EVALUATION OF HYPERPROLACTINAEMIA WITH THE USE OF THE INTERVALS FOR PROLACTIN AFTER MACROFORMS SEPARATION
2Department of Clinical Endocrinology, Chair of Endocrinology, Medical University of Lodz, Poland
INTRODUCTION
The elevated concentration of prolactin (PRL) in the blood serum, called hyperprolactinaemia, is a commonly encountered clinical condition. It is worth to note that it is in fact not a disease but the effect of some abnormalities occurring in the human body. Hyperprolactinaemia in majority of cases is caused by functional disturbances that affect PRL secretion, for example primary hypothyroidism, liver or kidney failure and some drugs intake. The increase in PRL level may be also due to the tumors of hypothalamic-pituitary region - most often a pituitary adenoma - prolactinoma (1-4). On the other hand, the laboratory result indicating hyperprolactinaemia may be the effect of macroprolactin (MaPRL) occurrence in the blood. Macroprolactin is a large PRL isoform (MW about 150 kDa) that consists mainly of the particle of monomeric hormone connected with immunoglobulin G. However, other macroforms of the hormone also may occur in the blood - those are dimers (big-PRL, MW 50–60 kDa) and conglomerates of monomeric or modified (e.g. with glycosylation) hormone particles (big-big-PRL, MW>100 kDa, even 600 kDa) (5).
In comparison with monomeric PRL (mPRL), MaPRL and probably other hormone macroforms have limited bioactivity, however, they may react with the components of immunoassays designed for the measurement of PRL concentration. Therefore, the presence of large amounts of MaPRL can lead to the overestimation of PRL level (pseudohyperprolactinaemia) and sometimes to the unsuitable treatment (6-12). To quantify MaPRL in the blood the precipitation with polyethylene glycol (PEG) is the most often used method (8-15). This screening method of MaPRL detection has a simple and cheap procedure but some unclear results may require confirmation by gel filtration chromatography (GFC) which is known as a “gold standard” for the separation of molecules on the basis of their size. However, GFC can not be routinely used because it is a time-consuming as well as expensive method (16).
Precipitation method involves calculating the recovery of mPRL - that means the percentage ratio of the hormone concentration measured after and before the separation of PRL macroforms. The cut-off value of recovery differs broadly from paper to paper which creates some difficulties in the proper evaluating of macroprolactinaemia (5, 11, 17-20). Therefore, assessing not only the recovery value but also the concentration of PRL after removing MaPRL (real PRL concentration reflecting the concentration of mPRL) may be a better way to distinguish true and pseudohyperprolactinaemia as some authors have proposed (9, 21-23). Moreover, it has been suggested that each laboratory undertaking MaPRL screening, has to establish a method - specific reference intervals derived by the use of PEG-treated sera from healthy individuals (21-23).
For that reason the aim of our study was to check if the intervals for PRL after macroforms separation differ from manufacturer’s reference ranges and to compare the results of PEG precipitation with the use of the criterion of recovery value and the criterion of real PRL concentration.
MATERIAL AND METHODS
The study protocol was approved by Bioethical Committee of the Medical University of Lodz (RNN/378/08/KB).
Hyperprolactinaemic sera
The study included 245 patients (224 women and 21 men) hospitalized in Department of Clinical Endocrinology, Medical University of Lodz. The inclusion criterion was the concentration of PRL exceeding 30 ng/mL measured in the fasting state. The study group consisted of 45 patients with a pituitary tumor (14 macroprolactinoma, 20 microprolactinoma and 11 non-functioning pituitary adenoma) and 200 persons with functional hyperprolactinaemia (the idiopathic origin, group of hyperandrogenic women with polycystic ovary morphology, primary hypothyroidism and drugs-induced hyperprolactinaemia).
Normoprolactinaemic sera
In order to check the difference between concentrations of PRL before and after the separation of macroforms in normoprolactinaemic sera, the samples were collected in the fasting state from apparently healthy people (with no evidence of endocrine disorders and without intake of drugs that may change PRL level). Finally, 120 sera were included into the study (89 from women aged 21–61 years and 31 from men aged 21–62 years).
Prolactin immunoassay
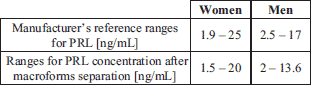
The prolactin concentration was measured by enzyme-amplified chemiluminescent immunoassay (Immulite 1000, Siemens). Analytical sensitivity of the assay is 0.5 ng/mL. Intra-assay and inter-assay coefficients of variation (CV) are respectively 6.1% (PRL concentration 6.3 ng/mL) and 9.6% (PRL concentration 14.1 ng/mL). Reference ranges are 1.9–25.0 ng/mL for women and 2.5–17.0 ng/mL for men. Prolactin Immulite kit was standardized to the World Health Organization’s third international standard for prolactin, 84/500.
