ONE SESSION OF EXERCISE OR ENDURANCE TRAINING DOES NOT INFLUENCE SERUM LEVELS OF IRISIN IN RATS
INTRODUCTION
Recently, skeletal muscle was identified as an endocrine organ that produces and releases a wide range of cytokines and other peptides. These cytokines and peptides are collectively named myokines. Myokines exert their effects in autocrine, paracrine, and endocrine manners and play an important role in driving adaptive changes to physical exercise. Myokines provide the basis for understanding cross-talk between exercising muscles and other organs, such as adipose tissue, liver, heart and vessels, and brain and could explain the beneficial effects of physical activity in preventing non-communicable diseases (1-3). Physical inactivity is associated with higher BMI and greater risk for excessive accumulation of fat in the central part of the body (4).
Recently, a new myokine, irisin was discovered, which is a downstream product of the transcriptional co-activator, PGC-1α (peroxisome proliferator-activated receptor-γ coactivator-1α). PGC-1a is increased in muscles by physical exercise and mediates many adaptive changes related to energy metabolism, for instance the expression of mitochondrial proteins encoded in both nuclear and mitochondrial genes. It stimulates also the expression of several skeletal muscle genes, including FNDC5 (fibronectin type III domain containing 5). The FNDC5 gene encodes a type I membrane protein that is subsequently proteolytically processed to form irisin. Irisin is secreted from muscles into the blood and induces browning of adipose tissue and UCP-1 (uncoupling protein 1) expression, and improves systemic metabolism by increasing energy expenditure (5-8). Irisin serum levels are positively correlated with body mass index, body weight, fat mass, and free fat mass (9).
Because of the short time since the discovery of irisin, there are very few experimental studies regarding the influence of physical exercise on FNDC5 expression in muscles and irisin protein levels in muscle and serum, and the results are controversial. The studies of Bostrom et al. (6) showed that FNDC5 mRNA is increased in muscles and irisin protein is increased in plasma following 3 weeks of training in mice, and after 10 weeks of training in humans, but Timmons et al. (10) confirmed the increase in muscle FNDC5 mRNA only in the case of highly active elderly human subjects.
The goal of the present study was to investigate the influence of a single session of acute exercise in untrained rats compared with trained rats, and prolonged endurance training on FNDC5 mRNA and irisin protein levels in white and red muscle and serum. Additionally, to determine whether endurance training, similar to that recommended for people, induces irisin in the signalling pathway, and subsequently directly induces the browning of adipose tissue.
MATERIALS AND METHODS
All procedures in the study were approved by the Ethical Committee of the Medical University in Bialystok and were performed according to EU regulations governing the treatment of laboratory animals.
This study used 60 male Wistar rats. The rats had access to water ad libitum, were fed Labofeed B, and were maintained in a 12/12 h light/dark cycle. The experimental protocol was as previously described (11-13).
During the first 5 successive days of the experiment, the rats were subjected to exercise adaptation, which consisted of 10 min of treadmill running at 15 m/min per day. Following adaptation, rats were randomly assigned to two groups: untrained (UT, n=30) and trained (T, n=30). Rats from the T group were subjected to prolonged endurance training consisting of treadmill running 5 days a week for 6 weeks. During the first week, the exercise time was increased by 10 min each day, starting from running 10 min/per day at a speed of 1200 m/h. During subsequent weeks of training, running time was a constant 60 min/day. During week 2, the running speed was 1500 m/h, and then it was increased to 1680 m/h during weeks 3–6. No additional stimulus was applied to enhance running.
