MATRIX METALLOPROTEINASE 9/NEUTROPHIL GELATINASE ASSOCIATED LIPOCALIN/TISSUE INHIBITOR OF METALLOPROTEINASES TYPE 1 COMPLEXES ARE LOCALIZED WITHIN CARDIOMYOCYTES AND SERVE AS A RESERVOIR OF ACTIVE METALLOPROTEINASE IN PORCINE FEMALE MYOCARDIUM
INTRODUCTION
Matrix metalloproteinase type 9 (MMP-9) is crucial for both physiological tissue repair and pathophysiological tissue remodeling (1-3). Despite the considerable progress in our understanding on the role of MMPs in cardiac remodeling (4-7), there are still several unexplained issues (8).
MMPs are synthesized as pro-enzymes and released to the extracellular space, where they mature to proteolytically active molecules due to the enzymatic cleavage of propeptide domains (4). Although MMPs are abundantly present in myocardial tissue (4), it still remains unclear which cells (cardiomyocytes or cardiofibroblasts) are the major source of these enzymes, as the available data is conflicting (9-12). Moreover, the regulation of the MMP activation in myocardium is complex and not completely understood (8).
In neutrophils, the regulation of MMP functioning has been shown to be regulated through the formation of complexes with tissue inhibitor of metalloproteinases type 1 (TIMP-1) and neutrophil gelatinase associated lipocalin (NGAL) (13), but such a mechanism has been demonstrated in neither myocardium nor any solid tissue. Importantly, there are gender differences in both the MMP-9 activity and the TIMP-1 expression in rat aortic smooth muscle cells (14) and murine myocardium after myocardial infarction (15). Also, circulating MMP-9 levels differ between male and female patients with diabetes mellitus (16), coronary artery disease (17) and heart failure (HF) (18), among other disorders.
Therefore, we performed the study in order to investigate the mRNA and protein expression of MMP-9, TIMP-1 and NGAL, the formation of complexes, their proteolytic activity and cellular localization in left ventricle (LV) myocardium of female pigs with and without right ventricle (RV) pacing induced systolic HF, and to confirm these findings in failing myocardium of a woman with severe non-ischaemic HF.
MATERIALS AND METHODS
Applied porcine model of right ventricle pacing induced systolic heart failure
The study was performed in 15 adult female pigs of Polish Large White breed (sibling 8-month-old females, initially weighing from 70 to 90 kg). All animals received human care in compliance with the Guide for the Care and Use of Laboratory Animals as published by the National Institutes of Health (NIH publication No. 85-23, revised in 1996). All experiments were performed in compliance with the Bioethical Committee of the Wroclaw University of Environmental and Life Sciences guidelines for the experimentation on animals.
All procedures and echocardiography measurements were performed during anesthesia administered according to the same protocol described below, with food restriction for 12 hours and water restriction for 4 hours before. Pigs were anesthetized using a modified protocol described by Goldmann et al. (19). Briefly, animals were premedicated with an intramuscular injection of 1 mg/m2 body surface area (BSA) medetomidine hydrochloride (Cepetor, CP-Pharma, Germany), 5 mg/m2 BSA of midazolam (Midanium, WZF Polfa, Poland) and 264 mg/m2 BSA of ketamine (Bioketan, Vetoquinol Biowet, Poland) in a mixing syringe. An ear vein was punctured for placement of the catheter for intravenous induction with 2–5 mg/kg body weight (BW) propofol (Propofol 1% MCT/LCT Fresenius, Fresenius Kabi, Germany). Following intubation (8.5 Charriere tubes), anesthesia was maintained by continuous infusions of 1–3 µg/h per kilogram BW fentanyl (Fentanyl WZF, WZF Polfa, Poland) and inhalation of isoflurane (1.5–2% vol) (Aerrane, Baxter, Poland). Monitoring of the basal life functions (ECG, end-tidal CO2, oxygen saturation, noninvasive blood pressure) was based on LIFEPAK 12 Defibrillator/Monitor (Medtronic, Poland). Single-chamber pacemaker (SENSIA SESR01, Medtronic, Poland) was implanted in each of 15 pigs under control of a fluoroscope (Ziehm 8000, Germany). A bipolar screw-in pacing transvenous lead (CAPSUREFIX NOVUS 58 cm, Medtronic, Poland) was inserted into the left internal jugular vein and positioned in the myocardium at the right ventricular apex. Lead was attached to pacemaker and the pacing system was placed in a subcutaneous pocket. Every pig was administered antibiotic intramuscularly for infection prophylaxis for 10 days. The animals were allowed a 2-weeks recovery period and then the pacemakers were programmed for sequential right ventricular pacing at 170 bpm in 10 randomly chosen animals, whereas 5 sham-operated animals served as controls.
All animals remained under everyday clinical care. There were differences in neither the clinical care nor the applied protocol of performed measurements between the RV paced and non-paced female pigs. The clinical assessments of HF signs and symptoms were performed regularly at the end of subsequent months. The following signs and symptoms of HF occurring in examined pigs were evaluated semi-quantified using a 0–3 scale (0 – no sign/symptom, 3 – very severe intensity of a particular sign/symptom): appetite, interest in surroundings, willingness to undertake physical activity (after forcing), dyspnea after exertion, lying down after exertion (fatigue), dyspnea at rest, ascites, redness of snouts and ears after exertion, snout and ears cyanosis at rest. All scores were averaged for each pig for the particular time point. The following ranges of averaged scores were used for the categorization of pigs to subsequent clinical stages of HF: mild HF (≥0 and ≤1), moderate HF (>1 and ≤2), severe HF (>2 and ≤3). After clinical assessment each animal was anesthetized and the pacemaker was deactivated for approximately 30 min. In this time a non-invasive measurement of resting arterial blood pressure, heart rate, body weight and transthoracic echocardiography was performed.
