THE PREVENTION OF ISCHEMIA/REPERFUSION INDUCED OXIDATIVE DAMAGE BY VENOUS BLOOD IN RABBIT KIDNEYS MONITORED WITH BIOCHEMICAL, HISTOPATOLOGICAL AND IMMUNOHISTOCHEMICAL ANALYSIS
INTRODUCTION
The ischemia/reperfusion process (IR) occurs during kidney transplants and various urological surgical procedures and can lead to serious kidney damage (1). Adenosine triphosphate, produced by aerobic tissues, is fragmented into adenosine monophosphate, adenosine, inosine and hypoxanthine. The produced hypoxanthine is metabolised by xanthine dehydrogenase to form xanthine and uric acid. Since nicotinamide dinucleotide is used in this process, no toxic oxygen radicals are formed (2, 3). One important feature of ischemia is the conversion of xanthine dehydrogenase to xanthine oxidase (XO) (4). Because molecular oxygen (O2) is used in the metabolism of hypoxanthine with XO (via the XO + O2 reaction), toxic oxygen radicals are produced as intermediate products. However, due to the lack of O2 in ischemic tissue, the accumulated hypoxanthine cannot be converted to xanthine, and no toxic oxygen radicals are produced as intermediate products (2). During prolonged ischemia, the discharge of cellular energy stores and the accumulation of toxic metabolites can lead to cell death (5). For this reason, the first intervention in tissue ischemia is to provide re-vascularisation of the tissue. However, as soon as reoxygenation occurs through reperfusion, the XO formed during ischemia converts the accumulated hypoxanthine into xanthine using O2 (4, 6), leading to the formation of excessive free oxygen radicals (7). These free oxygen radicals, which are known as mediators of reperfusion, oxidise the cell membrane lipids, resulting in the formation of toxic products such as aldehyde and malondialdehyde (MDA) (8). Furthermore, the oxygen radicals react with DNA and cause oxidative damage (9), including base changes in nucleic acids and DNA chain breakage. If these changes are not repaired, they result in mutations; such as, hydroxyguanine 8-(8-OHGua), which is formed by the oxidation of the guanine base, is accepted to be a mutagenic type of DNA (9, 10). As noted above, excessive free radical formation occurs due to the excess O2 released into the tissue during the reperfusion at the site of ischemic injury (11, 12). Thus, the return of the oxygen-rich arterial blood to the ischemic tissue initiates the XO + O2 reaction, which causes further damage. Therefore, IR damage can be prevented by reperfusion with venous blood containing less O2, by either sudden stabilisation of the XO + O2 reaction, or the direct neutralisation of the products of the XO + O2 reaction. This suggests that the accelerated XO + O2 reaction can be inhibited by venous blood, which contains 60% less oxygen than arterial blood (13). The direct neutralisation of the products of the XO + O2 reaction can be achieved by melatonin or any other antioxidant. Melatonin is known to neutralise free oxygen radicals directly (14, 15). However, there is no information in the literature describing the protective effect of the venous blood in IR-induced renal injury in rabbits.
Therefore, the purpose of this study was to investigate the biochemical immunohistochemical and histopathological effects of venous blood on IR-induced oxidative damage and DNA mutation compared to those of melatonin treatment, which has a proved protective effect on IR injuries.
MATERIALS AND METHODS
The protocols and procedures were approved by the local Animal Experimentation Ethics Committee (Date: 22. 02. 2012, meeting no: B.30.2.ATA.0.01.02/854).
Animals
The 30 albino New Zealand male rabbits (weighing 3.5–3.8 kg each) used in this study were supplied from the Ataturk University Medical Experimental Research and Application Center. The animals were kept at room temperature (22°C) under a standard 12/12 light/dark cycle with relative humidity of 50 to 60% in groups and were fed.
Drugs and chemicals
Ketamine HCl was obtained from Interhas (Austria) and melatonin was purchased from Przedsiebiorstwo Farmaceutyczne LekAm (Poland).
Study design
General procedure
The rabbits were divided into five groups as follows: renal ischemia (RI), renal ischemia-reperfusion (RIR), renal ischemia-venous blood-reperfusion (RIVR), melatonin + renal ischemia-reperfusion (MRIR), and healthy sham-treated control (HG). The surgical interventions were performed in the laboratory under sterile conditions using ketamine HCl (25 mg/kg via intramuscular injection) for anesthesia.