The UT group remained at rest during the training period. Twenty-four hours after the last training session, each group (UT and T) was randomly divided into three subgroups. Ten rats from each subgroup (UTpre, n=10 and Tpre, n=10) were sacrificed under anaesthesia (intraperitoneal chloral hydrate, 1 ml/100 mg body mass). The remaining rats in each UT and T subgroup were subjected to an acute exercise session consisting of 60 min of treadmill running at 1680 m/h. Relative effort was higher in the UT group compared with the T group. The rats from the two subgroups subjected to acute exercise were sacrificed either immediately following the acute exercise (UT0h, n=10 and T0h, n=10) or 3 hours after the acute exercise (UT3h, n=10 and T3h, n=10). Immediately after sacrifice, samples from the gastrocnemius (red and white portions) muscle were collected according to the described redistribution of specific fibres (14) from all rats in each group and stored at –80°C for subsequent analyses.
The mean body mass of rats in the UT group on the day of acute exercise was 271±11.6 g and 283.17±24.67 g in the T group.
mRNA isolation
Approximately 50 mg of skeletal muscle tissue (red and white portions of the gastrocnemius) was homogenized in a TissueLyser bead mixer (Qiagen, Germany) at a frequency of 25 Hz for 5 minutes. Total mRNA isolation was performed using an EZ1 RNA Universal Tissue Kit and Biorobot EZ1 (Qiagen, Germany) according to the manufacturer’s instructions. Total RNA concentrations were measured at 260 nm using NanoDrop spectrophotometry (ND-1000 Spectrophotometer, NanoDrop Technologies, Inc). Samples were then frozen and stored at –80°C for further analyses.
Reverse transcription
Reverse transcription of total mRNA into cDNA was performed using the Thermomixer Comfort (Eppendorf, Germany) with the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, USA) according to the manufacturer’s instructions.
Real-time PCR to quantify FNDC5 in mRNA
Detection of mRNA was performed using an ABI-Prism 7500 Sequence Detection System (Applied Biosystems, USA). Specific primers and probes and TaqMan Universal Master Mix for rat FNDC5 were purchased from Applied Biosystems. The relative amount of specific mRNA was normalized to rat GAPDH. The relative expression of mRNA was calculated using the 2-ΔΔCt method (15).
Protein quantization in skeletal muscle and serum
The evaluation of irisin concentrations in serum and in tissue homogenates was performed using enzyme-linked immunosorbent assay (ELISA). Each sample of skeletal muscle was homogenized in a TissueLyser bead mixer (Qiagen,USA) and centrifuged (10,000 rpm for 10 minutes, 4°C). The supernatant was collected and frozen at –80°C until analyses. Total protein concentration was measured at 562 nm on a Bio-Tek Power Wave XS spectrophotometer (Bio-Tek Instruments, USA) using bicinchoninic acid (BCA) Protein Assay Reagent (Pierce, Holland), according to the manufacturer’s instructions. Tissue and serum irisin protein concentrations were measured using an Irisin/FNDC-5 ELISA kit (Phoenix Pharmaceuticals, INC, USA). The results are presented as the ratio of irisin concentration/protein concentration.
Statistical analyses
Results are provided as the mean ±S.D. and as relative fold changes. Differences in mRNA levels (for statistics, DCT was used) and protein levels between groups were analysed using Kruskal-Wallis non-parametric ANOVA followed by post hoc Duncan’s Test. Pre- and post-training values of mRNA and protein levels in skeletal muscle tissue were compared using the Student’s t test. p<0.05 was considered to be statistically significant.
RESULTS
The relative changes in FNDC5 mRNA and irisin protein levels in red skeletal muscle (gastrocnemius, red portion) after an acute bout of exercise in UT and T rats are shown in Fig. 1. In UT rats, there was a significant decrease in FNDC5 mRNA in the UT0h and UT3h groups compared with the UTpre group (p=0.0003 and p=0.001, respectively). In T rats, FNDC5 mRNA levels were similar between groups between time points. Irisin protein levels decreased, but not significantly, in the UT0h group compared with the UTpre group, and there was a significant increase in the UT3h group compared with the UT0h group (p=0.002). In T rats, irisin protein levels were similar between time points.