Precipitation with polyethylene glycol
The precipitation with PEG was performed similarly to a method proposed by Olukoga and Kane (11) and also followed a protocol recommended by Diagnostic Products Corporation (nowadays owned to Siemens). Equal volumes of PEG (Sigma) and serum were mixed, incubated at room temperature for 10 minutes and next centrifuged at 3000 rpm for 30 minutes. The supernatant obtained after this procedure was diluted 10-fold and checked for PRL concentration (PRLPEG). The result was compared with PRL concentration in 10-fold diluted, untreated serum (PRLtotal). In accordance with the majority of literature data, we assumed that the recovery of monomeric prolactin (the percentage ratio PRLPEG/PRLtotal) equal or below 40% means that MaPRL dominates in the serum sample (macroprolactinaemic subjects) (5, 11, 19, 24-26).
Statistical analysis
The data obtained from the experiment was recorded on Excel (MS Office 2007) worksheets. The McNemara test was used to analyze the diagnostic concordance between the recovery ratio values compared with the real PRL concentration evaluated both in the context of manufacturer and new ranges for PRL.
RESULTS
Comparison of recovery values after precipitation with real prolactin concentrations (manufacturer’s reference ranges)
The calculation of recovery of monomeric PRL after macroforms separation with the use of the precipitation method showed macroprolactinaemia in 21/245 samples (8.6%) - in four persons with prolactinoma and in 17 subjects with functional hyperprolactinaemia. We noted that in the patients with recovery ≤40% real PRL concentration was elevated above manufacturer’s reference ranges (it means that those persons have indeed true hyperprolactinaemia) in 9 cases - in almost all (3/4) persons with organic and in 6/17 patients with functional hyperprolactinaemia (Table 1).
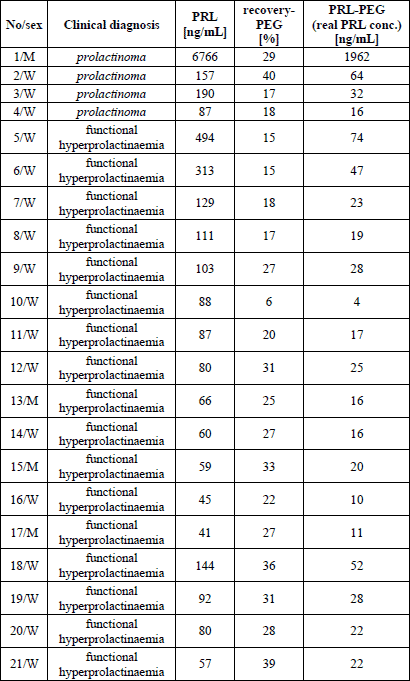
Among 224 patients without macroprolactinaemia based on recovery values (ratio >40%), real PRL concentrations were within the manufacturer’s reference range (it means that these patients have pseudohyperprolactinaemia - elevation of “native” PRL level was due to macroforms) in 36 cases. Those were six women with hypothalamic-pituitary area disease other than prolactinoma and 30 with functional hyperprolactinaemia. The remaining 188/224 persons had PRL concentration above the manufacturer’s reference range - that is true hyperprolactinaemia.
New intervals for prolactin after macroforms separation
In order to check the difference between concentrations of PRL before and after the separation of macroforms in normoprolactinaemic subjects, we performed the precipitation in 120 normoprolactinaemic (according to manufacturer’s reference ranges for PRL) sera. The mean recovery after the precipitation was 79.3±0.62%. It means that PRL concentration in the sera of potentially healthy people decreases by about 20%. Therefore, we modified the manufacturer’s reference range by decreasing limit values by 20% and we obtained new ranges for PRL concentration after macroforms separation (Table 2).
Comparison of recovery values after precipitation with real prolactin concentrations (new ranges for prolactin after macroforms separation)
Applying new ranges for PRL in the group of patients with macroprolactinaemia (recovery value ≤40%), we observed elevated PRL level (true hyperprolactinaemia) in 14 persons - 3/4 with organic and 11/17 subjects with functional hyperprolactinaemia (Table 1). In 224 patients without macroprolactinaemia (recovery value >40%), real PRL concentration was within new ranges for PRL (pseudohyperprolactinaemia) only in five persons. It means that the usage of new values for PRL showed true hyperprolactinaemia in 219/224 persons, including 31 among 36 patients with the normal hormone level according to the manufacturer’s reference range. The summary of the obtained results has been comprised in Table 3.
