ROLE OF NITRERGIC AND ENDOTHELIN PATHWAYS MODULATIONS
IN CISPLATIN-INDUCED NEPHROTOXICITY IN MALE RATS
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Alexandria University, Aleksandria, Egypt;
3Department of Clinical Pathology, Faculty of Medicine, Alexandria University, Aleksandria, Egypt
INTRODUCTION
Although second and third generation platinum agents are now available with improved toxicity profiles, cisplatin remains a highly effective treatment for many malignancy (1). Adverse effects of cisplatin include marked emesis and ototoxicity; however nephrotoxicity is considered as the main dose-limiting toxicity of cisplatin (2). Multiple mechanisms have been implicated in the pathogenesis of cisplatin-induced renal tubular injury including hypoxia, oxidative stress, inflammation, and apoptosis. Tumor necrosis factor-α (TNF-α) is a main cytokine that participates in both an early, vascular phase and a later, proinflammatory phase of renal injury in a murine model of cisplatin nephrotoxicity (3).
Interestingly, nitric oxide (NO) has been suggested to play a modulatory role in cisplatin-induced nephrotoxicity (4). Sildenafil is a selective inhibitor of phosphodiesterase-5 (PDE5), which degrades cyclic guanosine monophosphate (cGMP), a downstream product in the NO-soluble guanylate cyclase cascade in endothelial cells. Recently, several studies have shown that, in addition to treating erectile dysfunction, sildenafil can prevent or decrease tissue injury. In vivo and in vitro, early treatment with sildenafil ameliorated the progression of renal damage in the “5/6 nephrectomy” model (5), and sildenafil has been reported to provide cardioprotection against ischemic injury when infused at the onset of reperfusion in rabbits (6). In addition, sildenafil was found to reduce cisplatin-induced nephrotoxicity in rats (7).
Endothelins (ETs) cause dose-dependent vasoconstriction in most vascular beds. There is increasing evidence that endothelins participate in a variety of cardiovascular diseases (8), including hypertension, cardiac hypertrophy, as well as in renal failure (9). Interestingly, increased level of both circulating ET-1 and urinary excretion of ET-1 have been observed in patients treated with nephrotoxic agents as cyclosporine A and tacrolimus (10). Moreover, expressions of ET-1 have been found to be elevated in mice with cisplatin-induced nephrotoxicity, suggesting that ET-1 might play a role in the pathogenesis of such a disease (11).
Although the protective role of either NO or endothelin receptors modulation on the severity of cisplatin-induced nephrotoxicity has been recognized in previous studies including our own, the possible interaction between the two pathways remains obscure. Therefore, the current study tested for the first time the possible interaction between the nitrergic and endothelin pathways in cisplatin-induced nephrotoxicity in male rats. And furthermore, whether this interaction might be beneficial or not.
MATERIALS AND METHODS
Chemicals
Cisplatin: Cisplatine® Mylan 10 ml vial, Oncotec Pharma production, Germany; the endothelin (ET)-A receptor antagonist BQ-123: BQ-123 sodium salt 5 mg, purchased from Peptides International, Louisville, Kentucky, USA; sildenafil: viagra® tablets (sildenafil citrate equivalent to 50 mg Sildenafil, Pfizer Egypt S.A.E Cairo, A.R.E under authority of Pfizer INC, USA). A 2 mg/ml working solution was freshly prepared by triturating viagra® tablets (sildenafil citrate equivalent to 50 mg Sildenafil) in sterile physiological saline. Other chemicals and reagents used in biochemical tests were of analytical grade. Details of quantitative analysis kits are under biochemical analysis section.
Experimental design
The research experiments protocol was approved by the Animal Care & Use Committee (ACUC) at the faculty of Pharmacy, Alexandria University, Egypt.
