MODERATE-INTENSITY INTERVAL TRAINING INCREASES SERUM
BRAIN-DERIVED NEUROTROPHIC FACTOR LEVEL AND DECREASES INFLAMMATION IN PARKINSON'S DISEASE PATIENTS
INTRODUCTION
It is well documented that regular physical activity has beneficial effect on health status of people and it is recommended in prevention and treatment of various pathological conditions especially cardio-pulmonary metabolic and locomotors insufficiencies (1). Beginning with the pioneer discovery by Neeper et al. (2) showing that physical exercise up-regulates the brain-derived neurotrophic factor (BDNF) gene expression in the brain the popularity of research concerning the effect of physical exercise on central nervous system has significantly increased. It has been shown that BDNF which is widely expressed in various parts of the human brain (3) is involved in several processes determining its functioning including: synapse development and plasticity, neuronal connectivity as well as promotes the development of immature neurons and enhances the survival of adult neurons (4, 5). Moreover, it has been shown that the exercise-induced enhancement of brain BDNF level in the hypothalamus was related to an improvement of cognitive function in rodents (6).
It has been demonstrated that BDNF can cross the blood-brain barrier in both directions i.e. from the side of brain to the periphery and from the blood to brain via the high capacity saturable transporter system (7). Therefore, it is considered that serum BDNF levels reflect the BDNF concentration in the brain (8, 9). It has been shown, that physical training can significantly increase plasma and/or serum BDNF level in healthy people (10, 11), although some researchers reported no effect of various kind of physical exercises on basal BDNF level (12, 13). Little is known about the basal BDNF level in blood of patients suffering from neurodegenerative diseases such as Parkinson disease (PD) although it has been recently reported that basal serum BDNF level in the PD patients is significantly lower than in controls (14). PD is a neurodegenerative disorder particularly characterized by the loss of dopaminergic neurons in the substantia nigra (15) resulting in resting tremor rigidity, bradykinesia and gait disturbances in PD patients (16, 17). The background of the dopaminergic neurons loss in substantia nigra is poorly understood, however it is considered, that oxidative stress and inflammation are involved in the pathogenesis of Parkinson’s disease (18, 19). The importance of the neuroinflammatory mechanisms has been confirmed in the post-mortem and in vivo studies (20). It has been demonstrated, that in the PD patients an increased expression of pro-inflammatory mediators is accompanied by a presence of activated microglial cells and T lymphocytes in substantia nigra, which clearly show an involvement of innate and adaptive immunity in the affected brain regions (21). As suggested the activated microglial cells through a release of pro-inflammatory cytokines and through a direct or indirect release of ROS have negative impact on the substantia nigra (22). Moreover, in the substantia nigra of PD patients higher level of biomarkers of reactive oxygen species oxidative stress such as 4-hydroxy-2,3-nonenal, 8-hydroksyguanosine and lower glutathione (GSH) levels has been found when compared to the controls (23). Based on the post mortem studies it is suggested that the earliest sign of Parkinson disease is the axon degeneration and therefore the axon re-growth might be the most appropriate goal of the early therapy in PD (24).
It has been also reported that enhanced oxidative stress and inflammation can decrease BDNF level (25). Moreover, a relationship between the inflammation and BDNF level has been reported in varied experimental models (26-28). Therefore, it seems to be the case that physical activity potent to modify the level of oxidative stress and inflammation can influence serum BDNF level and the clinical picture of Parkinson’s disease. It has been reported that various kinds of rehabilitation programs involving physical activities seem to have beneficial effect on PD patients (29-32), however, the underlining mechanism remains unknown. It is postulated, based on animal model, that neuroprotective effect of physical activity in PD is related to activation of BDNF signaling pathway (33). However, surprisingly the knowledge concerning the effect of physical exercise training on serum BDNF level in the PD patients is very poor. Therefore, in the present study we have aimed to evaluate the effect of 8 weeks of regular physical training in PD patients on serum BDNF level. Furthermore, we have hypothesized that the training-induced elevation of basal serum BDNF level will be accompanied by an improvement of health status and attenuation of inflammation and lipid peroxidation in the Parkinson's disease patients.
PATIENTS AND METHODS
Participants
The subjects were informed about the aim of the study, familiarized with training environment and gave their written consent prior to the study that had been approved by the local Ethics Committee and according with the Declaration of Helsinki.