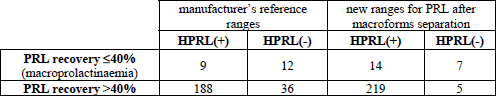
With the use of the McNemara test, we showed that the diagnostic concordance of recovery ratio values compared with real PRL concentration assessed in the context of new ranges for PRL after macroforms separation (89.4%) is statistically significantly higher (p<0.05) than the diagnostic concordance of recovery ratio values compared with the real PRL concentration evaluated in the context of the manufacturer reference range (76.7%).
DISCUSSION
Macroprolactinaemia is a common problem in a medical practice because, according to our previous study, it occurs in at least every tenth patient with hyperprolactinaemia (17). Some studies indicate that this frequency may be much higher and may achieve over 40% (27, 28). For that reason the proper estimation of MaPRL amounts in serum is very important. The precipitation with PEG - method that may be used to quantify MaPRL in blood, involve the separation of macroforms and the calculation of recovery of mPRL. The value of recovery indicates whether MaPRL predominates in serum or occurs in an insignificant amount. However, the most often proposed cut-off value for precipitation is 40%, but the values equal to 30 or even 50% were used (11, 13, 18-20). Therefore, the diagnosis if serum sample is macroprolactinaemic or not differs depending on the accepted cut-off value. Moreover, the most often used 40% cut-off value has unsatisfactory diagnostic specificity and can lead to misinterpretation when both an excess amount of macroprolactin and a very high concentration of monomeric prolactin are present simultaneously (9, 23).
Hence, in cases of hyperprolactinaemic sera it seems to be reasonable to assess not only the recovery value but also the concentration of PRL after the separation of macroforms. Real PRL concentration will indicate if the amount of the monomeric - biologically active (responsible for symptoms) form of the hormone in serum is elevated (9, 23). Thus, while evaluating the studied group of subjects we observed that in above half of persons (57%) with recovery less than 40% (significant macroprolactinaemia), real PRL concentration was within the reference ranges proposed by the manufacturer. It means that these patients indeed had pseudohyperprolactinaemia - the presence of MaPRL was responsible for elevated PRL level obtained in a laboratory. On the other hand, 43% of patients with macroprolactinaemia - mainly people with prolactinoma and very high PRL levels, had the raised concentration of mPRL (according to manufacturer’s reference ranges) and had, in fact, true hyperprolactinaemia. These are specific cases in which using only the criterion of recovery and finding macroprolactinaemia, which is thought to be responsible for elevated PRL level, could lead to a misdiagnosis and the withdrawal from treatment. Our results showed that it may be a significant problem.
Conversely, in 224 patients without macroprolactinaemia based on cut-off value, 16% persons had normal PRL concentration according to manufacturer’s reference ranges. They were persons with a slightly increased PRL level in whom the separation procedure led to the obtainment of correct real hormone concentration. Bearing in mind that precipitation has disadvantage of depleting serum not only from macroforms but also from smaller than MaPRL forms of PRL (including mPRL), it seems possible that even in serum with no MaPRL, the recovery value will be a little below 100% and real PRL concentration will be lower than “native”. Moreover, some studies have shown that small amounts (1–3%) of MaPRL are present in the sera of healthy people with normal PRL level (29, 30). Therefore, it would be more appropriate to assess a real PRL concentration not in the context of reference ranges designed by manufacturer for “native” hormone level but referring it to a new range of values established for PRL concentration after macroforms separation. Our results are rather similar to those reflecting nonspecific precipitation of mPRL and to the new intervals for PRL after macroforms separation presented by some other authors (decrease in serum PRL about 20–30%) (21, 29, 31, 32). We would like to accentuate that our study was performed with the use of Immulite 1000 that is thought to be medium-reactive towards MaPRL (33). Possibly when using immunoassay with higher or lower effectiveness, the percentage decrease of PRL level after macroforms separation may be a little different. Therefore, we also would like to underline that each laboratory or at least each region should elaborate their own reference values for tested parameters.