Experiments were carried out on thirty Sprague Dawley male rats (200–220 g) obtained from the Animal House of the Faculty of Pharmacy and Drug Manufacturing, Pharos University in Alexandria. Animals were divided into four groups:
I) control, given a single dose of normal saline, i.p., n=7;
II) cisplatin, given a single dose of cisplatin, 6 mg/kg, i.p., n=7 (12);
III) cisplatin + sildenafil, 2 mg/kg, i.p., a submaximal dose chosen from preliminary studies, n=8;
IV) cisplatin + sildenafil + BQ-123, 1 mg/kg, i.p., n=8 (13).
Each of the co-admininstered drugs was given in two doses; one hour before and one day after the cisplatin dose. The rationale of this schedule of dose administration was the maintenance of a steady sufficient plasma concentration of each of the drugs before, during and after the critical period of cisplatin-induced toxicity, since the biochemical changes that occur in the kidney after cisplatin administration are of crucial importance in determining the extent of a nephrotoxic lesion (14).
Biochemical analysis
Under mild anesthesia, blood samples were collected from the rat orbital plexus 96 hours after the cisplatin injection using non-heparinized capillary tubes. Serum was recovered and stored at –70°C until analyzed for determination of blood urea nitrogen (BUN) and serum creatinine using the Randox® assay kit (Randox Laboratories Ltd., United Kingdom). After collecting blood samples, rats were euthanized with an over dose of the anesthetic drug, rat kidneys were quickly removed, washed in ice cold saline, blotted dry, weighed, then homogenized with ice-cold saline to produce 40% kidney homogenate using a polytron (Kinematica Gmbh PT 45180). The kidney homogenates were divided into portions and stored at –70°C until analyzed for determination of renal malondialdehyde (MDA) (15), superoxide dismutase (SOD) (16), and nitrite/nitrate (17), tumor necrosis factor-α (TNF-α) was determined using RayBio® Rat Enzyme-Linked Immunosorbent Assay (ELISA) kit (Ray Biotech Inc., Georgia, USA), and caspase-3 was determined using Bender MedSystems Caspase-3 instant ELISA kit (Bender MedSystems, Vienna, Austria, Europe) according to the manufacturer’s instructions.
Histological evaluation
Following the animals’ sacrifice, tissues from the kidneys were carefully removed and preserved in 10% formalin for histological examination with haematoxylin and eosin (H&E) stain under a light microscope (18, 19). Histological evaluation of tubular necrosis was determined semi-quantitatively using the methods modified from McWhinnie and coworkers (20). Random fields were observed from each section using ×100 magnification. Ten sections were examined for each kidney and a score from 0 to 3 was given to the tubular profile such that:
0 – normal histology,
1 – for interstitial nephritis and inflammation,
2 – for tubular atrophy and intratubular dilation with or without intralumenal hyaline casts,
3 – for renal tubular necrosis with “sloughing off” of the renal tubular lining cells.
The total score for each kidney was calculated by addition of all 10 scores (21).
Statistical analysis
Results were expressed as mean ±S.E.M. of 7–8 experiments. The one-way analysis of variance (ANOVA or F test) followed by Student-Newman-Keuls post test was utilized. The criterion for statistical significance was set at the 0.05 level.
RESULTS
Kidney functions parameters
Compared to control (saline-treated) group, there were significant increases in BUN and serum creatinine levels in rats treated with cisplatin (6 mg/kg) at 96 h following cisplatin injection (20.69±1.45 vs. 62.63±4.74 mg/dl and 0.25±0.012 vs. 1.23±0.037 mg/dl, respectively). Co-administration of sildenafil (2 mg/kg, i.p.) alone or in presence of BQ-123 (1 mg/kg, i.p.) with cisplatin resulted in significant reductions in BUN levels compared to rats treated with cisplatin alone. However, the levels of BUN in the two groups still remained significantly higher than their corresponding value in the control group (Fig. 1). Similar result concerning serum creatinine level was obtained upon the concurrent administration of sildenafil alone with cisplatin. On the other hand, co-administration of both sildenafil and BQ-123 with cisplatin resulted in a significant reduction in serum creatinine level back to control value (Fig. 2).