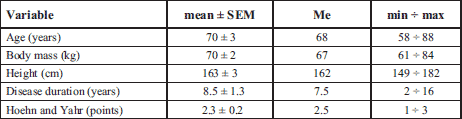
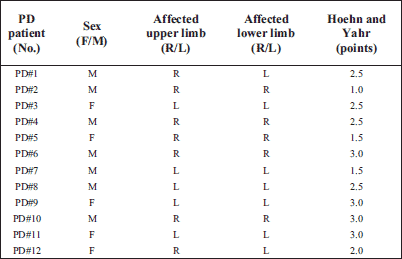
Twelve patients with idiopatic PD (women: n=5 men: n=7: mean ± S.E.M: age 70 ± 3 years; body mass 70 ± 2 kg; height 163 ± 3 cm, Table 1) participated in the present study. According to the Hoehn and Yahr scale (34) PD patients were mildly to moderately affected (score of 1–3) and one of them severely affected with score 4.0 (PD#7 during the post-training session). Detailed clinical characteristics of the PD patients are given in Table 2. The exclusion criteria for participation in the training program were: any cardiovascular and respiratory systems' symptoms (based on physician's subjects examination) and also motor deficits (related to PD stage or orthopedic and post-traumatic symptoms) that could limit the participation of PD patients in training sessions.
Clinical assessment of Parkinson's disease
PD patients were tested clinically by experienced neurologist blinded to other patients' results based on the Hoehn and Yahr scale and Unified Parkinson's Disease Rating Scale (UPDRS) (34).
Experimental procedure
The group of studied PD patients was tested twice: in April and in July (before and after 8 weeks of training). Before and after 8-weeks of training blood samples were taken from all subjects for an appropriate analysis. During both testing sessions the PD patients were in their medication off-phase (during intensification of PD symptoms after 12 hours (overnight) of anti-parkinsonian drugs withdrawal), however, they were in their medication on-phase during training sessions (beneficial effect of anti-parkinsonnian medication: mainly levodopa and within some patients piribedil, ropinirol). Medication doses for each PD patient remained the same as normally administered by their leading neurologist (disease stage-adjusted) and were constant throughout the whole 8-weeks training period.
Training protocol
The PD patients performed 8-weeks training program which consisted of three 1-hour training sessions weekly (total 24 training sessions). This training was performed on a stationary cycle ergometer (MONARK Ergomedic 874E, Sweden) that allowed to measure cadence (rpm) and power output (W). Each 1-hour training session consisted of 10-minutes warm-up (at slow voluntary speed), 40-minutes of moderate-intensity interval exercise and 10-minutes of cool-down phase (with slow voluntary speed). The moderate-intensity interval training session (IT) consisted of 8 sets of 5 minute interval exercise including 3-minutes cycling at 80–90 rpm (fast phase of IT) and 2-minutes cycling less than 60 rpm (slow phase of IT). The heart rate (HR) was measured by Polar system (Polar Electro Oy, Kempele, Finland) cadence and power were monitored and collected during each training session (warm up, IT, cool down phases). Training supervisor adjusted the resistance at the cycle ergometer for each patient to ensure cycling at each patient's target heart rate (THR) and with appropriate cadence. The patients cycled at 60–75% of their individualized HRmax, which was predicted for each patient based on the formula developed by Tanaka et al. (35). The PD patients were encouraged to cycle faster (80–90 rpm or 30% faster than their freely chosen pedaling rate) during the fast part of the IT. Each patient increased its THR every 2 weeks by 5% (60% of the HRmax during the first two weeks, 65% during the third and fourth week, 70% during the fifth and sixth week and 75% of HRmax during the seventh and eighth week of training period). Since the HRpeak in the PD patients was reported to be by about 5% lower than the HRpeak in the healthy individuals at similar age (36), most likely our patients during the applied training exercised at slightly higher percentage of HRmax than that calculated from the formula developed by Tanaka et al. (35). Nevertheless, taking also into consideration the results reported by Protas et al. (36) the highest HR reached by our patients during this training was still by about 20% lower than their predicted HRmax.