Based on these comprehensive clinical evaluations, each animal was assigned to a particular category of HF severity (i.e. mild – 10±3 weeks of pacing, n=4; moderate – 19±2 weeks of pacing, n=3 and severe HF – 26±10 weeks of pacing, n=3) and particular animals were euthanized at subsequent stages of HF. Control animals underwent euthanasia parallel to tachycardia induced cardiomyopathy (TIC) pigs (16±8 weeks, n=5) and were selected for this procedure in a random manner. None of the pigs was killed prematurely.
As demonstrated before, such a protocol of an applied RV pacing resulted in a gradual development of symptomatic HF, along with the LV dilatation and systolic dysfunction confirmed using transthoracic echocardiography, and the neurohormonal activation (an increase in circulating B-type natriuretic peptide (BNP)). This clinical phenotype of systolic HF was accompanied by the histological and molecular changes seen in LV myocardium, which were characteristic for myocardial remodeling (20).
Tissue sections from the free wall of LV myocardium were taken directly after the pigs were euthanized, and were immediately frozen in liquid nitrogen and stored at –80°C for the further molecular analyses. At the same time, separate sections for standard histology were immersed in a 4% paraformaldehyde solution and stored for the further assessments.
Human samples of left ventricle myocardium from a woman with severe systolic heart failure
Sample of LV myocardium was obtained at autopsy from a heart of a woman who died due to severe non-ischaemic systolic HF in Department of Heart Diseases, Wroclaw Medical University (Wroclaw, Poland). Section of the free wall of LV myocardium was dissected, snap-frozen in liquid nitrogen, and stored at –80°C until the further molecular analyses.
Overview of the performed molecular assessments
- Quantification of the mRNA expression of MMP-9, TIMP-1 and NGAL in the homogenates of LV myocardium of female pigs with and without HF, and the confirmation of the mRNA expression of these genes in a woman with severe HF (RT-PCR).
- Protein expression and the presence of monomers and various complexes containing MMP-9, TIMP-1 and NGAL in the homogenates of LV myocardium of female pigs with and without HF, and a woman with severe HF assessed in non-reducing and non-denaturing conditions (Western blotting and co-immunoprecipitation).
- Gelatinolytic activity (assessed in statu nascendi) of MMP-9 in the homogenates of LV myocardium of female pigs with and without HF, and a woman with severe HF assessed in non-reducing and non-denaturing conditions (gelatin zymography).
- Gelatinolytic activity of the homogenates of porcine and human LV myocardium preincubated with EDTA (known to inhibit specifically enzymatic activity of MMPs (21) in order to prove the specificity of gelatinolytic activity related to the presence of MMP-9 (gelatin zymography).
- Gelatinolytic activity of the samples from porcine and human LV myocardium depleted of active MMP-9 during gelatin affinity chromatography in order to identify gelatinolytically active MMP-9 not being the consequence of the potential autoactivation due to the interaction with Triton X-100 used during gelatin zymography (22) (gelatin zymography of the extracts remaining from gelatin affinity chromatography, where gelatin Sepharose 4B bound active MMP-9 (23).
- Spontaneous release of gelatinolytically active MMP9 from the homogenates of porcine LV myocardium during the incubation at 37°C (Western blotting and gelatin zymography).
- Release of gelatinolytically active MMP-9 from the homogenates of porcine LV myocardium (previously depleted of active MMP-9 during gelatin affinity chromatography) during the trypsin digestion (gelatin zymography).
- Localization of MMP-9 proteins in particular cellular components of porcine LV myocardium (immunohistochemistry).
Quantitative real time-PCR
Total RNA was prepared from 30-mg samples of porcine LV myocardium using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Poland) according to the manufacturer’s instructions. The protocol included an on-column DNAse digestion to remove the genomic DNA. First-strand cDNA was synthesized using a SuperScript III First-Strand Synthesis System with oligo(dT)20 primer (Invitrogen, Poland).
Based on the genomic and cDNA sequences, the primers for MMP-9, TIMP-1, NGAL and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were designed using the Molecular Beacon Software (Bio-Rad, Poland) (Table 1). The primers spanned exon junctions to prevent the amplification of genomic DNA. The GAPDH gene was chosen as a reference to normalize the differences in the amount of RNA and in the efficiency of reverse transcription.

The relative amounts of porcine MMP-9, TIMP-1 and NGAL in LV myocardium were determined using the quantitative real-time PCR using the iQ5 Optical System (BioRad, Poland) with the Kapa Mix (KapaBiosystems, USA) as appropriate. The reactions were performed under the following conditions: an initial denaturation of 94°C for 10 min, 35 cycles of 94°C for 30 s, 65°C for 30 s, followed by 72°C for 1 min. All measurements were performed in triplicates. The specificity of PCR was determined using a melt-curve analysis for each reaction. PCR products for all subsequent investigated genes were sequenced (Genomed, Poland) to confirm their identity.
The amplification efficiency was established by running a template at successive dilutions. Successive dilutions were plotted against the appropriate Ct values to generate a standard curve. The slope calculated from the standard curve was used to determine the amplification efficiency (E) according to the formula: E=101/slope. Since the amplification efficiencies for the target amplicons and GAPDH were not comparable, the Pfaffl method was used to determine the relative expression (24). mRNA expression was presented in arbitrary units (AU), where the sample of LV myocardium from one of the control pigs was chosen as the calibrator, and its mRNA expression was considered as 1.