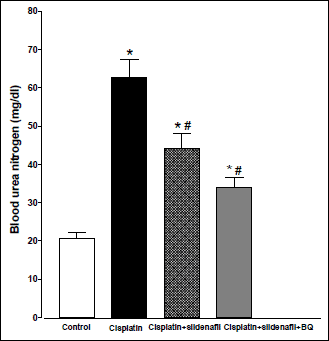 |
Fig. 1. Blood urea nitrogen (mg/dl) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. * and # denote significant difference (P<0.05) vs. control and cisplatin values, respectively. |
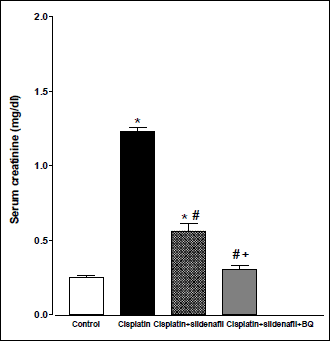 |
Fig. 2. Serum creatinine concentration (mg/dl) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. *, # and + denote significant difference (P<0.05) vs. control, cisplatin and cisplatin + sildenafil values, respectively. |
Renal oxidative stress
Acute administration of cisplatin (6 mg/kg, i.p.) to male rats resulted in a significant increase in MDA level in kidney homogenate compared to saline-treated rats (43.51±2.17 vs. 32.67±1.03 nmol/g tissue). Co-administration of sildenafil alone or in presence of BQ-123 with cisplatin resulted in significant reductions in MDA levels back to control value (Fig. 3). Compared with saline-treated rats, there were significant decreases in renal SOD activity and nitrate/nitrite level in the cisplatin-treated group. Co-administration of sildenafil alone or in presence of BQ-123 with cisplatin resulted in significant increases in SOD activity and nitrate/nitrite levels back to control values (Figs. 4 and 5, respectively).
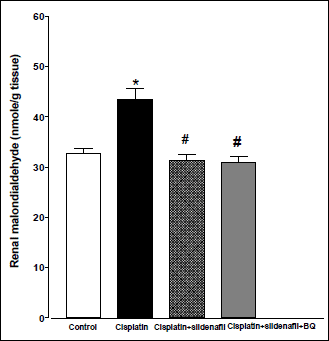 |
Fig. 3. Malondialdehyde concentration in kidney homogenate (nmol/g tissue) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. * and # denote significant difference (P<0.05) vs. control and cisplatin values, respectively. |
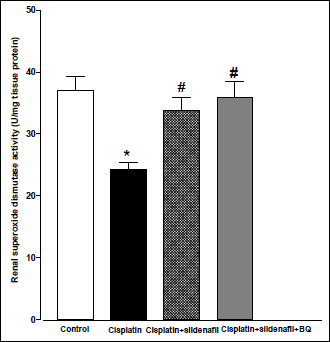 |
Fig. 4. Superoxide dismutase activity in kidney homogenate (U/mg tissue protein) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. * and # denote significant difference (P<0.05) vs. control and cisplatin values, respectively. |
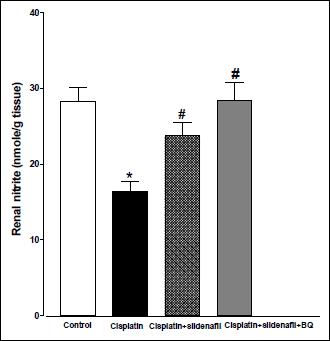 |
Fig. 5. Nitrite concentration in kidney homogenate (nmol/g tissue) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. * and # denote significant difference (P<0.05) vs. control and cisplatin values, respectively. |
Changes in tumor necrosis factor-α and caspase-3 levels
Compared to control (saline-treated) group, there were significant increases in the renal levels of caspase-3 and TNF-α in cisplatin-treated rats. Co-administration of sildenafil alone or in presence of BQ-123 with cisplatin resulted in significant decreases in caspase-3 levels back to control value (Fig. 6). On the other hand, co-administration of sildenafil alone with cisplatin resulted in a significant decrease in TNF-α level compared to rats treated with cisplatin alone. However, the level of TNF-α in cisplatin + sildenafil group still remained significantly higher than its corresponding value in the control group. Co-administration of both sildenafil and BQ-123 with cisplatin resulted in a significant reduction in TNF-α level back to control value (Fig. 7).