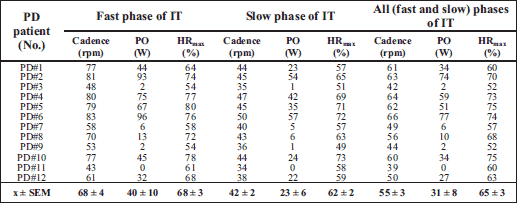
The supervisor provided water and any additional help during training session. The eight intervals' averaged values of the HR, cadence and power of each training session for each subject during the fast and slow phases of IT were calculated. Then the average value of the 24 training sessions for each of the parameter was calculated. Detailed information about moderate-intensity interval forced training parameters for each PD patient is given in Table 3. This interval training was well tolerated by the patients and it could be called interval training with moderate-intensity.
Blood sampling and measurements
In the studied groups blood samples for measurements of blood variables were taken from the antecubital vein at rest in the morning hours between 8:00–10:00 a.m. in the fasting state. Blood for plasma measurements was collected in tubes (SARSTEDT AG & Co, Germany) containing the appropriate anticoagulant solution (potassium EDTA) and centrifuged at 435 × g for 15 min at 4°C. Blood for serum measurements was collected in anticoagulant-free tubes with the clotting activator (SARSTEDT AG & Co, Germany) centrifuged at 653 × g for 10 minutes at 4°C. Plasma and serum were stored at –80°C for further analysis.
Hematological parameters
The total and differential blood cell counts were analyzed using standard hematological procedures and a dedicated analyzer - model ADVIA 60 (Bayer Corporation, 511 Benedict AV, Tarrytown, New York, USA).
Serum brain-derived neurotrophic factor measurements
Blood for serum BDNF measurements [BDNF]s was collected in anticoagulant-free tubes with the clotting activator (SARSTEDT AG & Co, Germany) and kept for 1 hour on ice (at a temperature of about 4°C). After that blood was centrifuged to isolate the serum at 653 × g for 10 minutes at 4°C. The serum was assay for BDNF with an enzyme-linked immunosorbent assay (ELISA) Kit (Promega, Wallisellen, Switzerland) after appropriate dilution with Block and Sample solution (provided with the kit). Amicroplate reader (BioTek Instruments, USA) set at 450 nm was used to determine BDNF values (intra-assay and interassay variation were less than 9% and 15% respectively) (37).
Serum soluble vascular cell adhesion molecule 1 and tumor necrosis factor-α
Soluble vascular cell adhesion molecule 1 (sVCAM-1, human Quantikine ELISA kit, DVC00, R&D System) and tumor necrosis factor-α (TNF-α, human Quantikine HS ELISA, HSTA00D, R&D Systems) concentrations were assayed in serum according to manufacturer's instructions.
Serum cortisol
Serum cortisol level was measured by means of electrochemiluminscent immunoassay (ECLIA) by Roche Diagnostics Ltd (Switzerland) using cobas e411 Roche Diagnostics Ltd analyzer (Switzerland).
Plasma 8-epi-prostaglandin F2α (F2isoprostanes)pl
8-epi-prostaglandin F2α concentration in plasma samples [F2isoprostanes]pl was assayed by 8-Isoprostane EIA Kit 516351, Cayman Chemical according to the manufacturer's instructions.
Plasma syndecan-1
Syndecan-1 concentration in plasma samples was assayed using human CD138/Syndecan ELISA kit 950.640.192, Diaclone according to manufacturer's instructions.
Statistical analysis
The results are presented as means ± S.E.M., median (Me), minimum ÷ maximum (min÷max). The significance was set at P<0.05. Statistical significance of the differences for paired samples was tested using the non-parametric Wilcoxon-signed-rank test (before vs. after the training). Non-asymptotic exact two-sided P - values are presented. The statistics were done using the statistical packet StatXact 9 (Cytel software, Corporation Cambridge, MA, USA) and STATISTICA 10.0 (StatSoft, Tulsa, OK, USA).
RESULTS
Total Unified Parkinson's Disease Rating Scale in Parkinson's disease patients group before and after the training
Unified Parkinson's Disease Rating Scale (UPDRS) total score in patient before training amounted to 48.9 ± 4.3 points and it decreased significantly after the training to 38.1 ± 3.9 points (P=0.01) (Fig. 1).