Surgical and pharmacological procedures
After injection of ketamine HCl, the rabbits were observed until ready for surgery. An hour before the anesthesia, the MRIR group received 2.5 mg/kg melatonin, delivered intra-peritoneally (i.p.) (16). The surgery was performed with animals held motionless in the supine position (17). The kidneys of the rabbits were accessed through a unilateral dorsal incision on the left kidney when rabbits were at that situation mentioned above. Subsequently, a vascular clip was placed on the lower portions of the renal artery (except for animals in the HG group), and resulting ischemia was maintained for one hour. After this period, in the RI group, both kidneys (left and contralateral kidneys) were removed for XO, malondialdehyde (MDA), glutathione (GSH), and hydrox yguanine 8-OHGua measurements and, immunohistochemical and histopathological studies. In the RIVR group, immediately prior to the reperfusion, 1 ml of venous blood withdrawn from the ear vein was injected slowly into renal artery of the left kidney for 5 minutes with a thin needle. To prevent coagulation, 0.2 ml of freshly withdrawn venous blood was administered per minute. In the RIR, RIVR and MRIR groups, the vascular clip was removed to allow reperfusion for three hours. Afterwards, all animals were sacrificed by high dose-anaesthesia, and both kidneys were removed for analyses. Before the animals were sacrificed, venous blood samples were withdrawn from the ear veins to measure blood urea nitrogen (BUN) and creatinine levels. The biochemical and histopathological results of all groups were compared.
Biochemical procedures
Biochemical analysis of the kidney tissue
To measure the enzyme activity in the kidney tissue, renal tissue homogenates were prepared. In the supernatants obtained from these homogenates, XO enzyme activity, MDA and total glutathione (tGSH) levels were measured, and DNA oxidation analysis were performed using appropriate literature-based methods (18-23).
Creatinine analysis
The quantitative determination of serum creatinine levels was performed by spectrophotometric assay using the Roche Cobas 8000 analyser, which uses a kinetic colorimetric test based on the Jaffe method.
Blood urea nitrogen analysis
The serum urea levels were quantified by spectrophotometric assay using the Roche Cobas 8000 analyser. The formula BUN=URE * 0:48 was used for the calculations.
Immunohistochemical procedures
Kidney tissues of rabbit stayed at 10% formaldehyde solution and then 4 mikron thickness of samples were taken onto positively charged lam. Following steps were applied to the samples positioned onto the Leica Bond-Max automatic immunohistochemistry instrument.
1) Keep the temperature at 60°C about 30 min. in order to melt parafin.
2) Hold the sample in dewax solution about 15 min to carry out deparafinization process.
3) Rehydration process was completed by keeping samples in 99% alcohol solution for 15 min.
4) Washing process was done in buffer solution about 3 min.
5) Antigen retrival process was maintained in Epitop 2 solution.
6) Washing process was done in buffer solution about 3 min.
7) Samples were held on 3% hydrogen peroxide solution about 10 min.
8) Washing process was done in buffer solution about 3 min.
9) Samples were incubated around 60 min by adding dropwise Nova Castra Bcl-2 antibody.
10) Washing process was done in buffer solution about 3 min.
11) 3 min incubation was done by adding Post Primer solution.
12) Washing process was done in buffer solution about 3 min.
13) 10 min incubation was done by adding Post Primer solution.
14) Washing process was done in buffer solution about 3 min.
15) Washing process was applied for 3 minutes in distile water.
16) 3 minutes incubation was carried out by dropwise addition of DAB + Choromogen.
17) Washing process was applied in distile water.
18) Staining was applied to the samples for 5 minutes.
19) Washing process was applied in distile water.
20) Samples that were washed with xylene and alcohol were closed by entellan.
The samples that were ready for screening by applying the procedure mentioned above were under investigation by two independent patalogists as blank samples who have no idea about the preview works. According to the investigation made with Olympus BX51, interstisyum, glomelurus and tubule structures were evaluated one by one with respect to their staining patterns against Bcl-2 by using the grade system mentioned below. In this evaluation, intensity and prevelance were seperately take into account. While prevalance shows the existence area of the dye, intensity shows its strogness.
Grading for prevalance:
+ lower than 10%, means weak;
++ 10–50% means intermediate intensity;
+++ means over than 50% signs a strong intensity.
Histopathological procedures
The kidneys were fixed in 10% neutral buffered formalin. After routine tissue follow-up, paraffin blocks were prepared, and 5 µm tissue sections were obtained. These sections were stained with haematoxylin-eosin (H&E) and periodic acid-Schiff (PAS). The light microscopic histopathological evaluation was performed by a pathologist who was blinded to the treatment (Olympus BX 51, Tokyo, Japan). The outer medulla and the cortex which are the most sensitive regions, of the kidney were evaluated for ischemic injury. Tubular necrosis, apoptotic bodies, swelling of tubular epithelium, tubular dilatation, intratubular cast formation, and loss of the hairy edge were evaluated on kidney sections.
