ELEVATED SERUM OSTEOPROTEGERIN IS ASSOCIATED WITH DECREASED OSTEOCLASTIC DIFFERENTIATION IN STENOTIC AORTIC VALVES
INTRODUCTION
The tripartite RANK/RANKL/OPG (receptor activator of nuclear factor κB, its ligand, and osteoprotegerin) signalling system plays a key role in the osteogenesis and bone remodelling by controlling recruitment, differentiation and function of the cells involved (1). OPG is a soluble member of the tumour necrosis factor receptor superfamily, expressed by cells (e.g. endothelial, vascular smooth muscle, osteoblastic) in various organs (2-4). It acts as a decoy receptor for RANKL, inhibiting its interaction with RANK on osteoclast precursor cell membranes, thus preventing their osteoclastic differentiation (1).
Recent studies suggest that calcific aortic valve stenosis (CAVS), the most frequent non-rheumatic heart valve disease and the main cause of valve replacement in the elderly is an actively regulated process that involves mechanisms of bone development (4-10). Altered expression of RANK, RANKL and OPG was observed in native stenotic valves as well as in calcified aortic valve allografts (10, 11). Moreover, serum levels of OPG are elevated in patients with CAVS (12).
The aim of the present study was to assess whether serum levels of OPG and sRANKL can be correlated with the character of histopathological changes in the valves of the patients with CAVS.
MATERIALS AND METHODS
Patients and tissue material
Stenotic aortic valves were obtained from patients with CAVS (n=27) undergoing valve replacement surgery (mean age 71.56±6.37 years; 16 males, 11 females). Only tricuspid valves without signs of infective endocarditis or rheumatic heart disease were included to the study. Control, macroscopically normal aortic valves (n=8) were collected upon autopsies from age- and sex-matched individuals. In CAVS patients, serum samples for measurements of OPG and sRANKL were collected twenty four hours before surgery and stored at –80°C until use. The respective controls (n=12) were serum samples collected from age- and sex-matched patients without aortic valve disease. Otherwise, the clinical parameters of the control group did not significantly differ from the group of stenotic patients. The study protocol was approved by the Bioethical Committee of the Jagiellonian University.
Assessment of osteoprotegerin and sRANKL
The concentration of OPG in serum was measured by multiplex Luminex technology using Human Bone Panel kit (Millipore, USA), according to the manufacturer’s instruction. Data were analyzed using a Flexmap 3D instrument (Luminex xPONENT® 4.0 Software) and Luminex Analyst Program (Luminex Corporation, Austin, Texas, USA).
The levels of sRANKL were measured using enzyme-linked immunosorbent assay (ELISA) kit (BioVendor, Brno, Czech Republic), according to the manufacturer’s instruction. Inter- and intra-assay coefficients of variation of both tests were between 7% and 11%.
Macroscopic analysis of valve cusps
The valve cusps were examined under Stemi 2000C stereomicroscope (Zeiss, Germany) coupled to Coolpix 990 digital camera (Nikon, Japan) and PC-class computer equipped with AnalySIS-FIVE® (Soft Imaging System GmbH, Munster, Germany) image analysis system. The following parameters were assessed: area of focal calcifications (measured at the aortic side and verified by von Kossa staining), expressed as percentage of the total cusp area, unilateral (aortic side only) or bilateral (aortic and ventricular sides) location of calcifications, and involvement of the cusp base (valve ring) in calcification.
Preparation of valve cups for miscoscopy
The cups of stenotic valves were dissected into two parts along the line passing from cusp base to its free margin through the most pronounced focal calcifications. The cusps of control valves were likewise divided into two halves. One part/half was fixed for 24 hours in 4% buffered paraformaldehyde, decalcified for 4 days in 10% EDTA and routinely embedded in paraffin. Six µm sections were mounted on polylysine-coated slides (Menzel-Glaser; Thermo Scientific, Germany). The other part/half was embedded in OCT (Jung, Nussloch, Germany) and frozen. Ten µm cryostat (Jung CM1800, Leica Instruments GmbH, Germany) sections were mounted on polylysine-coated slides, dried in air and fixed for 5 min in 4% buffered paraformaldehyde.