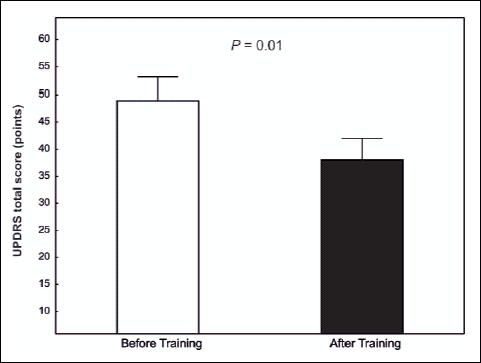 |
Fig. 1. Total Unified Parkinson's Disease Rating Scale (UPDRS) score in the PD patients (n=12) before and after 8 weeks of training. |
Brain-derived neurotrophic factor
In the group of patients (n=12) basal serum BDNF level [BDNF]s before training amounted to 10 977 ± 756 pg · mL-1 and after 8 weeks of training it has increased significantly (P=0.03) by about 34% to 14 206 ± 1 256 pg · mL-1 (Fig. 2).
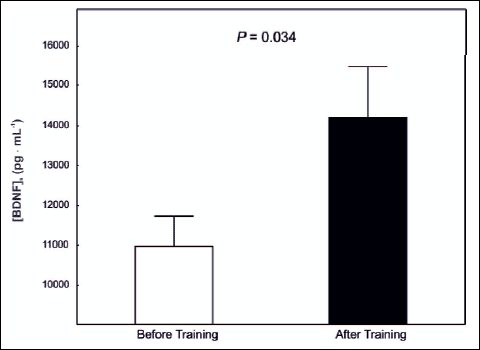 |
Fig. 2. Serum brain-derived neurotrophic factor level [BDNF]s in the PD patients (n=12) before and after 8 weeks of training. |
Blood platelets
Basal PLT level in PD patients before the training has amounted to 184 ± 13 K · L-1 and after 8 weeks of training its value amounted to 197 ± 12 K · L-1 and was not significantly different from pre-training level (P=0.91).
Serum cortisol level
No effect of the 8 weeks of training on the serum cortisol level (P=0.30) in the group of patients was found (514 ± 47 nmol · L-1 vs. 480 ± 43 nmol · L-1, respectively before and after training).
Plasma F2 isoprostanes level
Basal [F2 isoprostanes]pl after the training was not significantly different (P=0.38) than before training (39.8 ± 2.6 vs. 40.3 ± 2.9 pg · mL-1, respectively before and after the training).
Plasma syndecan-1 level
The 8 weeks of training did not affect (P=0.91) the basal plasma syndecan-1 level (22.28 ± 1.86 vs. 22.24 ± 2.23 ng · mL-1, respectively before and after the training) in the group of patients.
Serum vascular cell adhesion molecule 1 level
The 8 weeks of training resulted in a decrease in [sVCAM]ss level (P=0.001) in the studied group of patients by about 21% (805 ± 52 ng · mL-1 vs. 623 ± 31 ng · mL-1, respectively before and after training) (Fig. 3A).
Serum tumor necrosis factor
Basal serum TNF-α (P=0.03) significantly decreased after 8 weeks of training in the group of patients (by about 7%, Fig. 3B).
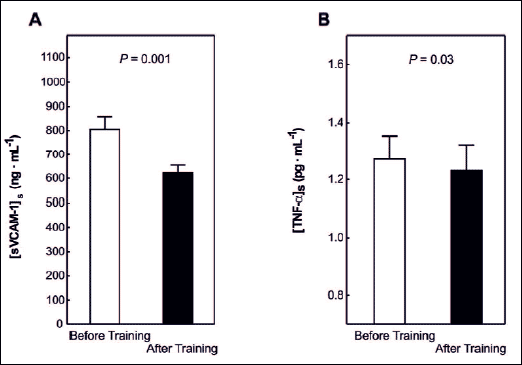 |
Fig. 3. Basal serum soluble vascular cell adhesion molecule-1 level [sVCAM-1]s in the PD patients before and after 8 weeks of training (panel A) and basal serum tumor necrosis factor level [TNF-α]s in the PD patients before and after 8 weeks of training (panel B). |
DISCUSSION
In this study we have found that moderate-intensity interval training (IT) performed 3 times per week for about an hour involving cycling at high pedaling rates improves the clinical status of Parkinson's disease patients as judged by a decrease in the UPDRS total score (Fig. 1). The main and original findings of this study are as follows: a) this training resulted in an increase of the basal serum BDNF level by about 34% (P=0.03) (Fig. 2) in PD patients; b) attenuated inflammation (a decrease in serum sVCAM-1 and serum TNF-α levels, Fig. 3) was not harmful to the endothelial glycocalyx (no changes in plasma syndecan-1 level) and did not resulted in enhanced oxidative stress as judged by no changes in [F2 isoprostanes]pl.