Immunochemical detection
1. Western blotting
Samples of LV myocardium (30 mg), either porcine or human ones, were homogenized in 200 µl of an ice-cold extraction buffer (50 mM Tris-HCl, 200 mM NaCl, 10 mM CaCl2, 1% Triton X-100, pH 7.6) (25) containing a 1:50 protease inhibitor cocktail (Sigma-Aldrich, Poland). After an incubation on ice (30 min) and a centrifugation at 9700×g, the supernatants were collected and stored on ice. Next, insoluble material was extracted twice during a 10 min incubation with 50 µl of ice-cold extraction buffer, and supernatants from both extractions were combined. Protein quantification was performed using the Bradford reagent (Sigma-Aldrich, Poland), according to the manufacturer’s instructions.
Protein samples (0.5–40.0 µg) were mixed with a non-reducing sample buffer (63 mM Tris, 10% glycerol, 2% SDS, 0.1% bromophenol blue, pH 6.8) and were incubated for 5 min at room temperature. Protein samples (25 µg) were also prepared in a reducing sample buffer (Pierce, Poland) (with a final dithiothreitol concentration of 0.1 M) followed by incubation for 5 minutes 95°C. LV homogenates were subsequently separated on a 10% SDS-PAGE and transferred onto the PDVF membrane (Millipore, Poland). The membrane was treated with Quentix Signal Enhancer (Pierce, Poland), blocked for 1 hour with 5% nonfat milk in the PBS containing 0.5% (v/v) Triton X-100 (Sigma-Aldrich, Poland), and incubated overnight with polyclonal goat antibodies against human MMP-9, TIMP-1 or NGAL (1:500) (R&D System, Poland). Primary antibodies were detected using an anti-IgG HRP-conjugated antibody. Blots were developed using the SuperSignal West Femto ECL substrate (Pierce, Poland). Recombinant proteins - NGAL (R&D System, Poland), TIMP-1 (R&D System, Poland), and the culture media from DH82 cell line (MMP9 source) prepared in the same way as LV homogenates were used as positive controls.
2. Co-immunoprecipitation
For the procedure of co-immunoprecipitation of HMW complexes, homogenates from LV myocardium, either porcine or human ones (suspended in an extraction buffer), were precleared by a 60 min incubation with 25 µl of protein G agarose (Sigma-Aldrich, Poland). The precleared supernatant was mixed with 25 µl of protein G agarose and one of the following antibodies, i.e.: anti-MMP-9, anti-TIMP-1 or anti-NGAL (5 µg) (the same antibodies which were used in Western blotting). After a 60 min incubation, the beads were washed 3 times, and proteins were eluted with 50 µl of Elution Buffer (pH 2.8, Pierce, Poland), and neutralized with 5 µl of 1 M Tris (pH 9.0). 10 µl of an eluate was electrophoresed on a 10% SDS-PAGE (non-reducing and non-denaturing conditions), followed by immunoblotting (the same conditions as in Western blotting). Samples immunoprecipitated with anti-MMP-9 antibody were blotted using anti-TIMP-1, and anti-NGAL antibodies, those immunoprecipitated with anti-NGAL antibodies were blotted using anti-TIMP-1 and anti-MMP-9 antibodies, and those immunoprecipitated with anti-TIMP-1 antibodies were blotted with anti-NGAL and anti-MMP-9 antibodies.
Gelatin zymography
Samples of porcine and human LV myocardium (30 mg) were homogenized in 200 µl of an ice-cold extraction buffer (50 mM Tris-HCl, 200 mM NaCl, 10 mM CaCl2, 1% Triton X-100, pH 7.6) (25). After an incubation on ice (30 min) and a centrifugation at 9700×g, the supernatant was collected and stored on ice. Insoluble material was then extracted twice for 10 min with 50 µl of ice-cold extraction buffer, and finally the insoluble material was removed using a centrifugation at 9700×g. Supernatants from all extractions were combined.
Protein quantification was performed using the Bradford reagent (Sigma-Aldrich, Poland), according to the manufacturer’s instructions. Protein samples (40 µg) were separated at 4°C in a non-reducing SDS-PAGE in 10% gels containing 1 mg/ml gelatin (25, 26). The gels were washed 3 times in 2.5% Triton X-100 for 30 min to renature the proteins by exchanging SDS by Triton X-100. Next, the gels were incubated overnight at 37°C in a collagenase buffer (50 mM Tris-HCl, 200 mM NaCl, 5 mM CaCl2, 0.2% Brij-35, pH 7.6) (27, 28). Subsequently, the gels were stained using 0.5% Coomassie Blue R-250 (Sigma-Aldrich, Poland) in 30% methanol and 10% acetic acid for 60 min, and destained in 30% methanol and 10% acetic acid (28). Gelatinase activity was identified as clear zones against a blue background.
Gelatin zymography of LV myocardial samples was performed twice, i.e.: first as described above, and secondly with a modification based on the preincubation of analyzed gels in collagenase buffer containing 10 mM EDTA, known to inhibit specifically enzymatic activity of MMPs (21) in order to prove the specificity of gelatinolytic activity associated with demonstrated bands to the presence of MMP-9.
Additionally, the culture media from canine macrophage-like DH82 cell line were used as a positive control of MMP-9 activity (29). Culture medium was incubated with 1 mM APMA (p-aminophenyl-mercuric acetate, Sigma-Aldrich, Poland) for 60 min to activate MMP-9 (23), and then the zymography procedure was proceeded. LV homogenates (100 µg) were also incubated with 1 mM APMA.
Gelatin zymography of the complexes containing active matrix metalloproteinases derived using gelatin affinity chromatography from porcine and human left ventricle myocardium
Because the exchange of the SDS with Triton X-100 (after electrophoresis) during gelatin zymography could cause the autoactivation of latent MMP forms without cleavage (22), the gelatin affinity chromatography was used to extract active MMP-9 from LV homogenates (23). The subsequent zymography in LV homogenates depleted of MMP activated during gelatin affinity chromatography allowed to prove whether the active MMP-9 were present in LV homogenates, regardless of the performed procedure.