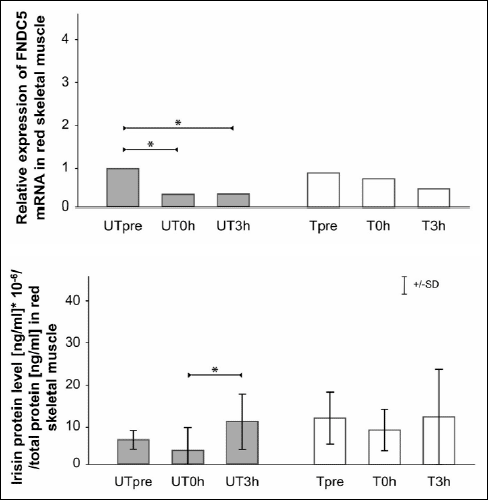 |
Fig. 1. Relative skeletal muscle (gastrocnemius, red) FNDC5 mRNA and irisin protein levels (mean ±S.D.) after an acute bout of exercise in untrained (UT) and trained (T) rats. Analyses were performed in samples collected from each group prior to an acute bout of exercise (UTpre, Tpre), just after the cessation of exercise (UT0h, T0h), and 3 hours post exercise (UT-3h, T3h). * Statistically significant difference (p<0.05). |
The relative changes in FNDC5 mRNA and irisin protein levels in white skeletal muscle (gastrocnemius, white portion) after an acute bout of exercise in UT and T rats are shown in Fig. 2. In UT rats, FNDC5 mRNA levels decreased in the UT0h group compared with the UTpre group (p=0.03). In the UT3h group, FNDC5 mRNA was increased in comparison with levels in the UT0h group (p=0.01). In T rats, there was a significant decrease in FNDC5 mRNA in the T0h group compared with the Tpre group (p=0.0007). The trend toward increased levels appeared 3 hours post exercise; however, this increase was not significantly different compared with the earlier time points.
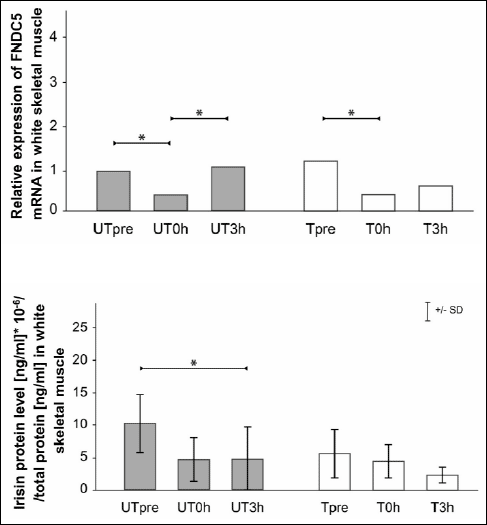 |
Fig. 2. Relative skeletal muscle (gastrocnemius, white) FNDC5 mRNA and irisin protein levels (mean ±S.D.) after an acute bout of exercise in untrained (UT) and trained (T) rats. Analyses were performed in samples collected from each group prior to an acute bout of exercise (UTpre, Tpre), just after the cessation of exercise (UT0h, T0h), and 3 hours post exercise (UT-3h, T3h). * Statistically significant difference (p<0.05). |
The irisin protein levels were decreased in the UT3h group compared with the UTpre group (p=0.02). In T rats, the irisin protein levels were similar between time points.
The relative changes in FNDC5 mRNA and irisin protein levels in red and white skeletal muscle following prolonged endurance training in T rats compared with UT rats are shown in Fig. 3. FNDC5 mRNA did not change after prolonged training in red or white muscle. Irisin protein levels increased in red muscle (p=0.03) and decreased in white skeletal muscle (p=0.02).
