EFFECTS OF GADOLINIUM CHLORIDE ON BASAL FLOW AND COMPRESSION-INDUCED RAPID HYPEREMIA IN THE RABBIT MASSETER MUSCLE
INTRODUCTION
Several studies have recently focused on the rapid vasodilation elicited by external muscle compression (1-3) suggesting that, like the classical myogenic response, it is at least partly mediated by mechanosensitive mechanisms responding to a reduction in transmural pressure (2, 4, 5).
Understanding the mechanisms of vascular mechano-sensitivity is of primary importance for its potential contribution to the regulation of vascular tone and, consequently, of vascular resistance and arterial blood pressure (6-8).
Mechano-sensitivity of blood vessels is considered to rely mostly on mechano-sensitive channels (MSC), largely expressed by both endothelium and vascular smooth muscle (2, 9). Selective blockade of these channels has been attempted in in vitro studies (10, 11) by means of different substances such as 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS), tarantula protein (GsMtx2), gadolinium (Gd3+) (10). Although being characterized by low specificity (12), gadolinium (Gd3+, a trivalent lanthanide), has been widely used as MSC blocker, to test mechano-sensitivity of single cells (11) and isolated vessel preparations (13). In particular Gd3+ was shown to provoke marked attenuation of the classical myogenic response (13), i.e. the slow (lasting about 1 min) vascular constriction/dilatation occurring in response to step increase/decrease in the vessel's transmural pressure. However, to our knowledge the effect of MSC blockade has never been tested on the rapid dilatory response induced by a short lasting muscle compression.
Aim of the present work is to test the role of mechano-sensitive ion channels on the rapid hyperaemic response induced by external compression of the masseter muscle in anesthetized rabbits, by assessing the effect of Gd3+ administration. A recently developed model has been employed, which allows for accurate monitoring of blood flow from the purely muscular masseteric artery (14). This vascular bed appears to be very reactive to mechanical stimuli and the ensuing hyperaemic events can be produced with high repeatability by muscle compression (5, 15).
One of the reason why Gd3+ has been little investigated in vivo is related to its toxic effect, possibly resulting in hypotension, cardiac arrhythmias, A-V bundle block, ventricular fibrillation when administered systemically (16). In order to overcome acute collateral effects and prevent disturbing cardiovascular changes at systemic level, Gd3+ was here locally administered in small doses through close arterial injection.
MATERIAL AND METHODS
The study was performed in accordance with the principles of laboratory animal care. Purposes and protocols were approved by the Ethical Committee for Animal Experiments at the University of Turin.
Surgical procedure
Experiments were carried out on 8 male European rabbits (Oryctolagus cuniculus) weighing between 3.1 and 3.9 kg, anesthetized with urethane (Urethane, Sigma Aldrich, 1.2 g/kg i.v.). Full surgical anaesthesia was maintained by injecting additional doses of the drug (0.4 g/kg i.v,) through a catheter inserted in the cannulated left femoral vein. In all animals trachea was intubated and the head was fixed in a stereotaxic frame by screws implanted in the nasal and frontal bones.
A telemetric pressure transducer (TA11PA-D70, DSI - St. Paul, MN, USA) was implanted, the catheter being inserted into the right femoral artery for monitoring arterial blood pressure (ABP).
A perivascular flow probe (model 0.7 PSB, Transonic Systems Inc, Itaha, NY, USA) was implanted on the masseteric branch of the facial artery (Ma). The Ma, which exclusively supplies the rostral portion of the masseter muscle, was isolated medially to the mandibular margin, immediately after its branching from the facial artery, for placement of the probe (14). In 5 of 8 animals a bilateral implant was performed so that blood flow could be recorded simultaneously from the left and right masseteric artery.
A silicone cannula (diameter 0.5 mm, CNC-3IS-LSA/LSP Access Technologies, USA) was inserted at the distal mandibular end of the facial artery and advanced proximally, up to the origin of the Ma, for close arterial injection of Gd3+ in the masseter muscle. The cannula was continuously perfused with saline solution at 2–3 ml/h (infusion pump Terumo Europe STC-521, Leuven, Belgium) to prevent clotting.
The experimental procedures started after stabilization of the hemodynamic variables, about 1 hour after the surgical preparation was completed. At the end of the experiments animals were killed with an i.v. injection of a lethal dose of urethane.