Statistical analysis
All data were subjected to one-way analysis of variance (ANOVA) using SPSS 18.0 software. The difference between the groups using one-way ANOVA test, the degree of significance of difference was determined by least significant difference (LSD) option, and significance was declared at p<0.05. The results are explained as the mean ± the standard error of the mean (S.E.M.).
RESULTS
Biochemical findings
Xanthine oxidase test results
As shown in Fig. 1, the xanthine oxidase activities in the left kidney tissues of the RI, RIR, RIVR, MRIR groups were found to be significantly higher than HG, RIK, RIRK, RIVRK and MRIRK groups.
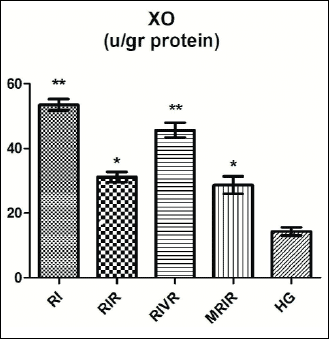 |
Fig. 1. XO activities in renal tissue of RI (renal ischemia), RIR (renal ischemia-reperfusion), RIVR (renal ischemia-venous blood-reperfusion), MRIR (melatonin + renal ischemia-reperfusion), HG (healthy sham control) groups. |
Malondialdehyde test results
The amount of MDA in the left kidney tissues of the RIR group was increased statistically significant than RIVR, MRIR and HG groups (Fig. 2).
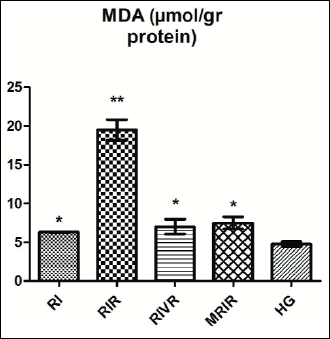 |
Fig. 2. MDA level in renal tissue of RI (renal ischemia), RIR (renal ischemia-reperfusion), RIVR (renal ischemia-venous blood-reperfusion), MRIR (melatonin + renal ischemia-reperfusion), HG (healthy sham control) groups. |
Total glutathione test results
As shown in Fig. 3, the amounts of tGSH decreased significantly in the left kidney tissue following reperfusion. The amounts of tGSH measured in the RIVR, MRIR groups were higher than RIR group.
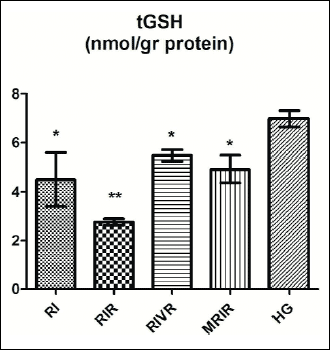 |
Fig. 3. tGSH level in renal tissue of RI (renal ischemia), RIR (renal ischemia-reperfusion), RIVR (renal ischemia-venous blood-reperfusion), MRIR (melatonin + renal ischemia-reperfusion), HG (healthy sham control) groups. |
Hydroxyguanine-8 test results
The amounts of 8-OHGua, a DNA mutagenesis product, were increased in the RIR group, whereas the amounts of 8-OHGua in the RIVRK and MRIRK groups were found to be similar to the levels measured in HG group (Fig. 4).
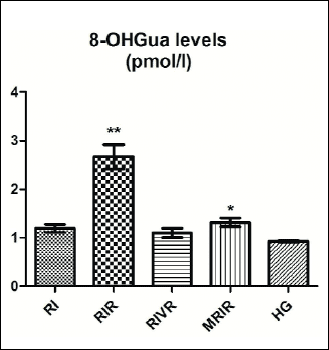 |
Fig. 4. 8-OHGua/Gua level in renal tissue of RI (renal ischemia), RIR (renal ischemia-reperfusion), RIVR (renal ischemia-venous blood-reperfusion), MRIR (melatonin + renal ischemia-reperfusion), HG (healthy sham control) groups. |
Blood urea nitrogen and creatinine test results
As shown in Table 1, the average BUN and the blood creatinine levels in the RIVR and MRIR groups were lower than RIR group.
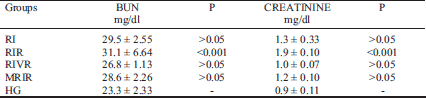
Histopathological findings
As shown in Fig. 5a, normal renal cortex histology was observed in the HG group. In the RI group, significant hemorrhaging was observed in the renal tubule lumen (Fig. 5b, asterisk) and mild swelling and intratubular cast formation in the tubular epithelial cells (Fig. 5b, arrows), whereas the contralateral kidney exhibited an almost normal histological appearance (Fig. 5c).