Histology and histochemistry
Deparaffinized sections were stained routinely with hematoxylin and eosin. Frozen sections were stained with Oil red O (ORO) to reveal lipids.
Immunohistochemistry
Deparaffinized sections were first boiled in citrate buffer (pH 6.0) for antigen retrieval and then preincubated for 40 min with 5% normal goat serum (Vector, Burlingame, CA; # S-1000) in PBS containing 0.01% sodium azide, 0.05% thimerosal, 0.1% bovine serum albumin and 0.5% Triton X-100 to reduce nonspecific binding and to increase penetration of the antibodies. The sections were next incubated overnight with the primary antibodies against: tartrate-resistant acid phosphatase (TRAP), dilution 1:50 (code no. NCL-TRAP; Novocastra, Newcastle, UK), to visualize cells with osteoclastic differentiation (13); CD68 antigen, dilution 1:1 (code no. IHCR2113-6; Chemicon, Temecula, CA), a marker of clasically activated (M1) macrophages (14); CD34 antigen, dilution 1:50 (code no. NCL-END; Novocastra, Newcastle, UK), a marker of endothelial cells, to reveal blood vessels (15). Next, sections were washed extensively in PBS and incubated for 90 min with goat anti-mouse Alexa555-conjugated antibody, dilution 1:200 (code no. A-21424; Molecular Probes, Eugene, OR). Cell nuclei were counterstained with DAPI (Sigma, Saint Louis, MO, USA). Sections were washed three times in PBS and mounted in glycerol/PBS solution (pH=8.6). Negative controls were performed by omitting the primary antibodies during the first incubation.
Microscopy and morphometry
Sections were examined under Olympus BX50 bright field/fluorescence microscope (Olympus, Japan). Images were recorded using DP-71 digital CCD camera (Olympus, Japan) coupled to PC-class computer equipped with AnalySIS-FIVE® (Soft Imaging System GmbH, Munster, Germany) image analysis system. Sections of valves containing mono- and multinuclear TRAP-positive cells were also examined in a laser scanning (confocal) fluorescence microscope (FluoView FV10i, Olympus, Tokyo, Japan). Images were collected using the FV10-ASW v.3.00 software (Olympus, Japan).
Microvessel density and the number of CD68-positive macrophages was assessed semiquantitatively in 10 randomly chosen high magnification (400×) fields per valve sample according to the following 0–3 scale:
macrophages: 0 – no cells; 1 – single cells per field; 2 – up to 3 cell groups or numerous single cells per field; 3 – numerous cell groups per field;
blood vessels: 0 – no blood vessels; 1 – 1-3 vascular profiles per field; 2 – 4-10 vascular profiles per field; 3 – >10 vascular profiles per field.
The area occupied by ORO-stained lipids was measured using the image analysis system and expressed as percentage of the total cusp section area.
Statistical analysis
The variables were expressed as mean ± S.D. or median (lower-upper quartile values) depending on their type and distribution. The Kolmogorov-Smirnov test was used to access conformity with a normal distribution. The statistical analysis included (in compliance with the specific category of collected data) t-Student test for unpaired data, Mann-Whitney U test, and χ2 or Fisher’s exact tests. The one-way ANOVA (followed by the Newman-Keuls post hoc test) or Kruskal-Wallis tests were used for multiple group comparison. Stepwise multivariate logistic regression analysis was performed to test whether the level of circulating OPG is a predictor of the valvular TRAP positive cells. Age, gender and serum sRANKL, were tested as potential covariates. Statistical analyses were performed using Statgraphics 5.1 Plus for Windows (Statpoint Technologies INC, Warrenton, USA) software. In all tests two-tailed p values <0.05 were considered statistically significant.