Drugs
Gadolinium chloride (Gd3+, Sigma, Steinhein, Germany), an antagonist of stretch-activated cation channels, was dissolved in HEPES buffer (pH 7.3–7.4) (17-19) and infused at increasing concentrations of 0.1 mM, 1.0 mM and 10 mM into the cannulated facial artery, through an infusion pump (Terumo STC-521, Terumo Co., Tokyo). The rate of injection was set to 50% of basal blood flow in the masseteric artery, ranging between 6 and 24 ml/h, for a duration of 2 minutes. This approach was aimed to obtain the same Gd3+ plasma concentration in all masseteric arteries. Given that rabbit haematocrit is about 40% (20), the estimated plasma concentration (EPC) of Gd3+ in masseteric artery during drug infusion approximately corresponds to 0.045–0.45–4.5 mM for the three dosages employed. The three administrations were separated by 1-h lasting intervals, during which changes in basal blood flow and vascular reactivity were assessed.
Mechanical stimulation
Mechanical compression of the masseter muscle was performed unilaterally by means of a shaker (model V2, Data Physics Corporation, San Jose, CA USA), driven by a computer-generated trapezoidal signal (rise time: 0.1 s; plateau: 1 s; return: 0.1 s), delivered through a 1401 micro I/O board (CED, Cambridge, UK). The shaker was armed with a cylindrical head (diameter of about 1.3 cm, amplitude of movement up to 5 mm) positioned so that it exerted appropriate and repeatable compressions of the cheek at the level of the anterior portion of the masseter muscle (5). Mechanical stimuli were delivered in series of 10 MCs separated by 30-s intervals. This inter-stimulus interval was maintained precisely constant (PC-controlled) since it was observed to affect the amplitude of the hyperemic response to MC (15).
One series was delivered before the first Gd3+ administration (control), then other series were periodically delivered at 3.5, 15, 30 and 45 min after the end of each Gd3+ infusion, as illustrated in Fig. 1.
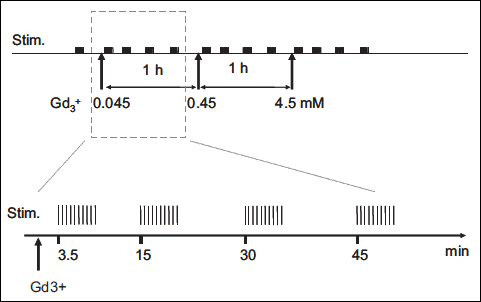 |
Fig. 1. Scheme describing the experimental protocol mechanical stimulation. It was composed by 5 series of 10 MC (30 second between each one). The series was started before Gd3+ administration (control), after 3.5 minutes (t3.5), after 15 minutes (t15), after 30 minutes (t30) and after 45 minutes (t45). This sequence was repeated (0.045 mM–0.45 mM–4.5 mM). |
Data acquisition and processing
Acquisition of all signals and off-line processing were performed with Spike2 (CED, Cambridge, UK). Simple algorithms were implemented in the Spike2 script language aimed at identifying single cardiac cycles (based on systolic peak detection on the ABP signal), from which time averages of the different signals (one value per cardiac cycle) were computed. Measurement of ABP, heart rate (HR) and masseteric artery blood flow ipsilateral (iMaBF) and contralateral (cMaBf) to the side of mechanical stimulation and of Gd3+ injection were continuously performed. The blood flow response to MC was characterized in terms of peak flow response and peak amplitude (=peak flow-basal low). These measurements were taken before and 4 times after each Gd3+ injection (at. 3.5, 15, 30, 45 min, after injection). Basal flow and peak flow of hyperaemic responses were normalized to the basal flow of the control condition (i.e., before the first Gd3+ injection). In addition, the peak amplitude of the hyperaemic response was normalized to the pre-stimulus level. For all variables the average effects were computed across all animals (n=8) and reported as mean ± standard deviations (S.D.).
Statistical significance of the effect of Gd3+ on the measured variables was assessed with a two-ways ANOVA and Duncan Post hoc test, with time from injection (time: 3.5, 15, 30, 45 min) and Gd3+ concentration (EPC: 0.045, 0.45, 4.5 mM) as factors.