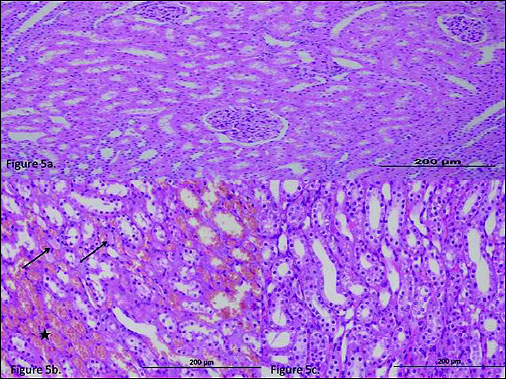 |
Fig. 5. (a) The histo-pathological examination of the renal tissue of HG (healthy control) group. (b), The histo-pathological exami-nation of the renal tissue of RI (renal ischemia) group. There was significant hemorrhage in the renal tubule lumen (asterisk), and mild swelling and intratubular cast formation in the tubular epithelial cells (arrows). (c) The histo-pathological examination of the renal tissue of RIC (renal ischemia contralateral) group. |
In the RIR group, the outer medulla of the kidney exhibited significant epithelial necrosis in tubules (Fig. 6a, asterisk) and numerous apoptotic bodies (Fig. 6a, arrows). The extensive accumulation of hyaline casts was observed in the dilated tubules in the cortex (Fig. 6b, open arrow). No microvilli were observed in the proximal tubules. There was moderate swelling in the contralateral kidney tubule epithelial cells of the RIR group (Fig. 6c).
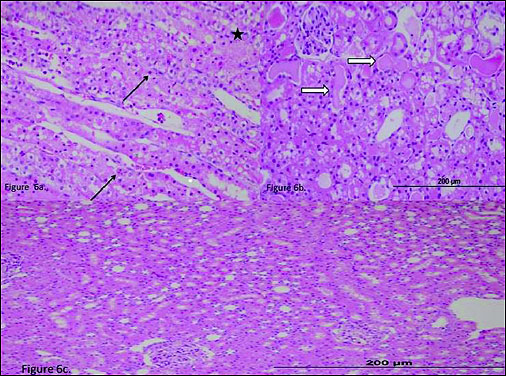 |
Fig. 6. (a) The histo-pathological examination of the renal tissue of RIR (renal ischemia-reperfusion) group. In the outer medulla of the kidney, there was significant epithelium necrosis (asterisk) and numerous apoptotic bodies (arrows). (b) The histo-pathological examination of the renal tissue of RIR (renal ischemia-reperfusion) group. A severe accumulation of hyaline casts were seen in the dilated tubules in the cortex (open arrow). (c) The histo-pathological examination of the renal tissue of RIRC (renal ischemia-reperfusion contralateral) group. |
In the kidney tissue of the RIVR group, mild swelling of the tubular epithelium (Fig. 7a) and a mild accumulation of hyaline casts (Fig. 7b, PAS, open arrow) were observed. The microvilli structures in the proximal tubules were preserved (Fig. 7b, PAS, arrow). Necrosis was rarely observed in the tubular epithelial cells, and apoptotic bodies were not encountered. In the epithelial cells of the contralateral kidney tubules of the RIVR group, only mild swelling was observed (Fig. 7c).
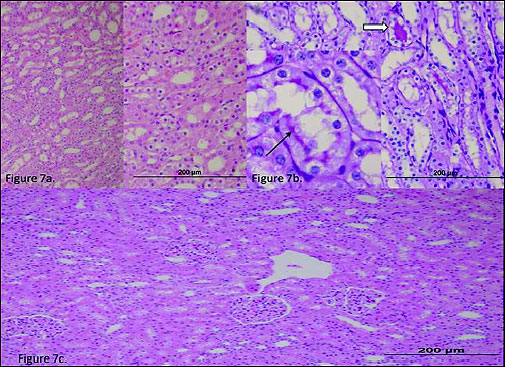 |
Fig. 7. (a) The histo-pathological examination of the renal tissue of RIVR (renal ischemia-venous blood-reperfusion) group. There was seen mild swelling of the tubular epithelium. (b) The histo-pathological examination of the renal tissue of RIVR (renal ischemia-venous blood-reperfu-sion) group. There was seen a mild accumulation of hyaline casts. (c) The histo-pathological examina-tion of the renal tissue of RIVRC (renal ischemia-venous blood-reperfusion contralateral) group. There was observed mild swelling. |
In the outer renal medulla of the MRIR group, a more pronounced swelling of the renal tubule epithelial cells and a small number of necrotic and apoptotic cells (Fig. 8a, arrow) were determined. The microvilli were partially preserved in the proximal tubules in the cortex (PAS, Fig. 8b, arrow). The contralateral renal tubular epithelial cells of the MRIR group exhibited mild swelling (Fig. 8c).