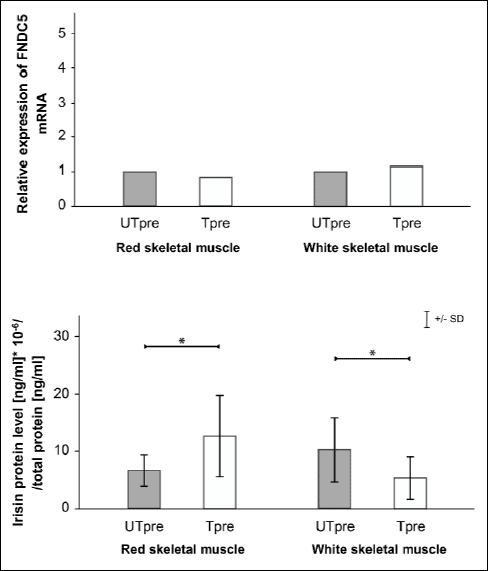 |
Fig. 3. Relative changes in skeletal muscle (gastrocnemius, red, panel A, and gastrocnemius, white, panel B) FNDC5 mRNA and irisin protein levels (mean ±S.D.) after prolonged endurance training (T vs. UT). * Statistically significant difference (p<0.05). |
Serum irisin levels did not change after an acute bout of exercise in either UT or T rats and after prolonged training (Fig. 4).
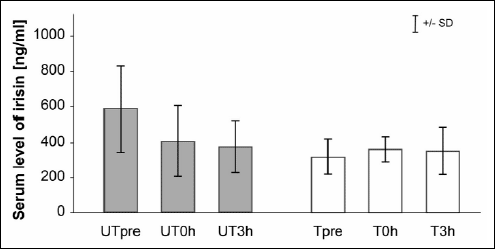 |
Fig. 4. Irisin protein levels in serum after an acute bout of exercise in untrained (UT) and trained (T) rats. 0j |
DISCUSSION
The most surprising effect of our experiment was the stable irisin serum levels after acute bouts of exercise in untrained and trained animals and after prolonged training. These findings are in contrast with the results reported by Bostron et al. (6); however, their results referred to humans and mice, and the results may not be transferable between species. Our results are in line with the recently published data obtained by Fain et al. (16), who reported that prolonged training do not result in increase of FNDC5 mRNA and protein in skeletal muscle and irisin level in serum in normal pigs. Huh et al. (17) reported an increase in irisin serum levels after an acute sprint in untrained humans and unchanged irisin levels after the same exercise in trained humans. The level of irisin increased when the level of ATP dropped, but remained unchanged when the level of ATP also remained unchanged. They proposed the hypothesis that when ATP concentrations in muscle are decreased, irisin production is upregulated. Thus, irisin could contribute to restoration of ATP homeostasis, but return to baseline levels soon after this restoration is completed. Based on Huh’s results and conclusions, we hypothesize that the mode of energy metabolism could explain the lack of increase in irisin serum levels in our study. Running at the speeds used in our experiment, the exercise was moderate for the rats so ATP depletion was low and primarily red muscle fibres were recruited (18). Myosin heavy chain composition of muscle fibres has an impact on the oxygen cost of exercise. (19). This could explain why we did not observe any immediate changes in irisin protein levels after an acute bout of exercise in red or white muscle in either group. However, other regulatory mechanism could be involved to induce an increase in irisin protein level in red muscle in the UT group 3 hours after the exercise compared with the time point immediately after exercising. Otherwise, in the UT group in white muscle, there was a decrease in irisin protein level 3 hours after an acute bout of exercise compare to pre-exercise level. Similar results were found after prolonged training as the increase in irisin protein levels were observed only in red muscle; in white muscle, a decrease in irisin protein levels was observed. It seems that small changes observed 3 hours after an acute bout of exercise occurred during the training period. The aforementioned increases in irisin protein levels in muscles seem too small to induce an increase in irisin serum levels after acute bouts of exercise or prolonged training.
In our experiment, FNDC5 mRNA expression was decreased right after an acute exercise session in red and white muscles in trained and untrained rats, however non significantly in trained group in red muscle. A trend towards a return to baseline levels was observed 3 hours following cessation of exercise, but only in white muscle in untrained group. Bostrom at al. (6) reported that acute exercise did not change FNDC5 mRNA levels in mice; however, they did not describe the parameters of the exercise and time points of samples taken in relation to the cessation of exercise. They observed increased FNDC5 mRNA following prolonged training in mice and humans. In our experiment, FNDC5 mRNA levels did not change after endurance training in red or white muscles. Timmons et al. found an increase in FNDC5 mRNA levels in humans, but only in active elderly subjects. They did not confirm Bostrom’s results with regard to young active subjects after an aerobic exercise, and found no relationship between the FNDC5 mRNA in muscle and diabetes status in humans (10).