RELULTS
Histopathological examination
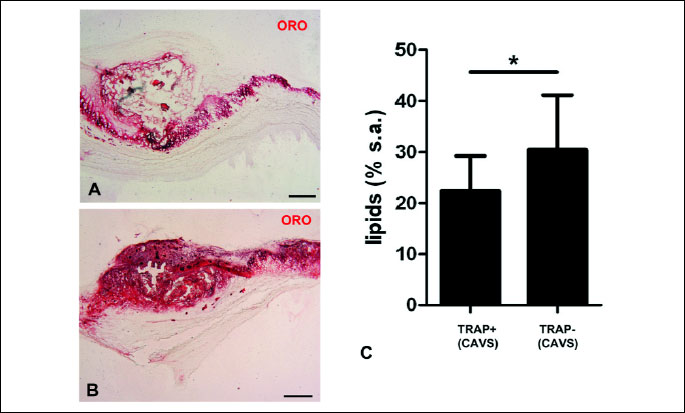
Microscopic examination of valve sections revealed more or less extensive focal calcifications in all stenotic valves and in 4 cases osteogenic metaplasia (bone trabeculae). Cells positive for TRAP (an enzyme expressed by cells undergoing osteoclastic differentiation) were found in 14 (51.9%) stenotic valves; no such cells were observed in the control valves (Fig. 1). These cells were mostly mononuclear, large multinuclear cells with typical osteoclastic morphology were found in valves of 4 (14.8%) patients. TRAP-positive cells were predominantly located in the vicinity of focal calcifications and in cases of valvular osteogenic metaplasia on the surfaces of bone trabeculae. All valves with osteogenic metaplasia contained TRAP-positive cells. CD68-positive macrophages also showed preferential location associated with focal calcifications, however, their distribution was more uniform, with relatively numerous cells located in non-affected parts of fibrosa and spongiosa. In the control valves, their number was significantly lower than in the stenotic valves (p=0.02). Blood vessels (mostly capillaries and just a few arterioles and venules) were observed close to focal calcifications in 20 (74.1%) stenotic valves and were absent from control valves. In stenotic valves, lipid deposits were located inside and in the vicinity of focal calcifications; lipid-rich strands of tissue were also observed in the superficial layer of fibrosa in other valve areas (Fig. 2). The control valves showed only trace lipid content: lipid-occupied area >1% was found only in two valves.
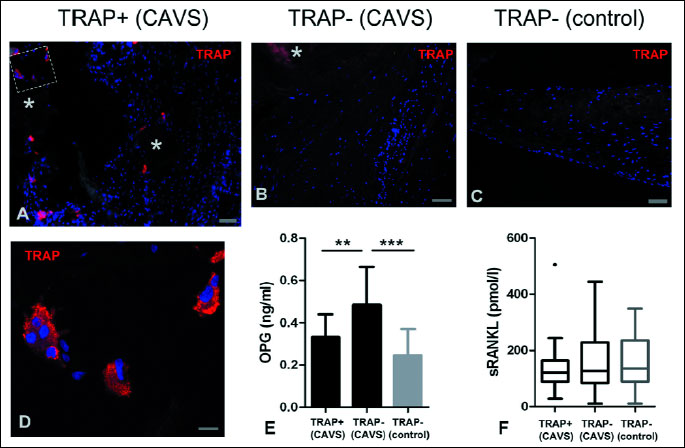
Circulating osteoprotegerin level influences osteoclastic differentiation in calcified aortic valves
Serum levels of OPG were significantly higher in patients with CAVS than in the control group (0.41±0.16 ng/ml vs 0.25±0.12 ng/ml; p=0.005), while no significant difference was found in case of sRANKL (126.1 [87.75-155.7] pmol/l vs 134.8 [89.36-235.2] pmol/l; p=0.5).
We examined the relation between OPG and sRANKL serum levels of CAVS patients and the histological parameters of their valves. The only significant association was observed in case of OPG and the presence or absence of TRAP-positive cells: significantly lower OPG serum concentration (p<0.01), comparable to that of the control group, was found in patients, whose valves contained such cells (Fig. 1).