By the comparison of the real PRL concentrations with a new reference ranges for PRL after macroforms separation, we showed that in group of 21 subjects with macroprolactinaemia, 14 persons had true hyperprolactinaemia. This result is higher than the one obtained with the use of manufacturer’s reference ranges (9/21 patients) and indicates that in as many as 66% of cases there is a risk of omission of hyperprolactinaemia due to the result of MaPRL dominance obtained with the use of recovery ratio which falsely suggests that there is no hyperprolactinaemia in the serum sample. Conversely, in patients without macroprolactinaemia the application of new ranges for PRL showed that only five persons had a normal hormone level. This result is much lower than that obtained with the use of the manufacturer’s reference range (36 persons, only women). However, in the cases of slightly elevated “native” hormone levels, real PRL concentration may be differently classified taking into account that the manufacturer’s and our new reference limit are close (25 and 20 ng/mL respectively for women). Therefore, in 5/224 persons results negative for MaPRL dominance and hence suggesting hyperprolactinaemia, turn out to be false because their real concentration of PRL was actually normal (pseudohyperprolactinaemia). The above data seem to indicate that with the use of the new ranges for PRL after macroforms separation the number of misleading results for MaPRL might be much lower than with the use of the reference ranges proposed by manufacturer.
It is rather obvious that the self-established new intervals would be more appropriate than reference ranges developed by the manufacturer in a distant country on a probably different population. Particularly as regards the values for PRL after macroforms separation procedure, the above statement seems very logical. Our results confirmed that the interpretation of real PRL concentration with the use of manufacturer’s and new intervals for PRL may differ significantly, mainly in subjects with functional hyperprolactinaemia. Obviously we are aware that, despite of suggestions, the elaboration of new intervals for PRL after macroforms separation by each laboratory or even each region is very difficult due to technical and financial aspects. Therefore, we would like to underline that while assessing the real PRL concentration, it should be kept in mind that sometimes using manufacturer’s reference ranges may be a little incompatible with the values of real PRL concentration.
In summary, we conclude that evaluating of macroprolactinaemia only with the use of the recovery ratio may be not sufficient for a proper diagnosis in hyperprolactinaemic patients. The laboratory result preferably should contain the recovery value given together with a real PRL concentration. To assess PRL level after macroforms separation, reference intervals suggested by the manufacturer of immunoassay might be used but better means to identify patients with true hyperprolactinaemia accurately is using the appropriate ranges for concentration of PRL after macroforms separation.
Acknowledgements: This study was financially supported by the Medical University of Lodz (Grant No 503/5-020- 02/503-01).
Conflict of interests: None declared.
REFERENCES
- Pawlikowski M, Karasek M. Hyperprolactinaemia. The Essentials. Touch Briefings: European Endocrine Disease 2006: 53-57.
- Karasek M, Pawlikowski M, Lewinski A. Hyperprolactinemia: causes, diagnosis, and treatment. Endocrinol Pol 2006; 57: 656-662.
- Cortet-Rudelli C, Sapin R, Bonneville JF, Brue T. Etiological diagnosis of hyperprolactinemia. Ann Endocrinol 2007; 68: 98-105.
- Kaluzny M, Bolanowski M. Hyperprolactinemia: etiology, clinical symptoms, and therapy [in Polish]. Postepy Hig Med Dosw 2005; 59: 20-27.
- Bartoszewicz Z. Makroprolaktynemia - hiperprolaktynemia charakteryzujaca sie obecnoscia wysokoczasteczkowej makroprolaktyny. http://www.roche.pl/fmfiles/ re7190002/ RDP /labforum/labforum14.pdf.
- Leite V, Cosby H, Sobrinho LG, Fresnoza MA, Santos MA, Friesen HG. Characterization of big, big prolactin in patients with hyperprolactinaemia. Clin Endocrinol (Oxf) 1992; 37: 365-372.
- Hattori N, Inagaki C. Anti-prolactin autoantibodies cause asymptomatic hyper-prolactinemia: bioassay and clearance studies of PRL-immunoglobulin G complex. J Clin Endocrinol Metab 1997; 82: 3107-3110.
- McKenna TJ. Should macroprolactin be measured in all hyperprolactinaemic sera? Clin Endocrinol 2009; 71: 466-469.
- Suliman AM, Smith TP, Gibney J, McKenna TJ. Frequent misdiagnosis and mismanagement of hyperprolactinemic patients before the introduction of macroprolactin screening: application of a new strict laboratory definition of macroprolactinemia. Clin Chem 2003; 49: 1504-1509.
- Jeske W. The main reasons behind variability in detection of prolactin and causes of true or apparent discordance with clinical state. Endocrinol Pol 2008; 59: 30-32.
- Olukoga AO, Kane JW. Macroprolactinaemia: validation and application of the polyethylene glycol precipitation test and clinical characterization of the condition. Clin Endocrinol (Oxf) 1999; 51: 119-126.