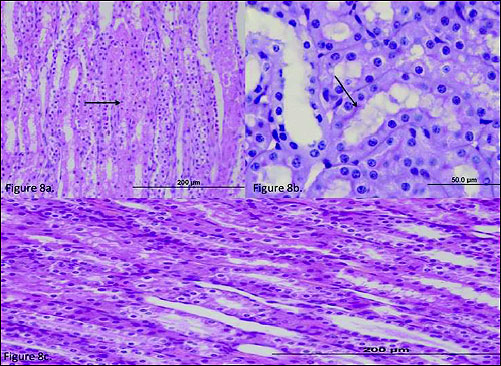 |
Fig. 8. (a) The histo-pathological examination of the renal tissue of MRIR (melatonin + renal ischemia-reperfusion) group. In the tubular epithelium were observed more pronounced swelling of the renal tubule epithelial cells and a small number of necrotic and apoptotic cells (arrow). (b) The histo-pathological examination of the renal tissue of MRIR (melatonin + renal ischemia-reperfusion) group. In the cortex, in the proximal tubules, the microvilli were partially preserved (arrow). (c) The histopathological examination of the renal tissue of MRIRC (melatonin + renal ischemia-reperfusion contralateral) group. |
Immunohistochemical findings
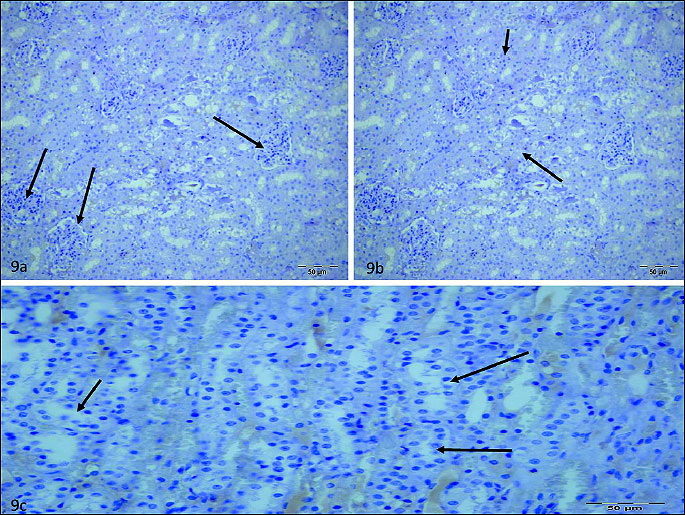
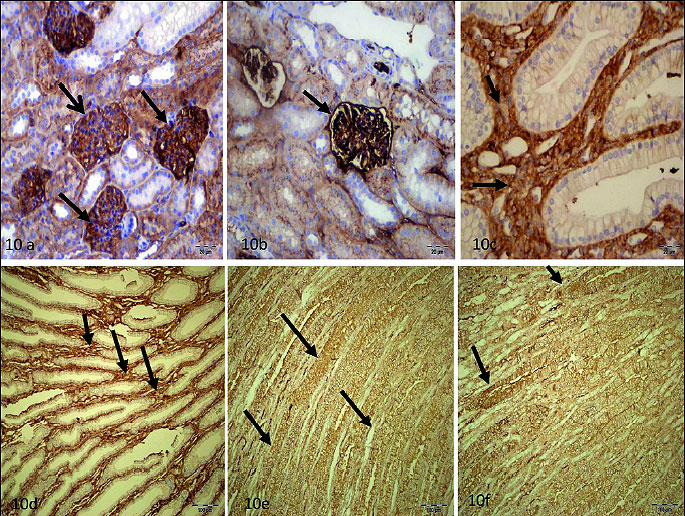
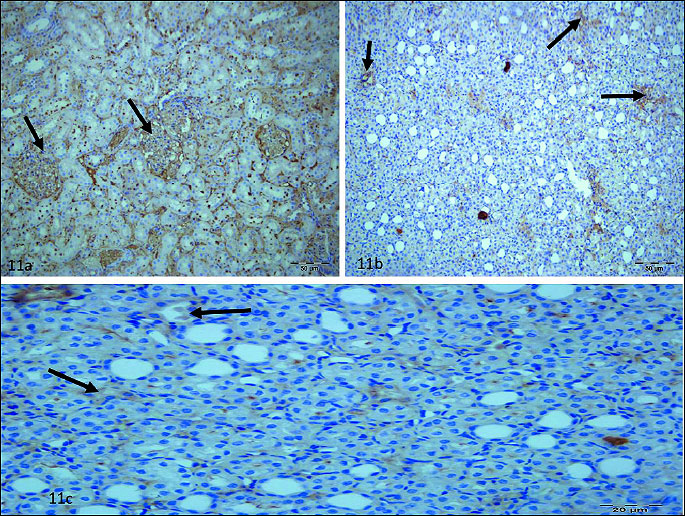
Any staining sign were detected in glomerulus (Fig. 9a), interstitium (Fig. 9b), tubule (Fig. 9c) of RIR group. Staining ratio and intensity of glomerulus cells was higher than 50% in RIVR (Fig. 10a, 10b) group and staining ratio and intensity of interstitium (Fig. 10c, 10d) and tubule cells (Fig. 10e, 10f) was also higher than 50% in that group. This value was found to be lower than 10% in MRIR group (Fig. 11a-11c) glomerulus, interstitium and tubule cells. Pathological findings for glomerulus, interstitium and tubule cells of contralateral kidney of RIR, RIVR and MRIR groups were in parallel with healthy group.