Training-induced increase in serum or plasma BDNF levels in healthy subjects has been published before (12, 13). However, the training-induced increase in serum BDNF level in the PD patients shown in this study to our best knowledge is the first reported finding in this area of research. The mechanism by which training increases serum BDNF level in humans remain unknown. Since the BDNF in blood is mainly stored in blood platelets (38) one could speculate that the training-induced increase in the serum BDNF level in the PD patients is simply caused by an increase in their blood platelets count. However, in the present study, blood platelets count in the PD patients before as well as after training did not change (P=0.91). Therefore, we can exclude an increase in blood platelets count as the explanation of the observed increase in serum BFNF level in the PD patients. More likely explanation of the training-induced increase in serum BDNF level in our patients is an increase in its production in the brain as well as in periphery. In has been recently shown by Seifert et al. (39) that endurance training lasting 3 months resulted in enhanced resting release of BDNF from the brain in young, healthy men, which suggest, that the observed in our study training-induced elevation of the basal serum BDNF level in the PD patients could be also caused by an increase of its release from the brain. Moreover, we cannot exclude an increase of BDNF release after training from other peripheral tissues including endothelium as reported recently by Prigent-Tessier et al. (40).
In view of literature data the training-induced increase in serum BDNF level might contribute to the improvement of patients health status in several ways. First of all, it is well documented that BDNF plays an important role in functioning of the brain (5, 41, 42). Moreover, a recent studies have shown that BDNF stimulates synthesis of prostacyline (PGI2) in cerebral arteries (43). Prostacyline plays an important role in endothelium-dependent relaxation and also possess anti-platelet, anti-atherogenic, vasculoprotective and cardioprotective properties (44). Indeed, it was reported that patients with acute coronary syndromes have reduced levels of BDNF in plasma (45). It has been also recently postulated that the already established role of BDNF in synaptic plasticity and synaptic growth could be a challenge for clinical therapies in neurodegenerative disorders (5). Therefore, the training-induced increase in the serum BDNF level in the PD patients (Fig. 2) could be directly linked to the observed improvement of health status of our patients (Fig. 1). Interestingly, Ziebel et al. (46) recently reported that serum BDNF levels in patients with parkinsonism correlates positively with striatal dopamine transporter (DAT) availability. These authors concluded that in patients with striatal dopaminergic neurodegeneration serum BDNF levels decrease along with loss in striatal DAT binding. This suggest that the training-induced increase in serum BDNF level might have protective effect on dopaminergic neurons in the PD patients.
It should be noticed that not all single bouts of exercise or training programs applied to varied groups of subjects resulted in a significant increase of serum or plasma BDNF levels in humans (12, 13). The reason for this is unknown, but it seems to be very likely that the intensity and training work load applied seem to play an important role in the magnitude of BDNF response to training (11-13, 47, 48). It seems to be the case that there is a threshold level of exercise intensity below which no effect of physical exercise on serum or plasma BDNF level can be found. Interestingly, it has been reported that in case of PD patients training programs involving high velocity cycling exercise is more effective than a program involving cycling at low pedaling rate only (49). In the present study, we have applied interval training composed of both low and high velocity cycling which for the PD patients was not always easy to follow. The choices of the high pedaling rate program was based on the earlier findings (49) showing that the physical training involving high pedaling rate is more beneficial to the PD patients than that based on low pedaling rates. Indeed, the previous human studies (40, 49) have suggested that physical activity exerts beneficial neuroplastic effects in PD patients' central nervous system. The study by Ridgel et al. (32) in which a period of eight weeks of training (3 session per week) involving pedaling on a tandem cycle ergometer applied in PD patients showed that an improvement in motor PD symptoms and upper extremities manual dexterity occurred only in these patients who pedaled with higher than voluntary rate (about 90 rpm) but it did not occurred in voluntary pedaling patients (about 60 rpm). Interestingly, the training program involving cycling at high pedaling rates applied in the present study, was indeed effective in increasing basal serum BDNF level and it resulted in an improvement of clinical picture of our patients, as judged by an attenuation of UPDRS total score (Fig. 1). However, it should be noticed that the pedaling rate developed by our patients (Table 3) was clearly lower than in case of the study by Ridgel et al. (32) but even so it was difficult to follow by several of our patients. It should be also noticed that this rather demanding training program had no effect on the basal serum cortisol level in the studied patients (P=0.30).