RESULTS
In control conditions MaBF ranged between 0.2 and 1 ml/min, on average 0.44±0.08 ml/min. Compression of the masseter muscle produced a transient reduction in iMaBF, followed by a rapid hyperaemic response, developing immediately after release of the stimulus (peak amplitude: 0.85±0.33 ml/min, time to peak: 2.2±0.8 s) and returning to control within 10–15 sec. A representative example of the hyperaemic response to subsequent stimuli is shown in Fig. 2A.
Neither ABP nor HR nor cMaBF were statistically dependent on the time from Gd3+ injection (p=0.74) or on the concentration of the drug (p=0.95), indicating that Gd3+ did not reach the general circulation at a concentration sufficient to be effective. On average, ABP, HR and cMaBF were 96.1±5.8 mmHg, 308±10 bmp and 0.36±0.13 ml/min, respectively.
Administration of Gd3+ at increasing concentration progressively decreased the amplitude of the hyperaemic response to MC, while also affecting basal blood flow. A representative example is shown in Fig. 2B in which the average responses to MC collected 45 min after Gd3+ administration at different concentrations (progressively lighter grey traces corresponding to increasing concentrations) are superimposed to the MC response in control condition (black trace). It can be observed that the decrease in the amplitude of response occurs independently of an effect on basal blood flow.
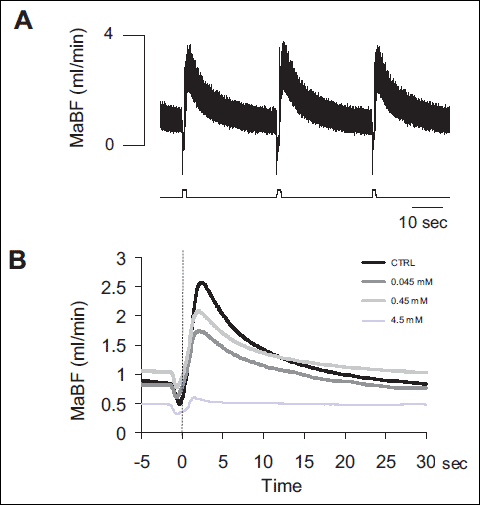 |
Fig. 2. (A) Original recording of masseteric artery blood flow (MaBF) in response to masseter muscle compressions (MC). Each compression induced a stereotyped hyperaemic response that followed the transient reduction in flow. The recording is extracted from the sequence of 10 stimuli delivered before Gd3+ administration, in one animal. (B) Effect of Gd3+ administration at increasing concentration on the MC response. Each curve is the average of the responses to the last 8 MCs in the 10-MC sequence delivered during control (black) and 45 min after each Gd3+ infusion (grey traces, see EPC in the legend), in the same animal of Fig. 2A. |
The time course of peak amplitude (light grey) and basal flow (dark grey) is reported in Fig. 3A, the full height of each bar thus representing peak flow. None of the variables exhibited a dependence on time from Gd3+ administration (peak flow: p=0.51; basal flow: p=0.20; peak amplitude: p=0.45) but all showed a dependence on concentration (p<0.01).
After averaging over time, peak flow at the different EPCs was 1.2±0.2 ml/min at 0.045 mM, 1.4±0.2 ml/min at 0.45 mM and 0.25±0.1 ml/min at 4.5 mM (not shown), only at the latter concentration being significantly different from the control condition (1.3±0.2) (p<0.01).
Basal masseter blood flow was 0.4±0.08 ml/min at the control, increased to 0.5±0.11 ml/min at 0.045 mM (p=0.9) and 0.73±0.18 ml/min (p<0.01) at 0.45 mM and decreased to 0.22±0.18 ml/min at 4.5 mM (p<0.01).
The peak amplitude decreased from 0.85±0.33 ml/min during the control to 0.64±0.25 ml/min at 0.45 mM (p<0.01) as shown in Fig. 3A. However, since basal flow was concomitantly increased, the relative peak amplitude (=peak amplitude / basal flow) was markedly attenuated by gadolinium at EPC=0.45 mM. This effect is represented in Fig. 3B in which the average relative peak amplitude is reported after normalization to control value, taken as 100%. Relative peak amplitude was reduced from 195±77% of baseline, in control condition, to 100±42 % (at 0.45 mM, EPC), corresponding to an average reduction of 45±28%.