DISCUSSION
Although there are many methods that target IR damage, IR still causes serious complications. To date, no specific protocol has been defined for the use of venous blood in the prevention of IR injury. In this study, XO activity was significantly higher only in the kidney tissue of the RI group compared to all other groups. The increased XO activity in the kidney tissue in the RI group compared to the other groups indicates that the XO metabolism is slower in the ischemic tissue. Furthermore, MDA levels in the RI group were significantly higher than those in the HG group and lower than those in the RIR group. This suggests that XO metabolism and the XO + O2 reaction are more passive in the RI group than in the RIR group. It is known that there is a slight increase in production of free radicals during ischemia (24). Isaoglu et al. have also shown that MDA levels are high and GSH levels are low in ischemic tissues (25). In our study, the maximum increase of MDA and the maximum decrease of GSH were observed in the RIR group. Several studies show that an increase in MDA and deficiency in GSH lead to oxidative stress (26). Reduced GSH plays an important role in protecting cells against oxidative damage from radicals such as hydrogen peroxide (H2O2), hydroxyls (OH●), superoxides (O2●-), and alkoxyls (RO●) (27).
The insignificant increase in the 8-OHGua levels in the RI group indicates the production of low level of free oxygen radicals which appear to be insufficient to cause DNA damage (28). In contrast, the RIR group exhibits excessive 8-OHGua production, and hence an increase in oxygen free radical production. The OH● radicals, which are formed by the reaction of H2O2 with Fe-Cu ions in the nucleus, also cause DNA damage. The OH● radicals react with the carbon atoms of guanine at positions 4, 5 and 8 and create DNA product radicals. The C8-OH radical, which is formed by the participation of the OH● radical to C-8, is oxidized to form 8-OHGua by losing one electron and one proton (29). Mutagenic products, such as 8-OHGua and FAPyGuanin (FAPyG), are formed as a result of the DNA damage caused by the OH● and the O2 radicals (28).
Paller et al. (24) have shown that a small amount of free radicals are formed during ischemia, and a greater amount of free radicals are produced during reperfusion after tissue oxygenation has been re-established. IR injury is the product of the reaction between the XO accumulated during ischemia and the O2 that is abundantly available during reperfusion (7, 11). Therefore, XO is produced during ischemia, and O2 becomes available during reperfusion, and the XO + O2 reaction occurs during IR, which in turn causes free radical production and oxidative stress. Therefore, the stabilisation of the XO + O2 reaction is very important for reducing the IR damage. This increased reaction can be stabilised either by inhibiting XO activity or decreasing the O2 supply to the environment.
Melatonin is able to directly neutralise oxygen radicals (14, 30-32). Melatonin was shown to neutralise the products of the XO + O2 reaction without suppressing the activity of XO, thus protecting the tissue from oxidative stress. Parlakpinar et al. have shown that the kidney damage that occurs due to increased MDA levels and decreased GSH levels after pinealectomy can be corrected by the addition of melatonin (33). These data indicate that melatonin neutralises the products of the XO + O2 reaction without suppressing the activity of XO, thus protecting the tissue from oxidative stress.
