceral pain.
Previously, we found that the administration of exogenous GLP-1 or exendin-4 inhibits colonic circular muscle contraction, especially in an IBS-C group, suggesting that GLP-1 may relieve pain by improving abnormal GI motility (23). In the present study, we investigated the effects of GLP-1 analogue, exendin-4, on visceral hypersensitivity.
Visceral hypersensitivity is currently the leading hypothesis that may explain painful symptoms experienced by IBS patients (3). Most IBS patients present high sensitivity to pain, or discomfort during CRD (24). The exact location of abnormality of visceral pain processing is still unknown. Theories of its etiology have range widely from disturbance of gut mucosa to a complex disordered interaction between the digestive tract and nervous systems. The effects of chemical compounds on visceral pain at a peripheral or a central level should be of interest (25).
In this study, both AWR and EMG methods were used to assess visceral sensitivity in a neonatal acetic acid-induced colonic sensitization model. This model has been largely shown to be reproducible, and stable, without colonic inflammation in adult rats (16). We found that the plasma level of GLP-1 in the colonic sensitization model decreased compared with the control. This finding suggests that GLP-1 may be involved in visceral hypersensitivity. Furthermore, we analyzed whether exendin-4 affected visceral hypersensitivity in the colonic sensitization rats. Both AWR and EMG data showed that the exendin-4 at the dose of 5 mg/kg was nearly as effective as the dose of 10 µg/kg in ameliorating visceral hypersensitivity. This suggests that the dose of 5 µg/kg of exendin-4 may be the optimal dose to ameliorate visceral hypersensitivity. The dose of 1 µg/kg only led to a non-significant trend of mitigation of visceral hypersensitivity. These findings suggest that exendin-4 dose-dependently decreased visceral hypersensitivity in colonic sensitized rats.
5-HT is an important signaling molecule in the gastrointestinal tract. Recently, various studies revealed that 5-HT is predominantly produced in the EC and interneurons, and is involved in the control of GI secretion, motility, and visceral perception (14). To date, several studies have indicated that 5-HT plays an important role in the pathophysiology of visceral hypersensitivity. Here, we found that the 5-HT levels, both in colon tissue and in plasma, were significantly decreased in colonic sensitized rats after treatment with exendin-4. This finding suggests that GLP-1 analogue exendin-4 may attenuate visceral hypersensitivity in colonic sensitized rats through a serotonergic pathway.
It is now known that synthetic and metabolic events are tightly controlled by 5-HT availability and effectiveness. 5-HT is synthesized by the actions of the rate-limiting enzyme TPH, including TPH-1 and TPH-2. TPH-1 is found in EC cells, mast cells, and pinealocytes (26, 27), whereas TPH-2 is restricted to central and enteric neurons (28). The TPH-1-dependent 5-HT in EC cells is released in response to a variety of signals, including increased intraluminal pressure and acid, which initiate peristaltic and secretory reflexes (29). 5-HT can also stimulates extrinsic primary afferent neurons to convey noxious signals, including pain and nausea, to the central nervous system (30, 31). Within the enteric nervous system, TPH-2-dependent 5-HT is a neurotransmitter that mediates excitatory pathways regulating motility and secretion (2). After release of 5-HT from EC cells or neurons, it is inactivated by uptake into enterocytes or neurons through SERT, followed by conversion to 5-hydroxyindoleacetic acids which are subsequently excreted in the urine. Enterocytes express SERT, which terminate the action of 5-HT by removing it from the interstitial space (32). Abnormalities in serotonergic signaling, including altered expression of TPH-1 and SERT, and altered release of 5-HT, have been implicated in IBS pathogenesis (33, 34). In our study, the expression of SERT was significantly reduced in colonic sensitized rats compared to controls, which is consistent with other studies (34-36). However, there was no change in TPH-1 expression between colonic sensitized rats and the controls counterparts. Exendin-4 dose-dependently increased SERT expression in colonic sensitized rats. This effect was in contrast to findings in other studies (29, 35). A possible explanation for this discrepancy is due to differences in species or the anatomical region studied. Interestingly, the expression of TPH-1 in colon tissue was significantly decreased in colonic sensitized rats after treatment with exendin-4 for one week. These results indicated that GLP-1 analogue exendin-4 reduced the level of 5-HT in the colon through inhibiting 5-HT synthesis and promoting the reuptake of 5-HT, which attenuates visceral hypersensitivity in rats with neonatal colon sensitivity. The study from Chojnacki et al. showed that tianepine (serotonin reuptake enhancer) diminished IBS symptoms (37). This suggests that GLP-1 influences visceral hypersensitivity in part through promoting serotonin reuptake like serotonin reuptake enhancer.
Since the major source of bio-available 5-HT in the human body is located in the bowel, primarily in epithelial cells (38), we carried out double-immunofluorescence staining and found that 5-HT and GLP-1R co-localize. GLP-1 analogue exendin-4 affected 5-HT uptake, likely by coupling with the GLP-1 receptor in intestinal epithelial cells. In addition, the expression of TPH-1 in colon tissue was significantly decreased in colonic sensitized rats after treatment with exendin-4, this indicated that GLP-1 analogue exendin-4 affected 5-HT release, likely by coupling with the GLP-1 receptor in EC cells. Further studies are needed to investigate the molecular mechanisms of GLP-1 analogues on colon 5-HT synthesis and reuptake.