Gelatin (Sigma Aldrich, Poland) was covalently linked to the Cyanogen-Bromide-Activated Sepharose 4B (Sigma Aldrich, Poland). Porcine and human LV myocardial samples were prepared as described in the section Immunochemical detection. The extracts of LV myocardium (0.5 mg) were mixed with an equal volume of Brij buffer (0.5 M NaCl, 5 mM CaCI2, 50 mM Tris, 0.05% Brij, pH 7.6) (30) and 40 µl of Gelatin-Sepharose 4B (Sigma-Aldrich, Poland) in order to allow to bind active MMPs (provided they were present in the extracts from LV myocardium) to Gelatin-Sepharose 4B during a 60 min incubation at a room temperature. Afterwards, Gelatin-Sepharose 4B was washed 3 times using Brij buffer (30), and was incubated for 10 min with an elution buffer (0.5 M NaCl, 0.05% Brij, 10% DMSO) at a room temperature.
15 µl of eluted material (containing potentially active MMPs) was analyzed in gelatin zymography (as described in details above).
Spontaneous release of active matrix metalloproteinase 9 and after trypsine digestion (with and without its inhibitor)
Extracts from the homogenates of porcine LV myocardium (1 mg) were prepared as described in the section Gelatin zymography.
The spontaneous release of active MMP-9 from HMW complexes from porcine LV myocardium (without depleting of active MMPs) was investigated after an incubation at 37°C lasting 0, 1, and 2 hours. Afterwards, the protein samples (40 µg) were collected, and analysed using Western blotting (with anti-MMP-9 antibody) and gelatin zymography.
The release of active MMP-9 from HMW complexes in porcine LV myocardium was also analysed after the depleting of active MMPs during gelatin affinity chromatography. In order to deplete the homogenates of porcine LV myocardium from active MMPs, the extracts of LV myocardium (1 mg) were mixed with an equal volume of Brij buffer (0.5 M NaCl, 5 mM CaCI2, 50 mM Tris, 0.05% Brij, pH 7.6 (30), 40 µl of Gelatin-Sepharose 4B (Sigma-Aldrich, Poland) and incubated at a room temperature for 1 hour. Next, the mixtures were centrifuged (2000 rpm, 30 s, at a room temperature), and Gelatin-Sepharose 4B-bound active MMPs were discarded.
The remaining supernatants (containing the extracts from porcine LV myocardium depleted of active MMPs) were incubated during a 60 min at 37°C in the following conditions:
a) without adding any enzyme (spontaneously);
b) in the presence of trypsine (3 µM, Sigma-Aldrich, Poland);
c) in the presence of trypsin (3 µM, Sigma-Aldrich, Poland) along with proteinase inhibitor cocktail (Sigma-Aldrich, Poland).
Afterwards, the protein samples (40 µg) were collected, and analysed using gelatin zymography.
Immunohistochemistry staining of porcine left ventricle myocardium
The expression of MMP-9 protein in the sections of porcine LV myocardium was assessed using immunohistochemistry with a polyclonal rabbit antibody (DakoCytomation, Poland). Briefly, the formalin-fixed, paraffin embedded tissue sections were deparaffinized, unmasked with heat and incubated for 1 hour at room temperature with anti-MMP-9 antibody at a 1:100 dilution in a Dako Antibody Diluent with Background Reducing Components (Dako, Poland). Staining was performed using the Dako Cytomatation LSAB+System-HRP (Dako, Poland). The sections were counterstained using Mayer’s haematoxylin. Negative control was performed by omitting the primary anti-MMP-9 antibody.
Statistical analysis
All molecular assessments were performed in triplicates. Comparisons of MMP-9, NGAL and TIMP-1 expression results between sham-operated and HF groups were assessed using a Mann-Whitney U test. p<0.05 was considered as statistically significant. Statistical analyses were performed using the Polish version of Statistica 9.1 (StatSoft, USA).
RESULTS
mRNA expression of matrix metalloproteinase 9, tissue inhibitor of metalloproteinases 1 and neutrophil gelatinase associated lipocalin in left ventricle myocardium
Analyses were performed in 10 female pigs with RV pacing induced systolic HF (numbers of pigs with mild/moderate/severe HF: 4/3/3) and 5 female sham-operated pigs (controls). There were no differences in the mRNA expression of MMP-9 and TIMP-1 in LV myocardium between healthy pigs, and animals with mild, moderate and severe HF (Fig. 1 panel A, all p>0.2). The mRNA expression of NGAL in LV myocardium was approximately 4-fold increased in pigs with moderate HF as compared to controls (p<0.05). The mRNA expression of MMP-9, TIMP-1 and NGAL was confirmed in the explanted heart from a woman with severe HF.
Protein expression of matrix metalloproteinase 9, tissue inhibitor of metalloproteinases 1 and neutrophil gelatinase associated lipocalin in left ventricle myocardium
Western blotting of LV homogenates from both healthy and diseased animals (performed in non-reducing and non-denaturing conditions) using anti-MMP-9 antibodies revealed the presence of 3 bands of 130 kDa, 170 kDa and 220 kDa (Fig. 1B). The same band pattern was observed when homogenates of porcine LV myocardium were blotted with anti-TIMP-1 or anti-NGAL antibodies (Fig. 1B). No bands corresponding to monomers of these molecules were detected. Furthermore, Western blotting of LV homogenates from the patient with severe HF demonstrated the analogous patterns of 3 HMW complexes (Fig. 1B).