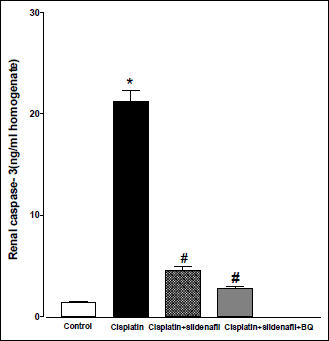 |
Fig. 6. Caspase-3 concentration in kidney homogenate (ng/ml homogenate) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. * and # denote significant difference (P<0.05) vs. control and cisplatin values, respectively. |
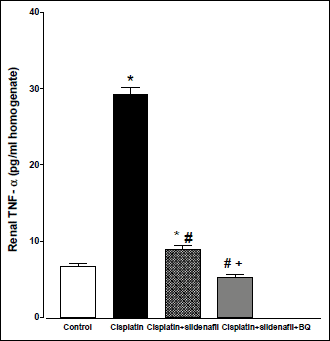 |
Fig. 7. Tumor necrosis factor-α concentration in kidney homogenate (pg/ml homogenate) obtained from male rats at 96 h following the administration of saline (control), cisplatin (6 mg/kg, i.p.), cisplatin + sildenafil (2 mg/kg, i.p. at two time intervals; one hour before and one day after the cisplatin dose), and cisplatin + sildenafil + BQ (1 mg/kg, BQ-123 i.p. at two time intervals; one hour before and one day after the cisplatin dose). Values are means of 7–8 observations expressed as mean ±S.E.M. *, # and + denote significant difference (P<0.05) vs. control, cisplatin and cisplatin + sildenafil values, respectively. |
Histological renal damage
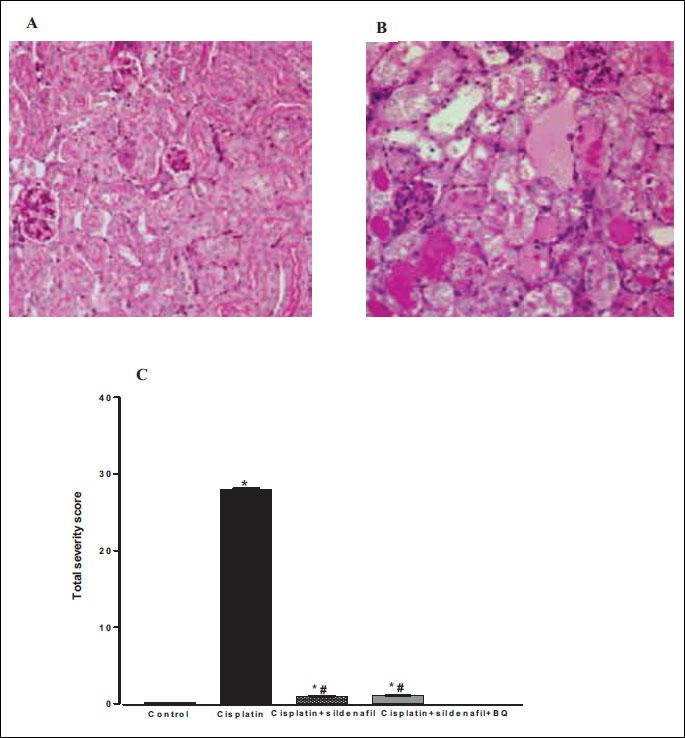
Histological examinations of the kidneys obtained from rats treated with cisplatin showed typical ‘acute tubular necrosis” pattern (4, 12); namely widespread tubular-cell necrosis, tubular dilatation, and “sloughing off” of the renal lining cells (Fig. 8B). The total severity score for tubular damage was significantly reduced in rats administered sildenafil alone or in presence of BQ-123 with cisplatin compared to the cisplatin-treated group (Fig. 8C).