- Ignacak A, Kasztelnik M, Sliwa T, Korbut RA, Rajda K, Guzik TJ. Prolactin - not only lactotropin. A new view of the old hormone. J Physiol Pharmacol 2012; 63: 435-443.
- Shimatsu A, Hattori N. Macroprolactinemia: diagnostic, clinical, and pathogenic significance. Clin Dev Immunol 2012; 2012: 167132. doi: 10.1155/2012/167132.
- Beda K, Winczyk K. Macroprolactin - prevalence, diagnostic methods and clinical significance. Fol Med Lodz 2009; 36: 87-110.
- Fahie-Wilson M, Smith TP. Determination of prolactin: the macroprolactin problem. Best Pract Res Clin Endocrinol Metab 2013; 27: 725-742.
- Gel Filtration. Principles and Methods. GE Healthcare, 2007.
- Beda-Maluga K, Pisarek H, Komorowski J, Pawlikowski M, Swietoslawski J, Winczyk K. The detection of macroprolactin by precipitation and ultrafiltration methods. Endocrinol Pol 2011; 62: 529-536.
- Vieira JG, Tachibana TT, Obara LH, Maciel RM. Extensive experience and validation of polyethylene glycol precipitation as a screening method for macroprolactinemia. Clin Chem 1998; 44: 1758-1759.
- Leslie H, Courtney CH, Bell PM, et al. Laboratory and clinical experience in 55 patients with macroprolactinemia identified by a simple polyethylene glycol precipitation method. J Clin Endocrinol Metab 2001; 86: 2743-2746.
- Rivero A, Alonso E, Grijalba A. Decision cut-off of the polyethylene glycol precipitation technique in screening for macroprolactinemia on Immulite 2000. Clin Chem Lab Med 2004; 42: 566-568.
- Beltran L, Fahie-Wilson MN, McKenna TJ, Kavanagh L, Smith TP. Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: evaluation and validation on common immunoassay platforms. Clin Chem 2008; 54: 1673-1681.
- Smith TP, Kavanagh L, Healy ML, McKenna TJ. Technology insight: measuring prolactin in clinical samples. Nat Clin Pract Endocrinol Metab 2007; 3: 279-289.
- Smith TP, Fahie-Wilson MN. Reporting of post-PEG prolactin concentrations: time to change. Clin Chem 2010; 56: 484-490.
- Kostrzak A, Warenik-Szymankiewicz A, Meczekalski B. Is the assessment of biological activity of prolactin essential in the diagnosis of hyperprolactinemia? Pol Merk Lek 2010; 28: 359-361.
- Germano L, Mormile A, Filtri L, et al. Evaluation of polyethylene glycol precipitation as screening test for macroprolactinemia using Architect immunoanalyser. Biol Spec 2005; 20: 402-407.
- Elenkova CA, Genov N, Abadzhieva Z, et al. Macroprolactinemia in patients with prolactinomas: prevalence and clinical significance. Exp Clin Endocrinol Diabetes 2013; 121: 201-205.
- Vallette-Kasic S, Morange-Ramos I, Selim A, et al. Macroprolactinemia revisited: a study on 106 patients. J Clin Endocrinol Metab 2002; 87: 581-588.
- Hauache OM, Rocha AJ, Maia AC Jr, Maciel RM, Vieira JG. Screening for macroprolactinaemia and pituitary imaging studies. Clin Endocrinol (Oxf) 2002; 57: 327-331.
- Kavanagh L, McKenna TJ, Fahie-Wilson MN, Gibney J, Smith TP. Specificity and clinical utility of methods for the detection of macroprolactin. Clin Chem 2006; 52: 1366-1372.
- Hattori N, Ishihara T, Saiki Y. Macroprolactinaemia: prevalence and aetiologies in a large group of hospital workers. Clin Endocrinol (Oxf) 2009; 71: 702-708.
- Schneider W, Marcovitz S, Al-Shammari S, Yago S, Chevalier S. Reactivity of macroprolactin in common automated immunoassays. Clin Biochem 2001; 34: 469-473.
- McCudden CR, Sharpless JL, Grenache DG. Comparison of multiple methods for identification of hyperprolactinemia in the presence of macroprolactin. Clin Chim Acta 2010; 411: 155-160.
- Smith TP, Suliman AM, Fahie-Wilson MN, McKenna TJ. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J Clin Endocrinol Metab 2002; 87: 5410-5415.
A c c e p t e d : February 13, 2014