In conclusion, GLP-1 analogue exendin-4 dose-dependently ameliorates visceral hypersensitivity and decrease 5-HT level by inhibiting 5-HT synthesis and promoting the reuptake of 5-HT in colonic sensitized rats. Further studies are warranted to better understand the molecular mechanisms responsible for amelioration of visceral hypersensitivity by GLP-1 analogues.
Author’s contribution: Yang Yan participated in the study concept and design; acquisition, analysis and interpretation of data, drafting the manuscript. Cui Xiufang participated in the acquisition of data, statistical analysis and writing the article. Chen Yan participated in the acquisition of data. Wang Ying participated analyzed the data and reading the manuscript. Li Xueliang participated in the technical and material support. Lin Lin participated in the interpretation of the data. Zhang Hongjie participated in the study concept and design, study supervision, interpretation of data, and critical revision of the manuscript for important intellectual content.
Yang Yan and Cui Xiufang contributed equally to this work.
Acknowledgements: This work was supported by the National Natural Science Foundation of China, No 81270469 and the Key Medical Personnel of Jiangsu Province (no. RC2011063).
Conflict of interest: None declared.
REFERENCES
- Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am 2005; 34: 189-204.
- Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut 2001; 49: 743-745.
- Heaton KW, Ghosh S, Braddon FE. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut 1991; 32: 73-79.
- Hellstrom PM, Hein J, Bytzer P, Bjornsson E, Kristensen J, Schambye H. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther 2009; 29: 198-206.
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409-1439.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132: 2131-2157.
- Hellstrom PM, Naslund E, Edholm T, et al. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil 2008; 20: 649-659.
- Schirra J, Nicolaus M, Roggel R, et al. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 2006; 55: 243-251.
- Hellstrom PM, Smithson A, Stowell G, et al. Receptor-mediated inhibition of small bowel migrating complex by GLP-1 analog ROSE-010 delivered via pulmonary and systemic routes in the conscious rat. Regul Pept 2012; 179: 71-76.
- Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellstrom PM. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. J Clin Invest 1998; 102: 764-774.
- Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996; 137: 2968-2978.
- Rotondo A, Amato A, Lentini L, Baldassano S, Mule F. Glucagon-like peptide-1 relaxes gastric antrum through nitric oxide in mice. Peptides 2011; 32: 60-64.
- Li ZY, Chen Y, Lin L, Wang MF, Hao B, Zhang HJ. Changes of serum glucagon-like peptide-1 receptor in rat models of irritable bowel syndrome subtypes. Chin J Dig 2012; 32: 249-255.
- Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut 2002; 51 (Suppl. 1): i81-i86.
- Tack J, Sarnelli G. Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut 2002; 51 (Suppl. 1): i77-i80.
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 2007; 132: 615-627.
- Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 2008; 57: 1230-1237.
- Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol 1995; 269: G119-G125.
- Bojanowska E, Radziszewska E. Combined stimulation of glucagon-like peptide-1 receptor and inhibition of cannabinoid CB1 receptor act synergistically to reduce food intake and body weight in the rat. J Physiol Pharmacol 2011; 62: 395-402.
- Chauveau J, Fert V, Morel AM, Delaage MA. Rapid and specific enzyme immunoassay of serotonin. Clin Chem 1991; 37: 1178-1184.
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 2011; 62: 139-150.
- Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2008; 6: 772-781.
- Yan Chen, Zhengyang Li, Yan Yang, Lin Lin, Hongjie Zhang. Role of glucagon-like peptide-1 in the pathogenesis of experimental irritable bowel syndrome rat models. Int J Mol Med 2013; 31: 607-613.
- Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 1990; 98: 1187-1192.
- Bonaz B. Visceral sensitivity perturbation integration in the brain-gut axis in functional digestive disorders. J Physiol Pharmacol 2003; 54 (Suppl. 4): 27-42.
- Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA 2003; 100: 13525-13530.
- Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003; 299: 76.
- Gutknecht L, Waider J, Kraft S, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm 2008; 115: 1127-1132.
- Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 1958; 140: 381-407.
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol 1998; 506: 551-561.
- Grundy D, Blackshaw LA, Hillsley K. Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity. Dig Dis Sci 1994; 39 (Suppl. 12): 44S-47S.
- Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci 2001; 21: 6348-6361.
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil 2005; 17: 863-870.
- Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004; 126: 1657-1664.
- Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol 2012; 302: G1053-G1060.
- Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 2006; 130: 34-43.
- Chojnacki C, Walecka-Kapica E, Mokwinska M, et al. Influence of tianeptine on melatonin homeostasis and psychosomatic symptoms in patients with irritable bowel syndrome. J Physiol Pharmacol 2013; 64: 177-183.
- Erspamer V. Occurrence and distribution of 5-hydroxytryptamine (enteramine) in the living organism. Z Vitam Horm Fermentforsch 1957; 9 :74-96.
A c c e p t e d : May 20, 2014