DISCUSSION
Cisplatin is a chemotherapeutic agent useful in the treatment of testicular, ovarian, bladder, breast, head, and neck cancers (22). Nephrotoxicity, however, is the dose-limiting factor for cisplatin clinical use (23). Cellular and molecular mechanisms responsible for cisplatin-induced nephrotoxicity involve formation of reactive species, hypoxia, inflammation, and apoptosis (24). Results of the current study demonstrated that acute administration of cisplatin (6 mg/kg) to male rats led to histological alterations of the renal tubular cells. These changes were associated with decline in renal functions, resulted in alteration in kidney functions parameters manifested as elevated serum creatinine and BUN with concomitant increases in levels of renal MDA, serum caspase-3 and TNF-α and decreases in the level of renal nitrate/nitrite and SOD activity.
The levels of serum BUN and creatinine correlate well with the decline and recovery of renal function and the evoked decline in these parameters in the current study are in line with previous studies (25-27) indicating cisplatin-induced nephrotoxicity. Furthermore, the findings of the current study point to the presence of oxidative stress and are in accordance with data in previous reports (28-30). Cisplatin was found to generate superoxide and hydroxyl radicals, and to stimulate renal lipid peroxidation (31). The role of NO in cisplatin nephrotoxicity is unclear. Previous studies had shown a decreased NO level measured as total nitrate/nitrite level following cisplatin administration (4, 32), which is in line with results of the current study. The decrease in NO synthesis induced by cisplatin can be explained by the detected pathological damage of glomerular endothelial cells. Previous studies suggested that changes in renal haemodynamics play an important role in cisplatin-induced nephrotoxicity (4, 32, 33).
Renal injury by cisplatin has been associated with oxidative stress, inflammation, and apoptosis (34-36). Specifically, apoptosis is an important mode of cell death in cisplatin nephrotoxicity. The binding of extracellular TNF-α to a cell surface receptor activates caspase 8. Caspase activation is thought to be important in the genesis of apoptosis (37). In particular, caspase-3, the execution caspase, is instrumental in the apoptotic process; it cleaves and activates poly (ADP-ribose) polymerase which leads to DNA fragmentation (38, 39).
Recently, several studies have shown that, in addition to treating erectile dysfunction, sildenafil can prevent or decrease tissue injury. Results of the current study revealed that the concurrent administration of sildenafil with cisplatin offered a moderate reno-protective effect against cisplatin-induced nephrotoxicity manifested as significant decrease in BUN and serum creatinine compared to cisplatin treated group. This was supported by the histological sections showing normal morphology. The obtained protective effect may be primarily attributable to the inhibition of oxidative stress; demonstrated by our findings that sildenafil treatment restored the elevated renal MDA and the reduced level of renal nitrate/nitrite and SOD activity, and the inhibition of inflammation and apoptosis manifested as reducing the level of TNF-α with subsequent decrease in the level of casapase-3 and thus inhibiting the apoptotic process, which is in line with the study conducted by Lee et al, (7).