It is still not clear why in case of the PD patients the high velocity cycling is more effective than the slow one. There could be simple explanation that cycling at a given power output using high pedaling rate requires more energy than cycling at low frequency (50). Moreover, while cycling at high vs. slow pedaling rates at a given power outputs the PD patients could generate the required power outputs at greater reserves of muscle force generating capabilities (50). This strategy could allow the PD patients to perform the training program for a longer period of time at relatively high metabolic rates without exhaustion, as in the present study, and could provide sufficient stimulus for adaptation to the applied training.
It has been shown that Parkinson disease is accompanied by inflammation (18, 20-22) and oxidative stress (19, 23). As recently shown by Goldberg et al. (51) mitochondrial oxidative stress plays a key role in neurodegeneration in Parkinson disease. In the group of our PD patients training had no impact on the oxidative stress as judged by no changes in [F2isoprostanes]pl, (P=0.38). However, it should be pointed out that the applied in this study training program resulted in a decrease of basal serum sVCAM-1 and plasma TNF-α levels (Fig. 3). This indicates that this training did induce some anti-inflammatory responses in the PD patients. We postulate, that the attenuation of the inflammatory responses after training could be at least partly responsible for the observed increase of serum BDNF level after the training (Fig. 2) and improvement of clinical picture of our patients (Fig. 1). Therefore, for deeper understanding of the potential link between the training-induced attenuation of inflammation with an increase of BDNF level it would be interesting to examine, on animal model, the effect of PPAR-γ agonists (rosiglitazone and troglitazone) administration, which mimic the effect of regular exercise (52) and possess anti-inflammatory activity (53, 54), on the BDNF level.
Interestingly, the applied in the present study high pedaling rate endurance training program in PD patients had no harmful effect on the endothelial glycocalyx, as judged by unchanged serum syndecan-1 level (P=0.91), considered as a marker of endothelial glycocalyx damage (55).
In conclusion, the moderate-intensity interval training performed 3 times per week (each session lasting 60 min) involving cycling at high pedaling rates improves the clinical status of Parkinson's disease patients as judged by a decrease in the UPDRS total score. This was accompanied by a significant increase of the basal serum BDNF level in the studied patients. The training resulted also in a decrease of serum sVCAM-1 and TNF-α levels indicating that this training program attenuated the inflammation in the PD patients. We have concluded that the improvement of health status of the Parkinson's disease patients after training could be related to the increase of serum BDNF level caused by the attenuated inflammation in those patients.
Acknowledgements: We thank the patients for their kind cooperation in this study.
We gratefully acknowledge the financial support of the Ministry of Science and Higher Education (Poland) (grant: no. 0247/p01/2010/70 awarded to Jaroslaw Marusiak). Dr. Jerzy A. Zoladz was supported by funds for the statutory research in 2012 and 2013 for the Chair of Physiology and Biochemistry, Faculty of Rehabilitation, University School of Physical Education in Cracow, Poland.
Conflict of interests: None declared.
REFERENCES
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16 (Suppl. 1): 3-63.
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Exercise and brain neurotrophins. Nature 1995; 373: 109.
- Murer MG, Boissiere F, Yan Q, et al. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain with particular reference to Alzheimer's disease. Neuroscience 1999; 88: 1015-1032.
- Ebadi M, Bashir RM, Heidrick ML, et al. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int 1997; 30: 347-374.
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 2013; 14: 401-416.
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis learning and long-term potentiation in mice. Proc Natl Acad Sci USA 1999; 96: 13427-13431.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998; 37: 1553-1561.
- Blugeot A, Rivat C, Bouvier E, et al. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci 2011; 31: 12889-12899.
- Sartorius A, Hellweg R, Litzke J, et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 2009; 42: 270-276.
- Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett 2010; 479: 161-165.
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008; 59 (Suppl. 7): 119-132.
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 2010; 40: 765-801.
- Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol 2010; 61: 533-541.
- Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. J Neurol 2010; 257: 540-545.
- Fitzmaurice AG, Rhodes SL, Lulla A, et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci USA 2013; 110: 636-641.
- Marusiak J, Jaskolska A, Budrewicz S, Koszewicz M, Jaskolski A. Increased muscle belly and tendon stiffness in patients with Parkinson's disease as measured by myotonometry. Mov Disord 2011; 26: 2119-2122.