Western blots performed with the same samples in reducing (i.e. incubated with DTT for 5 min at 95°C) revealed the presence of a 60 kDa band (blotted with anti-MMP9 antibody) corresponding to MMP-9 monomer form and a 50 kDa band (blotted with both anti-NGAL and anti-TIMP-1 antibodies), which corresponded to dimers of NGAL and TIMP-1 (Fig. 1C). Additionally for TIMP-1 a 25 kDa band was observed (TIMP-1 monomer).
Co-immunoprecipitation of porcine LV myocardium using a combination of anti-MMP-9 and anti-NGAL antibodies, as well as a combination of anti-TIMP-1 and anti-NGAL antibodies confirmed the presence of MMP-9, TIMP-1 and NGAL in these 3 aforementioned complexes. Additionally, a co-immunoprecipitation revealed the presence of a weak 115-kDa band containing MMP-9, TIMP-1 and NGAL (Fig. 1D). No bands corresponding to monomers of these molecules were detected.
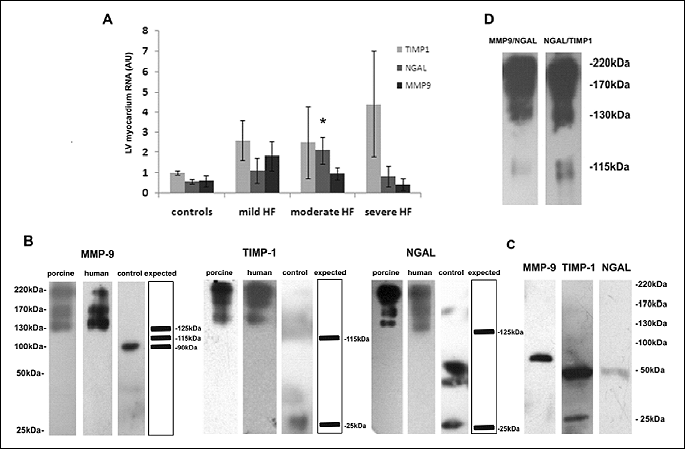
Semi-quantitative Western blotting using anti-MMP-9, anti-TIMP-1 and anti-NGAL antibodies demonstrated that the 220 kDa complex was the most abundant both in porcine LV myocardium (Fig. 2) and in human LV myocardium of a woman with severe HF (data not shown).
Gelatinase activity in the homogenates of left ventricle myocardium
Gelatin zymography (performed in the same conditions as Western blotting, i.e. in non-reducing and non-denaturing conditions) of the homogenates of LV myocardium revealed the presence of 80 and 115 kDa bands in both healthy and diseased animals, and a weak 130 kDa band in pigs with severe HF, but other HMW complexes had no proteolytic activity (Fig. 3A). Similar results were obtained in the LV sample from the patient with HF (Fig. 3B). No proteolytically active bands were detected at molecular masses higher than 130 kDa.
Gelatin zymography performed in the same way, but with the preincubation of these zymograms with EDTA (known to inhibit specifically enzymatic activity of MMPs), revealed no proteolytically active bands (data not shown). Also, a positive control (a culture medium from DH82 cells as well as LV homogenates activated by APMA) demonstrated that a 115 kDa band could be converted to 88 and 80 kDa gelatinolytically active bands (data not shown). Hence, the proteolytic activity of these 3 bands revealed in a standard gelatin zymography (Fig. 3A and 3B) was associated with the presence of active MMP-9 in these complexes (most likely MMP-9).
In order to eliminate the potential activating effects of SDS-PAGE on MMP proteolytic properties in analyzed samples of LV myocardium, the homogenates of LV myocardium underwent gelatin affinity chromatography, which allowed to obtain active MMPs. Gelatin zymography of these extracted complexes revealed the presence of active 80 kDa and 115 kDa bands in porcine LV myocardium (Fig. 3D) and the presence of active 115 kDa and 130 kDa bands in human LV myocardium (Fig. 3C).
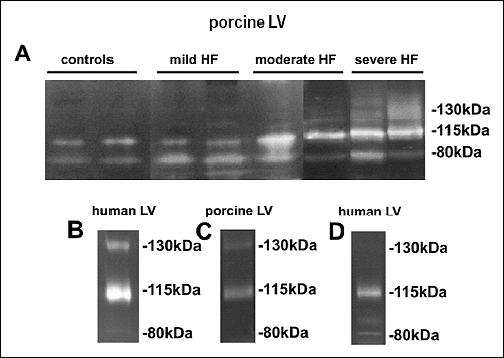 |
Fig. 3. Representative gelatin zymograms of the homogenates from LV myocardium of healthy female pigs, and female pigs with mild, moderate and severe HF, respectively (A). The figure was assembled from two different gels. Representative gelatin zymogram of the homogenate from LV myocardium of a woman with end-stage HF (B). Representative gelatin zymograms of the complexes containing (potentially) active MMPs derived during gelatin affinity chromatography of the homogenate of LV myocardium of a female pig with moderate HF (C) and a woman with end-stage HF (D). |
Spontaneous and trypsin induced release of proteolytically active matrix metalloproteinase 9
Incubation of the homogenates of LV myocardium from both healthy and diseased female pigs at 37°C resulted in a decrease in the intensity of 130, 170 and 220 kDa bands (Western blotting, Fig. 4A). Gelatin zymography performed on the same samples revealed a time-dependent appearance of a 88 kDa enzymatically active band (Fig. 4B), suggesting the release of active MMP-9 from MMP-9/TIMP-1/NGAL complexes. The decrease in the optic density of HMW complexes accompanied by an appearance of an 88 kDa gelatynolytically active band was markedly seen in pigs with severe HF (Fig. 4A and 4B).