Two endothelin receptor subtypes, termed ET-A and ET-B, have been cloned and sequenced. ET-A receptors have a high affinity for ET-1 and are located on smooth muscle cells, where they mediate vasoconstriction. ET-B receptors have approximately equal affinities for ET-1 and ET-3 and are located on vascular endothelial cells, where they mediate release of prostaglandin-I2 (PGI2) and NO (8). Previous reports had demonstrated that the renal damages associating cisplatin-induced nephrotoxicity were confined to the cortex, and that ET-1 and ET-A receptors were up-regulated without change of ET-B (40). Treatment with an ET-A receptor antagonist before or post-ischemia has been demonstrated to lessen glomerular dysfunction in rats (40). Buyukgebiz et al. mentioned that inhibiting function of ET via an ET-A antagonist might prevent reperfusion injury in kidney transplantation (41). Furthermore, the reno-protective effect of the ET-A receptor antagonist BQ-123 against cisplatin-induced nephrotoxicity was evident in a previous study conducted in our lab (42). The current study aimed at investigating the possible interaction between the nitrergic and endothelin pathways in cisplatin-induced nephrotoxicity. We, therefore, evaluated the effect of the concurrent administration of sildenafil and the ET-A selective antagonist BQ-123 with cisplatin. The present data demonstrated that the concurrent administration of both sildenafil and BQ-123 with cisplatin offered a reno-protective effect comparable to that obtained following the co-administration of sildenafil alone with cisplatin. Thus, the current study is the first to reveal that the cisplatin-induced nephrotoxicity can be attenuated via accumulation of cGMP following sildenafil administration even in the presence of ET-A-mediated vasoconstriction, suggesting the absence of obvious functional interaction between the nitrergic and endothelin pathways in cisplatin-induced nephrotoxicity in male rats.
In summary, the current study is the first to reveal that the presence of an intact NO/cGMP system may offer a moderate reno-protective effect against cisplatin-induced nephrotoxicity even in the presence of ET-A-mediated vasoconstriction, suggesting the absence of obvious functional interaction between the nitrergic and endothelin pathways in cisplatin-induced nephrotoxicity in male rats.
Conflict of interests: None declared.
REFERENCES
- Bennett PN, Brown MJ. Clinical Pharmacology. Churchill Livingstone, 2003, 606.
- Von-Hoff DD, Schilsky R, Reichert CM, et al. Toxic effects of cis-dichlorodiammine platinum in man. Cancer Treat Rep 1979; 63: 1527-1531.
- Schrier RW. Cancer therapy and renal injury. J Clin Invest 2002; 110: 743-745.
- Saleh S, El-Demerdash E. Protective effects of l-arginine against cisplatin induced renal oxidative stress and toxicity: role of nitric oxide. Basic Clin Pharmacol Toxicol 2005; 97: 91-97.
- Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, et al. Early treatment with cGMP phosphodiesterase inhibitor ameliorates progression of renal damage. Kidney Int 2005; 68: 2131-2142.
- Das A, Ockaili R, Salloum F, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 2004; 286: H1455-H1460.
- Lee KW, Jeong JY, Lim BJ, et al. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology 2009; 257: 137-143.
- Takaoka M, Kuro T, Matsumura Y. Role of endothelin in the pathogenesis of acute renal failure. Drug News Perspect 2000; 13: 141-146.
- Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol 2004; 24: 299-315.
- Slowinski T, Subkowski T, Diehr P, et al. Interaction of the endothelin system and calcineurin inhibitors after kidney transplantation. Clin Sci (Lond) 2002; 103 (Suppl. 48): 396S-398S.
- Lee S, Ahn D. Expression of endothelin-1 and its receptors in cisplatin-induced acute renal failure in mice. Korean J Physiol Pharmacol 2008; 12: 149-153.
- Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J Pharmacol Exp Ther 1980; 213: 551-556.
- Garrido MR, Israel A. Role of endothelin in stress-induced hypertension. J Hum Hypertens 2002; 16 (Suppl. 1): S29-S33.
- Borch RF, Pleasants ME. Inhibition of cis-platinum nephrotoxicity by diethyldithiocarbamate rescue in a rat model. Proc Natl Acad Sci USA 1979; 76: 6611-6614.
- Ottolenghi A. 2-Thiobarbituric acid (TBA) method. Arch Biochem Biophys 1959; 77: 355-360.
- Marklund S, Marklund G. Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay for SOD. Eur J Biochem 1974; 47: 469-474.
- Guevara I, Iwanejko J, Dembinska-Kiec A, et al. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta 1998; 274: 177-188.