In this study, the XO activity in the RIVR group was significantly higher than that in the RIR or MRIR group. However, the lowest amounts of MDA and 8-OHGua, as well as the highest amounts of GSH, were found in the RIVR group. These results indicate that venous blood stabilises the XO + O2 reaction due to the decreased delivery of O2 in the ischemic kidney tissue. The XO activity in the MRIR group was similar to that found in the RIR group. However, the MDA and 8-OHGua levels were lower and the GSH levels were higher in the kidney tissue of the MRIR group than in the RIR group, suggesting that melatonin protected the DNA in the kidney tissue from IR injury. Tan et al. (34) have shown that DNA damage is observed in pinealectomised rats. Radical species damage the DNA and activate the nuclear enzyme poly (ADP-ribose) synthase (PARS). This enzyme causes severe energy dysregulation in response to single strand DNA breaks and necrotic cell death. Melatonin has been reported to prevent the damage to organs through the inhibition of PARP activity in IR injury models (35).
In this study, renal function was evaluated by measuring BUN and creatinine levels. Slightly elevated BUN and creatinine levels were found only in the RIR group. The increases of BUN and creatinine in the RIR group suggest that the contralateral kidney is affected by IR injury. Severe elevation of BUN and creatinine is a sign of permanent damage to the renal tubule (36). However, in our study, because the IR injury was unilateral, there was no severe increase in BUN and creatinine levels.
The results of the biochemical parameters used in the evaluation of the kidney damage are also consistent with our histopathologic and immunohistochemical findings. In the RI group, histopathological findings, such as hemorrhaging, swelling of the tubular epithelial cells and a mild accumulation of intratubular casts, were observed in the lumen of the tubules. Tok et al. observed hemorrhage in ischemic kidney tissue (17). Siesjo et al. (37) have also shown that the swelling of the tubular epithelial cells in ischemic tissue occurs due to a decrease in O2 pressure, which results in a decrease in ATP levels and intracellular Na+ accumulation, the latter of which is associated with an isosmotic increase of water content, leading to acute cellular swelling. Many studies have reported that cast accumulation is observed in IR-induced kidney injury and can be reduced by antioxidant therapy (38-40).
The severe necrosis was observed in the tubular epithelial cells of the RIR group is consistent with the findings in the literature (41). Frega et al. (42) have reported that renal injury is much more severe after reperfusion than after ischemia. In the RIR group, numerous apoptotic bodies were found in the kidney tissue. Apoptosis is one key mechanism in renal IR injury (43). While there is no apoptotic cells were present in the tubular epithellial cells in RIVR group, several apoptotic cells were observed in MRIR group. These histopatological findings were agreed with immunohistochemical data. High level of Bcl-2 gene expression (b cell lymphoma-2) were seen in kidney glomerulus, interstitium and tubule tissues of RIVR group. Bcl-2 gene expression was determined to be mild in MRIR group and no gene expression was detected in RIR group. Bcl-2 gene is an oncoprotein that has a anti-apoptotic functions. It was reported that Bcl-2 gene inhibits caspases by direct and indirect mechanism and therefore prevents apoptosis (44). That is known that Bcl-2 inhibits cell death formed by free radicals (45). This protective effect of Bcl-2 has been shown to prevent the release of cytochrome C and apoptosis inducing factor (AIF) in mitochondria to cytoplasm (46). Prevention of microvillus loss caused by the oxidative stress, formation of tubular necrosis and suppression of apoptosis were exhibited (47).
In this study we have observed that renal damage due to reperfusion is much more severe than the damage caused by ischemia alone. The increased XO + O2 reaction in the kidney tissue after ischemia and reperfusion was stabilised by the use of venous blood, after which oxidant and antioxidant production was controlled protecting the kidney from IR injury. The experimental findings were gathered and examined using biochemical, histopathological and immunohistochemical techniques. Melatonin was thought to directly neutralise the products of the XO + O2 reaction. So, XO activity is not supressed and by this way it protects the tissue from oxidative stress. This study designed to understand the mechanism of IR damage formation has revealed a novel and useful approach to start reperfusion of ischemic tissue with venous blood.
Conflict of interests: None declared.
REFERENCES
- Kren V, Walterova D. Silybin and silymarin - new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2005; 149: 29-41.
- Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol 1988; 254: 768-774.
- Grace PA. Ischaemia-reperfusion injury. Br J Surg 1994; 81: 637-647.
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 2002; 282: 227-241.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72: 65-83.
- Lindsay TF, Liauw S, Romaschin AD, Walker PM. The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J Vasc Surg 1990; 12: 8-15.
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol 1986; 250: 749-753.
- Del Maestro RF. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl 1980; 492: 153-168.
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis 2000; 21: 361-370.
- Huang HY, Helzlsouer KJ, Appel LJ. The effects of vitamin C and vitamin E on oxidative DNA damage: results from a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2000; 9: 647-652.
- Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001; 94: 1133-1138.
- Orrenius S, Ankarcrona M, Nicotera P. Mechanisms of calcium-related cell death. Adv Neurol 1996; 71: 137-149; discussion 49-51.