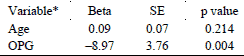
Stepwise multivariate logistic regression analysis was performed to evaluate OPG as a predictor of the occurrence of valvular TRAP-positive cells. Age, gender and serum sRANKL (factors known to be related to calcification/ ossification processes) were tested as potential covariates. OPG was the only variable predictive for the TRAP-positive cells (Table 1).
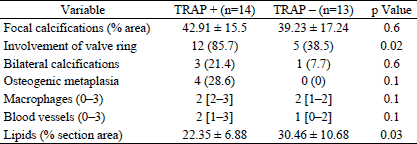
Then we checked whether the difference in the content of TRAP-positive cells between the patients with aortic stenosis is correlated with differences in the histopathological parameters of their valves (Table 2). Significant differences between the groups with and without TRAP-positive cells were observed in the presence of continuity between cusp and valve ring calcifications and in abundance of lipid deposits (Fig. 2). The presence of TRAP-positive cells was negatively correlated with the lipid content in the valves and positively correlated with valve ring calcification.
DISCUSSION
The calcific aortic valve stenosis (CAVS) is now widely regarded as an active biological process involving mechanisms similar to osteogenesis. Calcification of the valve is associated with the appearance of osteoblast-like cells expressing markers found also in skeletal osteoblasts (8, 16). Mononuclear and multinuclear cells showing osteoclastic differentiation are also observed in calcifying human valves (5, 17, 18). Hence, the involvement of the RANK/RANKL/OPG signalling system in the pathogenesis of CAVS has been postulated (19). Moreover, OPG, as a decoy receptor for TNF-related apoptosis-inducing ligand (TRAIL) (20), can also influence calcification of the valves via its interaction with TRAIL which was recently demonstrated to show high expression in calcific valves and to promote matrix synthesis and mineralization in cultured valvular interstitial cells (21).
We could not observe any relation between the occurrence of TRAP-positive cells and the area of calcification in the valve, although calcification of the valve ring was mostly associated with the presence of such cells. Moreover, true bone formation was found only in the valves that contained TRAP-positive cells. This might suggest that inhibited osteoclastic differentiation in the valve acts towards preventing osteogenic metaplasia and restricting the expansion of the calcification process in the valve. The concept that osteoclastic differentiation is important for osteogenic metaplasia is in line with results recently published by Chai et al. (22), who reported that in vivo ectopic bone formation is associated with osteoclastogenesis and osteoclastic activity.
Lipid accumulation was significantly lower in the valves with TRAP-positive cells. The neutral lipids detected by ORO staining are mostly oxidized LDLs (23). Enhanced content of LDLs in the valves of patients with elevated serum OPG and the reported effect of statins which were found to lower serum OPG level in patients with CAVS and carotid stenosis indicate a possible link between OPG and lipid accumulation in the stenotic valves (24, 25).
The main and to the best of our knowledge novel finding of this study is that elevated levels of circulating OPG is associated with inhibited osteoclastic differentiation in calcifying aortic valves. Recruitment of monocytes, precursors of macrophages and osteoclasts, to areas of atherosclerosis and calcification is controlled by several factors (26). The inhibition of osteoclast differentiation and recruitment is a well known effect of OPG in the skeletal system, but it remains unclear how it influences the local calcification process in the valve (1). On one hand, deficiency of osteoclasts which are capable of mineral resorption in bone and atherosclerotic arteries should promote calcification of the valve (27). On the other hand, in hypercholesterolemic mice OPG was demonstrated to attenuate pro-calcific processes in aortic valves (28). Moreover, in the animal model of atherosclerosis, which shares many features with calcific valve degeneration, OPG inhibits calcification. Mice deficient in both OPG and apolipoprotein E (ApoE) had larger calcified atherosclerotic lesions as compared to ApoE-knockout animals (29). Administration of recombinant osteoprotegerin to ldlr-deficient mice fed atherosclerotic diet reduced the size of calcified lesion area (30). Denosumab, an anti-RANKL antibody mimicking the action of OPG, reduced calcium deposition in aortic wall of glucocorticoid-treated human RANKL knock-in mice (31). In contrast, human studies provided some evidence of an opposite effect of OPG: high circulating OPG levels were demonstrated to enhance the severity and 10-year progression of carotid atherosclerosis and to enhance the severity of aortic calcification in hemodialysis patients (32, 33).