Homogenates of porcine LV myocardium containing 3 proteolytically active bands (80 kDa, 88 kDa and 115-kDa bands) (see the paragraph above) (Fig. 4C line 1) were efficiently depleted of active MMPs using the gelatin affinity chromatography, and these samples did not demonstrate any proteolytic activity appearing during a 60 min incubation at 37°C (Fig. 4C line 2). However, an analogous incubation at 37°C with trypsin resulted in an appearance of one strong proteolytically active 115 kDa band, and 2 weak proteolytically active 80 kDa and 88 kDa bands in gelatin zymograms (Fig. 4C line 3). This reaction was prevented, when the enzymatic activity of trypsin was inhibited using the proteinase inhibitor cocktail (Fig. 4C line 4).
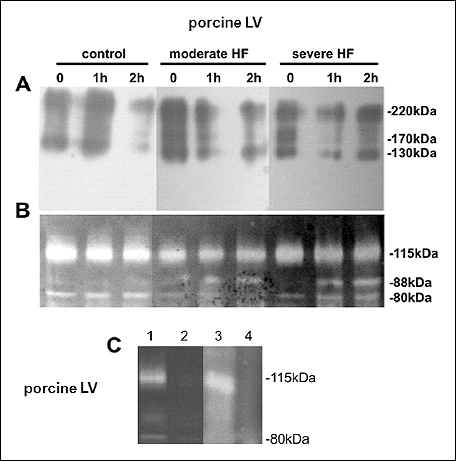 |
Fig. 4. Representative Western blots (A) and gelatin zymograms (B) of the homogenates of LV myocardium from healthy female pigs and female pigs with moderate and severe HF, respectively, incubated for 0, 1 and 2 hours at 37°C. Representative gelatin zymograms of the homogenates of LV myocardium of a female pig with moderate HF homogenate incubated for 1 hour at 37°C: a) without gelatin affinity chromatography (line 1); b) after gelatin affinity chromatography (which depleted samples of active MMP9) (line 2); c) after gelatin affinity chromatography with trypsin (line 3); d) after gelatin affinity chromatography with trypsin and protein inhibitor cocktail (line 4). Panel A and B were prepared from the same gels - the molecular weight markers (in case of panel A and B) were cut; in panel C there were two gels combined. |
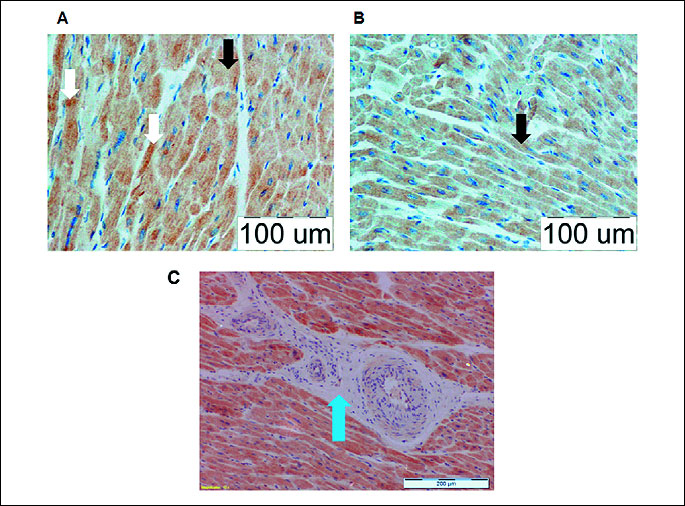
Matrix metalloproteinase 9 localization in porcine left ventricle myocardium
Immunohistochemical staining confirmed the presence of MMP-9 protein in the cytoplasm of cardiomyocytes in porcine LV myocardium (marked staining) (Fig. 5). Also, some particles stained with anti-MMP-9 antibody formed dense intracellular granules. The intensity of the anti-MMP-9 staining did not differ between LV myocardium of healthy and diseased female pigs (Fig. 5A and 5B). Furthermore, cardiofibroblasts of perivascular connective tissue from porcine LV myocardium did not stain with anti-MMP-9 antibody, suggesting that MMP-9 protein is not present in these cells (Fig. 5C). No expression of MMP-9 was observed in branches of coronary arteries as well as in endothelial cells (Fig. 5C).
DISCUSSION
Although there is a great amount of robust evidence on circulating levels of MMP-9 and associated proteins (e.g. TIMP-1 and NGAL) in patients with HF, which relate to disease progression and outcomes (31, 32), the available data on the presence of MMP-9 and associated enzymatic activity in myocardium is scarce and rather equivocal (8). In female pigs with tachycardia-induced cardiomyopathy and healthy sham-operated controls, we have demonstrated that MMP-9 forms the gelatinolytically inactive complexes with TIMP-1 and NGAL, which are present in both normal and failing myocardium and may serve as a reservoir of active MMP-9, which may get released spontaneously or after digestion by proteases. We have observed also that myocardial MMP-9 originates from cardiomyocytes, but not from cardiofibroblasts.
As patients with HF have high circulating MMP-9 levels (33), and these levels are particularly increased in subjects with accelerated myocardial remodeling and advanced HF (34), one might expect that the similar pattern of MMP expression would be seen within myocardial tissue. However, in our study the expression of mRNA and protein of MMP-9, TIMP-1 and NGAL in LV myocardium did not differ between healthy and diseased animals, including those with severe HF. Also, the results of other studies regarding the aforementioned issue are conflicting. For example, the mRNA MMP-9 was unchanged in infarcted and hypoxic rodent myocardium (35, 36), reduced in human failing myocardium (37), increased in canine failing myocardium (38) and myocardium from animals with hypertensive HF (39). The pattern of accompanying changes in the mRNA expression of TIMP-1 and NGAL was also not consistent (35-39). All these observations may indirectly suggest that the regulation of the myocardial MMP-9 activation and the subsequent release to the extracellular space and the peripheral circulation is not based on the simple modification of transcription and translation processes, but most likely, other mechanisms are involved, e.g. the formation of complexes with compounds modifying the stability and enzymatic activity of MMP-9 in target tissues (40).