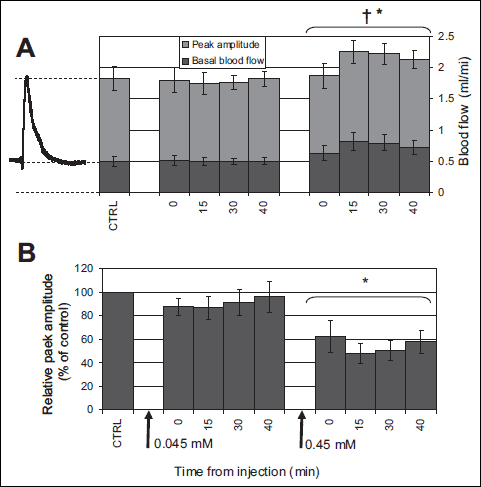 |
Fig. 3. Average effects of Gd3+ on basal flow and peak amplitude of the MC response. (A) average basal flow (dark grey) and peak amplitude (light grey) are reported before (CTRL) and at the different time intervals after close arterial injection of Gd3+ leading to estimated plasma concentration of 0.045, and 0.45 mM. As illustrated by the representative MC response reported on the left, the total height of each bar corresponds to average peak flow. (B) Relative peak amplitude, i.e., referred to current basal flow is reported in this bar diagram to highlight the reduction induced by Gd3+. All values have been normalized to control value (CTRL). The presentation of the bar diagram reflects the experimental protocol, with arrows at the bottom indicating Gd3+ administration and labels indicating the corresponding estimated plasma concentrations (0.045, 0.45 mM). Data corresponding to EPC of 4.5 mM were not reported (see Discussion). † and * indicate significant difference with respect to the control condition (p<0.01) for basal flow and peak amplitude, respectively. Error bars indicate standard error (n=8). |
DISCUSSION
For the first time the vascular effect of Gd3+ were investigated in vivo, by means of our recently-developed experimental model (5).
The results showed that close arterial injection of Gd3+, resulting in estimated plasma concentrations of 0.045 and 0.45 mM, progressively reduced the amplitude of the rapid hyperaemic response to external muscle compression (to 65% of control) while increasing basal blood flow (to 68% of control). The marked effects observed at the highest concentration (4.5 mM) deserve a more careful consideration being possibly produced by Gd3+ precipitation. Gadolinium administration did not affect systemic variables such as ABP and HR, nor blood flow in the contralateral masseteric artery.
Arterioles and small arteries usually develop and maintain some degree of active force at their normal intravascular pressure, allowing resistance to be modulated in two directions, vasodilatation and vasoconstriction. In his famous model Johnson (21) put forward the idea that arteriolar wall tension, rather than the vessel diameter, is the regulated variable so that vessel diameter is decreased in response to increased transmural pressure, according to the Laplace's law. This model also explains the "spontaneous" myogenic tone that develops in resting conditions, in relation to intraluminal pressure (22). The mechano-sensitive channels (MSC), largely expressed in both endothelium and smooth muscle of blood vessels, are considered to be involved in the vascular mechano-sensitivity (2, 9) that mediates the myogenic response (9) as well as in the maintenance of basal tone (12, 23-25).
Although a unique blocker of these mechano-sensitive channels has not been identified, gadolinium chloride is considered an effective mechano-sensitive channel blocker (26, 27) and its action on these channels has been documented by a large numbers of in vitro studies performed on different tissues, including vascular smooth muscle (26) and endothelium (28, 29).
In the present study, close arterial injection of Gd3+ resulted in increased resting blood flow (69±71% at 0.45 mM), in the absence of changes in arterial blood pressure. This reveals a dilatation of resistance vessels and, to our knowledge, is the first observation in vivo of the involvement of MSC channels in resting myogenic tone. This result is in agreement with the hyperpolarizing effect observed in isolated cerebral arteries upon exposure to Gd3+ (30 ±M), which was attributed to a depressant action exerted by the drug on non selective cation channels (30). However, a dilatory effect has not been consistently observed in in vitro studies (11, 23, 25), possibly due to the fact that single different vascular segments were investigated. Such a limitation does not apply to our in vivo model in which the whole muscolo-vascular tree of the masseter muscle has been exposed to the drug. Interestingly a dilatory effect of Gd3+ is compatible with the general hypotensive effect observed upon systemic administration by i.v. injection in cats (16), although in that study specific vascular effects were not investigated.