OPG and RANKL are also expressed in the valvular tissue and in myocardium, so local paracrine effects of these factors can not be excluded. However, an increased local expression of OPG and RANKL was observed in damaged myocardium of patients with myocardial infarction developing heart failure (34), in non-ischemic dilated cardiomiopathy (35) or in atrial fibrillation (36). In CAVS, most authors reported low or no expression of OPG in the calcified human valves (10, 11, 37), while OPG expression in myocardium seems to be a secondary effect, since myocardium and peripheral tissues can extract OPG and other factors from the circulation (38, 39). A contradictory result was reported by Pohjolainen et al. (7) who studied a wide spectrum of aortic valves from normal to heavily calcified and found progressive elevation of OPG gene expression and immunohistochemically detectable OPG content in the valves. Irrespective of this controversy, it seems that not only locally produced, but also circulating OPG can contribute to the calcification process in the valve (28).
In view of reports showing that drug therapies tested in CAVS (24) or used in other pathologies (25, 40, 41) may directly or indirectly influence serum OPG level, our observation can also be clinically relevant, although so far it is not clear whether the modification of the local valvular milieu by OPG reported in this study is beneficial or detrimental for CAVS patients.
In conclusion, our results suggest that elevated circulating OPG in patients with calcific aortic valve stenosis is associated with the inhibition of local osteoclastogenesis and osteogenic metaplasia in the stenotic valves. This aspect of OPG action should also be taken under consideration in planning treatment strategies that influence the RANK/RANKL/OPG system.
Acknowledgements: This work was supported by statutory grants (K/ZDS/000987 and K/ZDS/003823 to G.J.L.) from the Jagiellonian University Medical College.
Conflict of interest: None declared.
REFERENCES
- Trouvin AP, Goeb V. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging 2010; 5: 345-354.
- Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J Biol Chem 2000; 275: 20959-20962.
- Hofbauer LC, Shui C, Riggs BL, et al. Effects of immunosuppressants on receptor activator of NF-kappaB ligand and osteoprotegerin production by human osteoblastic and coronary artery smooth muscle cells. Biochem Biophys Res Commun 2001; 280: 334-339.
- Galeone A, Paparella D, Colucci S, Grano M, Brunetti G. The role of TNF-alpha and TNF superfamily members in the pathogenesis of calcific aortic valvular disease. ScientificWorldJournal 2013; 2013: 875363.
- Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 2001; 103: 1522-1528.
- Kaden JJ, Bickelhaupt S, Grobholz R, et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis 2004; 13: 560-566.
- Pohjolainen V, Taskinen P, Soini Y, et al. Noncollagenous bone matrix proteins as a part of calcific aortic valve disease regulation. Hum Pathol 2008; 39: 1695-1701.
- Rajamannan NM, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003; 107: 2181-2184.
- Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011; 124: 1783-1791.
- Steinmetz M, Skowasch D, Wernert N, et al. Differential profile of the OPG/RANKL/RANK-system in degenerative aortic native and bioprosthetic valves. J Heart Valve Dis 2008; 17: 187-193.
- Shetty R, Pepin A, Charest A, et al. Expression of bone-regulatory proteins in human valve allografts. Heart 2006; 92: 1303-1308.
- Akat K, Kaden JJ, Schmitz F, et al. Calcium metabolism in adults with severe aortic valve stenosis and preserved renal function. Am J Cardiol 2010; 105: 862-864.
- de Vries TJ, Schoenmaker T, Beertsen W, van der Neut R, Everts V. Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J Cell Biochem 2005; 94: 954-966.