- Carleton HM. Carleton’s Histological Techniques. Oxford Medical Publications, Oxford University Press, 1980; pp. 58-68, 90-95, 132-150, 182-198.
- Tagboto S, Griffiths AP. The evaluation of renal ischaemic damage: the value of CD10 monoclonal antibody staining and of biochemical assessments of tissue viability. BMC Clin Pathol 2007; 7: 5.
- McWhinnie DL, Thompson JF, Taylor HM, et al. Morphometric analysis of cellular infiltration assessed by monoclonal antibody labeling in sequential human renal allograft biopsies. Transplantation 1986; 42: 352-358.
- Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 2000; 58: 658-673.
- Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 1998; 34: 1522-1534.
- Daugaard G, Abildgaard U. Cisplatin nephrotoxicity. A review. Cancer Chemother Pharmacol 1989; 25: 1-9.
- Reiter RJ, Tan DX, Korkmaz A, Fuentes-Broto L. Drug-mediated ototoxicity and tinnitus: alleviation with melatonin. J Physiol Pharmacol 2011; 62: 151-157.
- Evenepoel P. Acute toxic renal failure. Best Pract Res Clin Anaesthesiol 2004; 18: 37-52.
- Kang DG, Lee AS, Mun YJ, et al. Butein ameliorates renal concentrating ability in cisplatin-induced acute renal failure in rats. Biol Pharm Bull 2004; 27: 366-370.
- Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet 2005; 365: 417-430.
- Antunes LM, Darin JD, Bianchi N de L. Effects of antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res 2001; 43: 145-150.
- Mora LO, Antunes LM, Francescato HD, Bianchi M. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res 2003; 47: 517-522.
- Weijl NI, Cleton FJ, Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 1997; 23: 209-240.
- Kuhlmann MK, Burkhardt G, Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant 1997; 12: 2478-2480.
- Saad SY, Najjar TA, Daba MH, Al-Rikabi AC. Inhibition of nitric oxide synthase aggravates cisplatin induced nephrotoxicity: effect of 2-amino-4-methylpyridine. Chemotherapy 2002; 48: 309-315.
- Offerman JJ, Meijer S, Sleijfer DT, et al. Acute effects of cis-diamminedichloroplatinum (CDDP) on renal function. Cancer Chemother Pharmacol 1984; 12: 36-38.
- Francescato HD, Costa RS, Scavone C, Coimbra TM. Parthenolide reduces cisplatin-induced renal damage. Toxicology 2007; 230: 64-75.
- Iseri S, Ercan F, Gedik N, Yuksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology 2007; 230: 256-264.
- Kiymaz N, Yilmaz N, Mumcu C, Anlar O, Ozen S, Kayaoglu CR. Protective effect of sildenafil (Viagra) in transient spinal cord ischemia. Pediatr Neurosurg 2008; 44: 22-28.
- Merline R, Lazaroski S, Babelova A, et al. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol 2009; 60 (Suppl. 4): 5-13.
- Tsuruya K, Ninomiya T, Tokumoto M, et al. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int 2003; 63: 72-82.
- Lau AH. Apoptosis induced by cisplatin nephrotoxic injury. Kidney Int 1999; 56: 1295-1298.
- Wilhelm SM, Stowe NT, Robinson AV, Schulak JA. The use of the endothelin receptor antagonist, tezosentan, before or after renal ischemia protects renal function. Transplantation 2001; 71: 211-216.
- Buyukgebiz O, Aktan AO, Haklar G, et al. BQ-123, a specific endothelin (ETA) receptor antagonist prevents ischemia-reperfusion injury in kidney transplantation. Transpl Int 1996; 9: 201-207.
- Helmy MM, Helmy MW, Abd Allah DM, Abo Zaid AM, Mohy El-Din MM. Selective ETA receptor blockade protects against cisplatin-induced acute renal failure in male rats. Eur J Pharmacol 2014; 730C: 133-139.
A c c e p t e d : March 12, 2014