The muscle vascular bed is known to have a large dilatory capacity and MaBF was frequently observed to increase above 10 times the basal value in this same preparation, during functional hyperaemia (14). On this basis, the decrease in the hyperaemic response to MC cannot be attributed to a saturation of the dilatory capacity associated to the increase in basal blood flow. Moreover, in some instances the reduction in the MC response was accompanied by negligible effects on basal blood flow, thus resulting in a net reduction of peak flow (e.g., Fig. 2, EPC=0.045 mM). These considerations indicate that the effect on MC response is distinct from the effect on basal flow.
It is known that an increase in transmural pressure activates the mechano-sensitive non-selective cation channels leading to depolarization of vascular smooth muscle cells and increase of intracellular [Ca++] which underlies the vasoconstriction according to the classical myogenic response (27). Opposite effects are considered to mediate the vasodilatation that follows a reduction in transmural pressure, although this phenomenon has been comparatively less investigated (9). Exposure to Gd3+ was shown to completely abolish the myogenic response in isolated afferent arterioles of the rabbit kidney at a concentration of 10 µM (13), while the same concentration was ineffective in the rabbit basilar artery (31), suggesting a large variability among different vascular compartments. In this respect, the musculo-vascular compartment is known to exhibit a prominent and rapid dilatation in response to mechanical stimulation, particularly to stimuli that decrease transmural pressure, e.g., compression of the muscle (1, 3, 4, 32, 33) and occlusion of the supplying artery (1). In addition, this rapid dilatory response has been observed also in isolated muscle feed arteries (2) and arterioles (25) subjected to external compression and to a drop in internal pressure, respectively. In the latter study the authors showed that the peak amplitude of the rapid dilatation following transient reduction of intraluminal pressure (80–10–80 mmHg) was significantly reduced by Gd3+ (10 µM), supporting the idea that MSCs mediate at least part of the response (25). The effect was however observed only for stimulus duration of 60 and 120 s and not for duration of 30 s. The MC stimulus adopted in the present study likely produces a similar decrease in transmural pressure of intramuscular blood vessels but, at difference with the above mentioned study it lasts only 1 s. Moreover the presently observed effects concern the response of the whole musculo-vascular network, not just the response of the arteriolar segment. Thus, although collected at higher theoretical Gd3+ concentration (EPC=450 µM, discussed below), the present results extend the observation of Koller & Bagi (25) to the response to 1-s lasting stimuli, in vivo, supporting the involvement of Gd3+-sensitive MSCs in the mechanically-induced rapid hyperaemia.
Blockade of mechano-sensitive structures is likely responsible for the decreased vascular reactivity to mechanical stimuli. It is presently not known whether these structures belong to smooth muscle cells as in the classical myogenic response, to the endothelium or both (34). The endothelium is known to release vasodilatory substances, such as NO, prostaglandins and K+, upon mechanical stimulation, not limited to shear stress (25, 35, 36). It is particularly interesting to consider the role of K+ that has been recently shown to have a role in the contraction-induced rapid dilatation (37, 38). In addition to being produced by skeletal muscle fibers during contraction, K+ may also be released by the endothelium upon activation of endothelial mechano-sensitive TRP channels and then produce hyperpolarization and relaxation of smooth muscle cells through activation of Kir channels and the 3Na+/2K+ pump (39, 40). That this pathway may underlie the MC hyperaemic response is also suggested by the recent observation that the dilatatory responses to K+ (36) and repetitive MC (15) share a transient nature.
On the other hand, about 55% of the response was not affected by Gd3+ at EPC=0.45 mM which suggests that other mechano-trasduction pathways, possibly involving non Gd3+-sensitive MSCs, extracellular matrix - integrins interactions and intracellular adhesion molecules, may concur to the rapid dilatory process (41).
In summary, different pathways may be hypothesized to account for the dual effect of Gd3+ of increasing basal blood flow and decreasing the hyperaemic response to MC. 1) The tonic vasodilator effect may be mediated by the blockade of NSCs in smooth muscle cells. The ensuing hyperpolarization and reduced calcium entry would result in a relaxation of smooth musculature and vessel dilatation (30). 2) The desensitization to mechanical stimulation could result from the blockade of endothelial mechano-sensitive channels resulting in reduced release of vasodilatory factors, presumably K+, and reduced vascular dilatations. In addition, other actions of Gd3+ on non mechano-sensitive structures might have contributed to these effects (see below). Further experiments are necessary to test these hypotheses.