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008; 13: 453-461.
- Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 2006; 54: 385-395.
- Miller JD, Weiss RM, Serrano KM, et al. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol 2010; 30: 2482-2486.
- Cappelli S, Epistolato MC, vianello A, et al. Aortic valve disease and gamma-glutamyltransferase: accumulation in tissue and relationships with calcific degeneration. Atherosclerosis 2010; 213: 385-391.
- Lis GJ, Litwin JA, Kapelak B, et al. Development of mature lamellar bone with a hematopoietic compartment in an aortic valve homograft. J Heart Valve Dis 2009; 18: 578-580.
- Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res 2011; 108: 1392-1412.
- Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 1998; 273: 14363-14367.
- Galeone A, Brunetti G, Oranger A, et al. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int J Cardiol 2013; 169: 296-304.
- Chai YC, Roberts SJ, Desmet E, et al. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials 2012; 33: 3127-3142.
- Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 1999; 19: 1218-1222.
- Dimitrow PP, Jawien M, Gackowski A. The influence of statins on levels of calcification biomarkers in patients with aortic sclerosis or mild aortic stenosis. J Heart Valve Dis 2011; 20: 18-22.
- Kadoglou NP, Gerasimidis T, Moumtzouoglou A, et al. Intensive lipid-lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur J Vasc Endovasc Surg 2008; 35: 661-668.
- Jawien J, Toton-Zuranska J, Gajda M, et al. Angiotensin-(1-7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol 2012; 63: 77-85.
- Doherty TM, Uzui H, Fitzpatrick LA, et al. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J 2002; 16: 577-582.
- Weiss RM, Lund DD, Chu Y, et al. Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice. PLoS One 2013; 8: e65201.
- Bennett BJ, Scatena M, Kirk EA, et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler Thromb Vasc Biol 2006; 26: 2117-2124.
- Morony S, Tintut Y, Zhang Z, et al. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation 2008; 117: 411-420.
- Helas S, Goettsch C, Schoppet M, et al. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol 2009; 175: 473-478.
- Kiechl S, Schett G, Wenning G, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004; 109: 2175-2180.
- Nitta K, Akiba T, Uchida K, et al. Serum osteoprotegerin levels and the extent of vascular calcification in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 1886-1889.
- Ueland T, Yndestad A, Oie E, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation 2005; 111: 2461-2468.
- Schoppet M, Ruppert V, Hofbauer LC, et al. TNF-related apoptosis-inducing ligand and its decoy receptor osteoprotegerin in nonischemic dilated cardiomyopathy. Biochem Biophys Res Commun 2005; 338: 1745-1750.
- Cao H, Li Q, Li M, et al. Osteoprotegerin/RANK/RANKL axis and atrial remodeling in mitral valvular patients with atrial fibrillation. Int J Cardiol 2013; 166: 702-708.
- Kaden JJ, Bickelhaupt S, Grobholz R, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol 2004; 36: 57-66.
- Helske S, Kovanen PT, Lindstedt KA, et al. Increased circulating concentrations and augmented myocardial extraction of osteoprotegerin in heart failure due to left ventricular pressure overload. Eur J Heart Fail 2007; 9: 357-363.
- Zheng L, Xu J, Qiu W, et al. Cardioprotection of exogenous erythropoietin in mice with ligature-induced aortic stenosis on maladaptive cardiac hypertrophy. J Physiol Pharmacol 2010; 61: 13-20.
- Fichna M, Zurawek M, Fichna P, Gryczynska M, Nowak J, Ruchala M. Increased serum osteoprotegerin in patients with primary adrenal insufficiency receiving conventional hydrocortisone substitution. J Physiol Pharmacol 2012; 63: 677-682.
- Mori K, Jono S, Emoto M, et al. Effects of pravastatin on serum osteoprotegerin levels in patients with hypercholesterolemia and type 2 diabetes. Angiology 2010; 61: 86-91.
A c c e p t e d : March 25, 2014