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology 2009; 72 (21 Suppl. 4): S1-136.
- Appel SH. Inflammation in Parkinson's disease: cause or consequence? Mov Disord 2012; 27: 1075-1077.
- Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 2013; 62: 132-134.
- Hirsch C, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 2009; 8: 382-397.
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord 2012; 18 (Suppl. 1): S210-S212.
- Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat Disord 2012; 18 (Suppl. 1): S207-S209.
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp Neurol 2005; 193: 279-290.
- Burke RE, O'Malley K. Axon degeneration in Parkinson's disease. Exp Neurol 2013; 246: 72-83.
- Zhao HF, Li Q, Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neuroscience 2011; 183: 189-202.
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25: 181-213.
- Gibney SM, McGuinness B, Prendergast C, Harkin A, Connor TJ. Poly I:C induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun 2013: 28; 170-181.
- Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun 2014; 38: 202-210.
- Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011; 77: 288-294.
- Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil 2007; 88: 1154-1158.
- Ridgel AL, Kim CH, Fickes EJ, Muller MD, Alberts JL. Changes in executive function after acute bouts of passive cycling in Parkinson's disease. J Aging Phys Act 2011; 19: 87-98.
- Ridgel AL, Vitek JL, Alberts JL. Forced not voluntary exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair 2009; 23: 600-608.
- Wu SY, Wang TF, Yu L, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun 2011; 25: 135-146.
- Fahn S, Elton R. Members of the UPDRS Development Committee. In: Recent Developments in Parkinson's Disease, Fahn S, Marsden CD, Calne DB, Goldstein M, (eds). Florham Park, NJ, Macmillan Health Care Information, 1987, pp. 153-163.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 2001; 37: 153-156.
- Protas EJ, Stanley RK, Jankovic J, MacNeill B. Cardiovascular and metabolic responses to upper- and lower-extremity exercise in men with idiopathic Parkinson's disease. Phys Ther 1996; 76: 34-40.
- Zoladz JA, Smigielski M, Majerczak J, et al. Hemodialysis decreases serum brain-derived neurotrophic factor concentration in humans. Neurochem Res 2012; 37: 2715-2724.
- Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 2002; 87: 728-734.
- Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 2010; 298: R372-R377.
- Prigent-Tessier A, Quirie A, Maguin-Gate K, et al. Physical training and hypertension have opposite effects on endothelial brain-derived neurotrophic factor expression. Cardiovasc Res 2013; 100: 374-382.
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 1996; 381: 706-709.
- Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA 2004; 101: 10827-10832.
- Santhanam AV, Smith LA, Katusic ZS. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke 2010; 41: 350-356.
- Gryglewski RJ. Prostaglandins platelets and atherosclerosis. CRC Crit Rev Biochem 1980; 7: 291-238.
- Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol 2005; 102: 169-171.
- Ziebell M, Khalid U, Klein AB, et al. Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiol Aging 2012; 33: 428.
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007; 39: 728-734.
- Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res 2006; 1121: 59-65.
- Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike it is about the pedaling: forced exercise and Parkinson's disease. Exerc Sport Sci Rev 2011; 39: 177-186.
- Zoladz JA, Rademaker AC, Sargeant AJ. Human muscle power generating capability during cycling at different pedalling rates. Exp Physiol 2000; 85: 117-124.
- Goldberg JA, Guzman JN, Estep CM, et al. Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson's disease. Nat Neurosci 2012; 15: 1414-1421.
- Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 2007; 31: 1302-1310.
- Celinski K, Dworzanski T, Fornal R, Korolczuk A, Madro A, Slomka M. Comparison of the anti-inflammatory and therapeutic actions of PPAR-gamma agonists rosiglitazone and troglitazone in experimental colitis. J Physiol Pharmacol 2012; 63: 631-640.
- Celinski K, Dworzanski T, Fornal R, et al. Comparison of anti-inflammatory properties of peroxisome proliferator-activated receptor gamma agonists rosiglitazone and troglitazone in prophylactic treatment of experimental colitis. J Physiol Pharmacol 2013; 64: 587-595.
- Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level a marker of endothelial glycocalyx degradation is associated with inflammation protein C depletion fibrinolysis and increased mortality in trauma patients. Ann Surg 2011; 254: 194-200.
A c c e p t e d : March 6, 2014