A major problem in the administration of Gd3+ (20-50 mg/kg) in vivo is related to its toxic effects, namely hypotension, cardiac arrhythmias, A-V bundle blockade and ventricular fibrillation, while no effects are reported below 0.5–1 mg/kg (16). However, with close arterial Gd3+ administration the total amount of drug injected was in the range 0.0017–0.17 mg/kg, i.e., well below the toxic threshold.
In addition Gd3+, is also prone to precipitate and was observed to lead to the formation of mineral emboli when administered at high concentration (0.14–0.35 mmol/Kg, corresponding to 3.1–7.8 mM plasma concentration) (42, 43). This possibility could not be avoided in the present study and was likely responsible for the sudden reduction of both basal blood flow and of responsiveness to mechanical stimuli observed at EPC of 4.5 mM.
Phenomena like Gd3+ precipitation (42, 43), binding to anions, phosphate, carbonate and EGTA (44), as well as diffusive processes in the interstitium all contribute to reduce the actual Gd3+ concentration as compared to the EPC. This may explain the reason why effects were presently observed at EPC considerably larger than (33) concentrations effective in in vitro studies.
Although Gd3+ is considered an effective MSC blocker, largely used in functional experiments on in vitro vessel preparation to study the role of stretch-activation cation channels (10, 11, 26, 27), it is not very specific, blocking effects of voltage-gated Ca++, Na+ and K+ channels having also being reported (16). It cannot be excluded that this side-effects have contributed to the observed increase of basal flow and decrease of rapid hyperaemia. In particular a blockade of calcium channels in smooth muscle cells could have contributed to the observed tonic vasodilatation while a blockade of K+ channels could have impaired a K+-based vasodilatory signalling from the endothelium, thus contributing to attenuate the dilatory response to MC.
The results suggest that Gd3+-sensitive MSCs take part in the maintenance of basal vascular tone and to the mechanically-induced rapid dilatation of muscle blood vessels. The fact that the hyperaemic response is only partly attenuated by Gd3+ suggests that other mechano-sensitive structures may also be involved.
Acknowledgements: We are particularly grateful Mrs Luisella Milano for her assistance in the surgical procedures.
This work was supported by grants from Istituto Nazionale Ricerche Cardiovascolari - Consorzio Interuniversitario (INRC).
Conflict of interests: None declared.
REFERENCES
- Turturici M, Mohammed M, Roatta S. Evidence that the contraction-induced rapid hyperemia in rabbit masseter muscle is based on a mechanosensitive mechanism, not shared by cutaneous vascular beds. J Appl Physiol 2012; 113: 524-531.
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 2006; 572: 561-517.
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 2007; 583: 861-874.
- Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol 1974; 227: 531-535.
- Turturici M, Roatta S. Compression-induced hyperaemia in the rabbit masseter muscle: a model to investigate vascular mechano-sensitivity of skeletal muscle. Physiol Meas 2013; 34: 307-314.
- Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2010; 298: H1769-H1775.
- Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol 2005; 289: H1917-H1922.
- Ahn DS, Choi SK, Kim YH, et al. Enhanced stretch-induced myogenic tone in the basilar artery of spontaneously hypertensive rats. J Vasc Res 2007; 44: 182-191.
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 1999; 79: 387-423.
- Baek EB, Jin C, Park SJ, et al. Differential recruitment of mechanisms for myogenic responses according to luminal pressure and arterial types. Pflugers Arch 2010; 460: 19-29.
- Park KS, Kim Y, Lee YH, Earm YE, Ho WK. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res 2003; 93: 557-564.
- Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol Sci 2009; 30: 363-374.
- Takenaka T, Suzuki H, Okada H, Hayashi K, Kanno Y, Saruta T. Mechanosensitive cation channels mediate afferent arteriolar myogenic constriction in the isolated rat kidney. J Physiol 1998; 511: 245-253.
- Roatta S, Mohammed M, Turturici M, Milano L, Passatore M. A model for investigating the control of muscle blood flow: the masseteric artery in conscious rabbits. Physiol Meas 2010; 31: N71-N77.
- Turturici M, Roatta S. Inactivation of mechano-sensitive dilatation upon repetitive mechanical stimulation of the musculo-vascular network in the rabbit. J Physiol Pharmacol 2013; 64: 299-308.