- Widmaier EP, Raff H, Strang KT. Respiratory physiology. In: Vander, Sherman, Luciano's Human Physiology: The Mechanisms of Body Function, EP Widmaier, H Raff, KT Strang (eds.). McGraw- Hill Companies; 2003. pp. 484-489.
- Reiter RJ. Cytoprotective properties of melatonin: presumed association with oxidative damage and aging. Nutrition 1998; 14: 691-696.
- Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res 2003; 58: 10-19.
- Brzezinska-Slebodzinska E, Slebodzinski AB, Styczynska E. Stimulatory effect of melatonin on the 5 '-monodeiodinase activity in the liver, kidney, and brown adipose tissue during the early neonatal period of the rabbit. J Pineal Res 1998; 24: 137-141.
- Tok A, Sener E, Albayrak A, et al. Effect of mirtazapine on oxidative stress created in rat kidneys by ischemia-reperfusion. Ren Fail 2012; 34: 103-110.
- Armstrong D. Free radical and antioxidant protocols. Introduction. Methods Mol Biol 1998; 108: 5-8.
- Mosca F, Fattorini D, Bompadre S, Littarru GP. Assay of coenzyme Q10 in plasma by a single dilution step. Anal Biochem 2002; 305: 49-54.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Prajda N, Weber G. Malignant transformation-linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Lett 1975; 59: 245-249.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968; 25: 192-205.
- Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2’-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol 1994; 234: 16-33.
- Paller MS. The cell biology of reperfusion injury in the kidney. J Invest Med 1994; 42: 632-639.
- Isaoglu U, Yilmaz M, Calik M, et al. Biochemical and histopathological investigation of the protective effect of disulfiram in ischemia-induced ovary damage. Gynecol Endocrinol 2012; 28: 143-147.
- Ross D. Glutathione, free radicals and chemotherapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol Ther 1988; 37: 231-249.
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983; 52: 711-760.
- Cadet J, Douki T, Gasparutto D, Ravanat JL. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat Res 2003; 531: 5-23.
- De Martinis BS, De Lourdes Pires Bianchi M. Methodology for urinary 8-hydroxy-2'-deoxyguanosine analysis by HPLC with electrochemical detection. Pharmacol Res 2002; 46: 129-131.
- Drobnik J, Tosik D, Piera L, et al. Melatonin-induced glycosaminoglycans augmentation in myocardium remote to infarction. J Physiol Pharmacol 2013; 64: 737-744.
- Gonciarz M, Gonciarz Z, Bielanski W, et al. The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. J Physiol Pharmacol 2012; 63: 35-40.
- Reiter RJ, Rosales-Corral S, Coto-Montes A, et al. The photoperiod, circadian regulation and chronodisruption: the requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin. J Physiol Pharmacol 2011; 62: 269-274.
- Parlakpinar H, Sahna E, Ozer M, Ozugurlu F, Vardi N, Acet A. Physiological and pharmacological concentrations of melatonin protect against cisplatin-induced acute renal injury. J Pineal Res 2002; 33: 161-166.
- Tan D, Reiter RJ, Chen L, Poeggeler B, Manchester LC, Barlow-Walden LR. Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis 1994; 15: 215-218.
- Cuzzocrea S, Reiter RJ. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur J Pharmacology 2001; 426: 1-10.
- Dickey DT, Muldoon LL, Doolittle ND, Peterson DR, Kraemer DF, Neuwelt EA. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol 2008; 62: 235-241.
- Siesjo B. Mechanisms of ischemic brain damage. Crit Care Med 1988; 16: 954-963.
- DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol 1997; 272: F292-F298.
- Slusser SO, Grotyohann LW, Martin LF, Scaduto RC. Glutathione catabolism by the ischemic rat kidney. Am J Physiol 1990; 258: F1547-F1553.
- Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dials Transplant 1999; 14: 923-929.
- Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol 1998; 18: 490-497.
- Frega NS, DiBona DR, Guertler B, Leaf A. Ischemic renal injury. Kidney Int Suppl 1976; 6: 17-25.
- Daemen MA, de Vries B, Buurman WA. Apoptosis and inflammation in renal reperfusion injury. Transplantation 2002; 73: 1693-1700.
- Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 1998; 3: 697-707.
- Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem 1997; 272: 11671-11673.
- Fennell DA, Cotter FE. Controlling the mitochondrial gatekeeper for effective chemotherapy. Br J Haematol 2000; 111: 52-60.
- Sener MT, Sener E, Tok A, et al. A Biochemical and histologic study of lethal cisplatin nephrotoxicity prevention by mirtazapine. Pharmacol Rep 2012; 64: 594-602.
A c c e p t e d : April 25, 2013