The methodology of the comprehensive assessment of complex structure and enzymatic activity of native MMP-9 in solid tissues is challenging. The application of different experimental protocols in distinct physicochemical conditions can affect the properties of MMP-9 molecule, which determine its stability, enzymatic activity and affinity to various cofactors (11, 40-42). This may significantly impede the precise interpretation of obtained results and their comparison with available evidence in the literature. For example, there are different estimates of a molecular weight of MMP-9 monomer (80–100 kDa) depending on the different experiment conditions (22, 43). The pattern of MMP-9 activity in human failing myocardium varies according to the applied conditions of samples preparation for gelatin zymography (11, 41, 42). According to Kolkenkrock et al. (13) neutrophils can form the MMP-9/NGAL/TIMP-1 complex, but the other studies denied the TIMP-1 synthesis by these cells (43, 44).
We have been able to demonstrate the presence of MMP-9, TIMP-1 and NGAL proteins in LV myocardium from pigs with and without systolic HF as well as in an explanted human failing heart only in a form of HMW complexes of estimated weights of 115, 130, 170 and 220 kDa. The similar HMW complexes have been isolated in the synovial fluid from osteoatrhtritis patients (45), in the urine of cancer patients (115–125 kDa MMP-9/NGAL complexes) (46) as well as in media of human cholangiocarcinoma cell cultures (130–220 kDa MMP-9/NGAL complexes) (47) and HT-1080 cell line cultures (a 115 kDa MMP-9/TIMP-1 complex) (48).
There are premises based on cell culture studies that these HMW complexes may stabilize MMP-9 molecules and serve as a tissue reservoir of active MMP-9 protecting the enzyme from a rapid degradation and enabling its slow release to the extracellular milieu (46, 47). Tschesche et al. (49) described the allosteric activation of proMMP-9 by NGAL, suggesting its involvement in the MMP activation. The presence of MMP-9 in HMW complexes could potentially contribute to the lack of any benefit of MMP-9 inhibitors in humans with HF (4). Although we found no differences in the amount of protein in the demonstrated HMW complexes containing MMP-9, TIMP-1 and NGAL, which was already found by Rouet-Benzineb et al. (11) in human myocardium, we demonstrated that HMW complexes could spontaneously release active MMP-9, and the efficacy of this release was markedly seen in pigs with severe HF. Additionally, we have proved that active MMP-9 can be released from HMW complexes due to trypsin digestion, similarly as Kolkenkrock et al. (13).
Finally, we have found that myocardial MMP-9 is present in porcine cardiomyocytes, but not in cardiofibroblasts. This is in accordance with the other immunohistological studies (9-11), although the in vitro studies on cultured cardiofibroblasts could lead to erroneous conclusions that these cells are the major source of MMP-9 in myocardial tissue (4, 12).
We have shown that MMP-9 originates from cardiomyocytes, forms the gelatinolytically inactive HMW complexes with TIMP-1 and NGAL, which are present in both normal and failing myocardium, and may serve as a reservoir of active MMP-9, released spontaneously or digested by proteases. Further studies are needed to elucidate the role of these HMW complexes in the extracellular matrix remodeling during the progression of HF, which presence should be considered when developing efficient strategies inhibiting myocardial MMPs.
Acknowledgements: This publication is part of project “WROVASC - Integrated Cardiovascular Centre” co-financed by the European Regional Development Fund within Innovative Economy Operational Program 2007-2013 realized in Regional Specialist Hospital, Research and Development Centre in Wroclaw. Seven LV tissue samples were obtained from experimental animals financed by the Ministry of Science and Higher Education grant no. NN308387837.
Conflict of interests: None declared.
REFERENCES
- Park SH, Hong H, Han YM, et al. Nonsteroidal anti-inflammatory drugs (NSAID) sparing effects of glucosamine hydrochloride through N-glycosylation inhibition; strategy to rescue stomach from NSAID damage. J Physiol Pharmacol 2013; 64: 157-165.
- Lekstan A, Lampe P, Lewin-Kowalik J, et al. Concentrations and activities of metalloproteinases 2 and 9 and their inhibitors (TIMPS) in chronic pancreatitis and pancreatic adenocarcinoma. J Physiol Pharmacol 2012; 63: 589-599.
- Brajer B, Batura-Gabryel H, Nowicka A, Kuznar-Kaminska B, Szczepanik A. Concentration of matrix metalloproteinase-9 in serum of patients with chronic obstructive pulmonary disease and a degree of airway obstruction and disease progression. J Physiol Pharmacol 2008; 59 (Suppl. 6): 145-152.
- Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007; 87: 1285-1342.
- Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J Mol Cell Cardiol 2010; 48: 558-563.
- Ducharme A, Frantz S, Aikawa M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 2000; 106: 55-62.
- Spinale FG, Coker ML, Heung LJ, et al. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 2000; 102: 1944-1949.
- Iyer RP, Patterson NL, Fields GB, Lindsey ML. The history of matrix metalloproteinases (MMPs): milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol 2012; 303: H919-H930.
- Aupperle H, Garbade J, Schubert A, et al. Effects of autologous stem cells on immunohistochemical patterns and gene expression of metalloproteinases and their tissue inhibitors in doxorubicin cardiomyopathy in a rabbit model. Vet Pathol 2007; 44: 494-503.
- Aupperle H, Baldauf K, Marz I. An immunohistochemical study of feline myocardial fibrosis. J Comp Pathol 2011; 145: 158-173.
- Rouet-Benzineb P, Buhler JM, Dreyfus P, et al. Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: potential role of MMP-9 in myosin-heavy chain degradation. Eur J Heart Fail 1999; 1: 337-352.
- Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 2000; 86: 1259-1265.
- Kolkenbrock H, Hecker-Kia A, Orgel D, Kinawi A, Ulbrich N. Progelatinase B forms from human neutrophils. complex formation of monomer/lipocalin with TIMP-1. Biol Chem 1996; 377: 529-533.
- Woodrum DT, Ford JW, Ailawadi G, et al. Gender differences in rat aortic smooth muscle cell matrix metalloproteinase-9. J Am Coll Surg 2005; 201: 398-404.
- Fang L, Gao XM, Moore XL, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol 2007; 43: 535-544.
- Thrailkill KM, Moreau CS, Cockrell GE, et al. Disease and gender-specific dysregulation of NGAL and MMP-9 in type 1 diabetes mellitus. Endocrine 2007; 37: 336-343.
- Tayebjee MH, Lip GY, Tan KT, Patel JV, Hughes EA, MacFadyen RJ. Plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-2, and CD40 ligand levels in patients with stable coronary artery disease. Am J Cardiol 2005; 96: 339-345.
- Samnegard A, Hulthe J, Silveira A, Ericsson CG, Hamsten A, Eriksson P. Gender specific associations between matrix metalloproteinases and inflammatory markers in post myocardial infarction patients. Atherosclerosis 2009; 202: 550-556.
- Goldmann C, Ghofrani A, Hafemann B, et al. Combination anesthesia with ketamine and pentobarbital: a long-term porcine model. Res Exp Med (Berl) 1999; 199: 35-50.
- Paslawska U, Gajek J, Kiczak L, et al. Development of porcine model of chronic tachycardia-induced cardiomyopathy. Int J Cardiol 2011; 153: 36-41.
- Galis ZS, Muszynski M, Sukhova GK, et al. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res 1994; 75: 181-189.
- Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005; 38 :73-83.
- Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem 1991; 198: 391-398.
- Pfaffl MW. A new mathematical model for quantification in real-time real time PCR. Nucleic Acids Res 2001; 29: e45.
- Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem 2003; 51: 821-829.
- Shapiro SD, Fliszar CJ, Broekelmann TJ, Mecham RP, Senior RM, Welgus HG. Activation of the 92-kDa gelatinase by stromelysin and 4-aminophenylmercuric acetate. Differential processing and stabilization of the carboxyl-terminal domain by tissue inhibitor of metalloproteinases (TIMP). J Biol Chem 1995; 270: 6351-6356.
- Yamashita C, Hayashi T, Mori T, et al. Angiotensin II receptor blocker reduces oxidative stress and attenuates hypoxia-induced left ventricular remodeling in apolipoprotein E-knockout mice. Hypertens Res 2007; 30: 1219-1230.
- Leber TM, Balkwill FR. Zymography: a single-step staining method for quantitation of proteolitic activity on substrate gels. Anal Biochem 1997; 249: 24-28.
- Barnes A, Bee A, Bell S, Gilmore W, Mee A, Morris R, Carter SD. Immunological and inflammatory characterisation of three canine cell lines: K1, K6 and DH82. Vet Immunol Immunopathol 2000; 75: 9-25.
- Moll UM, Youngleib GL, Rosinski KB, Quigley JP. Tumor promoter-stimulated Mr 92,000 gelatinase secreted by normal and malignant human cells: isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res 1990; 50: 6162-6170.
- Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, Squire IB. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodeling and prognosis after acute myocardial infarction. Eur Heart J 2008; 29: 2116-2124.
- Shrestha K, Borowski AG, Troughton RW, Thomas JD, Klein AL, Tang WH. Renal dysfunction is a stronger determinant of systemic neutrophil gelatinase-associated lipocalin levels than myocardial dysfunction in systolic heart failure. J Card Fail 2011; 17: 472-478.
- Yan AT, Yan RT, Spinale FG, et al. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail 2006; 12: 514-519.
- Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003; 107: 1579-1585.
- Hemdahl AL, Gabrielsen A, Zhu C, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol 2006; 26: 136-142.
- Yndestad A, Landro L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J 2009; 30: 1229-1236.
- Batlle M, Perez-Villa F, Garcia-Pras E, et al. Down-regulation of matrix metalloproteinase-9 (MMP-9) expression in the myocardium of congestive heart failure patients. Transplant Proc 2007; 39: 2344-2346.
- Rastogi S, Gupta RC, Mishra S, Morita H, Tanhehco EJ, Sabbah HN. Long-term therapy with the acorn cardiac support device normalizes gene expression of growth factors and gelatinases in dogs with heart failure. J Heart Lung Transplant 2005; 24: 1619-1625.
- Sakata Y, Yamamoto K, Mano T, et al. Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of angiotensin-converting enzyme inhibitor. Circulation 2004; 109: 2143-2149.
- Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J 2011; 278: 28-45.
- Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 1998; 97: 1708-1715.
- Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol 1996; 148: 1639-1648.
- Opdenakker G, Van den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 2001, 69: 851-859.
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA 2007; 104: 20262-20267.
- Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthitis Rheum 2007; 56: 3326-3335.
- Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL) modulation of MMP-9 activity by NGAL. J Biol Chem 2001; 276: 37258-37265.
- Nuntagowat C, Leelawat K, Tohtong R. NGAL knockdown by siRNA in human cholangiocarcinoma cells suppressed invasion by reducing NGAL/MMP-9 complex formation. Clin Exp Metastasis 2010; 27: 295-305.
- Okada Y, Gonoji Y, Naka K, et al. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells, purification and activation of the precursor and enzymatic properties. J Biol Chem 1992; 277: 21712-21719.
- Tschesche H, Zoelzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem 2001; 268: 1918-1928.
A c c e p t e d : March 26, 2014