- Adding LC, Bannenberg GL, Gustafsson LE. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc Drug Rev 2001; 19: 41-56.
- Hajduczok G, Chapleau MW, Ferlic RJ, Mao HZ, Abboud FM. Gadolinium inhibits mechanoelectrical transduction in rabbit carotid baroreceptors. Implication of stretch-activated channels. J Clin Invest 1994; 94: 2392-2396.
- Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol 2009; 587: 873-882.
- Matsukawa K, Nakamoto T, Inomoto A. Gadolinium does not blunt the cardiovascular responses at the onset of voluntary static exercise in cats: a predominant role of central command. Am J Physiol Heart Circ Physiol 2007; 292: H121-H1129.
- Marval E, Garcia L, Candela DE, Arocha-Pinango CL. Normal values of hemoglobin, hematocrit, blood coagulation factors, and fibrinolysis in New Zealand white rabbits [in Spanish]. Sangre (Barc) 1992; 37: 355-361.
- Johnson PC. The myogenic response. In: Handbook of Physiology, the Cardiovascular System, Vascular Smooth Muscle, Bohr DF, Somlyo AP, Sparks HV (eds). Sect II, Vol. 2, Chapt. 15. Bethesda, American Physiological Society, 1980, 409-442.
- Mellander S. On the control of capillary fluid transfer by precapillary and postcapillary vascular adjustments. A brief review with special emphasis on myogenic mechanisms. Microvasc Res 1978; 15: 319-330.
- Rapacon-Baker M, Zhang F, Pucci ML, Guan H, Nasjletti A. Expression of myogenic constrictor tone in the aorta of hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2001; 280: R968-R975.
- Cho YE, Ahn DS, Kim YH, Taggart MJ, Lee YH. Changes in stretch-induced tone induced by intracellular acidosis in rabbit basilar artery: effects on BKCa channel activity. Vascul Pharmacol 2007; 47: 74-82.
- Koller A, Bagi Z. On the role of mechanosensitive mechanisms eliciting reactive hyperemia. Am J Physiol Heart Circ Physiol 2002; 283: H2250-H2259.
- Hamill OP, McBride DW, Jr. The pharmacology of mechanogated membrane ion channels. Pharmacol Rev 1996; 48: 231-252.
- Schubert R, Brayden JE. Stretch-activated cation channels and the myogenic response of small arteries. In: Mechanosensivity in Cells and Tissues. Kamkin A, Kiseleva I (eds). Moscow, Academia, 2005.
- Yao X, Kwan HY, Huang Y. A mechanosensitive cation channel in endothelial cells. J Card Surg 2002; 17: 340-341.
- Yao X, Kwan HY, Dora KA, Garland CJ, Huang Y. A mechanosensitive cation channel in endothelial cells and its role in vasoregulation. Biorheology 2003; 40: 23-30.
- Welsh DG, Nelson MT, Eckman DM, Brayden JE. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J Physiol 2000; 527 (Pt 1): 139-148.
- Yeon DS, Kim JS, Ahn DS, et al. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovasc Res 2002; 53: 431-438.
- Clifford PS. Local control of blood flow. Adv Physiol Educ 2011; 35: 5-15.
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol 1996; 271: H1697-H1701.
- Clifford PS, Tschakovsky ME. Rapid vascular responses to muscle contraction. Exerc Sport Sci Rev 2008; 36: 25-29.
- Davis MJ. Perspective: Physiological role(s) of the vascular myogenic response. Microcirculation 2012; 19: 99-114.
- Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 2004; 11: 279-293.
- Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol 2007; 581: 841-852.
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation following a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 2013; 305: 29-40.
- Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 2011; 57: 133-139.
- Rath G, Dessy C, Feron O. Caveolae, caveolin and control of vascular tone: nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J Physiol Pharmacol 2009; 60 (Suppl 4): 105-109.
- Hill MA, Meininger GA. Arteriolar vascular smooth muscle cells: mechanotransducers in a complex environment. Int J Biochem Cell Biol 2012; 44: 1505-1510.
- Spencer A, Wilson S, Harpur E. Gadolinium chloride toxicity in the mouse. Hum Exp Toxicol 1998; 17: 633-637.
- Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol 1997; 25: 245-355.
- Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol 1998; 275: C619-C621.
A c c e p t e d : April 22, 2014