Changes in irisin protein levels following training in red or white muscle did not follow the changes inFNDC5 mRNA levels in the muscles. The possible reason for this could be the different proteolytic activities in muscle that are responsible for translating irisin protein from the product of the FNDC5 gene, or possible post-translational changes. In mammalian cells, the correlation coefficient between mRNA and protein levels is less than 0.5 (20).
In conclusion, serum level of irisin was stable after acute exercise or prolonged training and changes in irisin mRNA and protein levels after acute exercise and prolonged training in the muscles of rats are limited and depend on the training status and muscle type.
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; FNDC5, fibronectin type III domain containing 5; UCP-1, uncoupling protein 1
Acknowlwdgements: These studies were partially funded by the Medical University of Bialystok (grant no: 123-18810) and by Medical University of Warsaw (grant no: NZME/DYW/10/12).
Conflict of author’s: None declared.
REFERENCES
- Petersen AM, Pedersen BK. The role of Il-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol 2006; 57 (Suppl. 10): 43-51.
- Bilski J, Mazur-Bialy AI, Wierdak M, Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol 2013; 64: 143-155.
- Sadowska-Krepa E, Klapcinska B, Jagsz S, et al. Diverging oxidative damage and heat shock protein 72 responses to endurance training and chronic testosterone proprionate treatment in three striated muscle types of adolescent male rats. J Physiol Pharmacol 2013; 64: 639-647.
- Plonka M, Toton-Morys A, Adamski P, et al. Association of the physical activity with leptin blood serum level, body mass indices and obesity in schoolgirls. J Physiol Pharmacol 2011; 62: 647-656.
- Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol 2008; 59 (Suppl. 7): 5-18.
- Bostrom P, Wu J, Jedrychowski MP, et al. A PGC-1α - dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463-468.
- Kelly DP. Medicine. Irisin, light my fire. Science 2012; 336: 42-43.
- Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013; 240: 155-162.
- Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity - correlation with body mass index. Peptides 2012; 39: 125-130.
- Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature 2012; 488: E9-E10.
- Czarkowska-Paczek B, Zendzian-Piotrowska M, Bartlomiejczyk I, Przybylski J, Gorski J. The influence of physical exercise on the generation of TGF-b1, PDGF-AA, and VEGF-A in adipose tissue. Eur J Appl Physiol 2011; 111: 875-881.
- Czarkowska-Paczek B, Zendzian-Piotrowska M, Bartlomiejczyk I, Przybylski J, Gorski J. Skeletal and heart muscle expression of PDGF-AA and VEGF-A after an acute bout of exercise and endurance training in rats. Med Sci Monit 2010; 16: BR147-BR153.
- Czarkowska-Paczek B, Zendzian-Piotrowska M, Bartlomiejczyk I, Przybylski J, Gorski J. The effect of acute and prolonged endurance exercise on transforming growth factor-beta1 generation in rat skeletal and heart muscle. J Physiol Pharmacol 2009; 60: 157-162.
- Armstrong RB, Laughlin MH. Metabolic indicators of fibre recruitment in mammalian muscle during locomotion. J Exp Biol 1985; 115: 201-213.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–delta delta C(T)) method. Methods 2001; 25: 402-408.
- Fain JN, Company JM, Booth FW, et al. Exercise training does not increase muscle FNDC5 protein and mRNA expression in pigs. Metabolism 2013; 62: 1503-1511.
- Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentration in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012; 61: 1725-1738.
- Lambert MI, Noakes TD. Dissociation of changes in Vo2max , muscle QO2, and performance with training in rats. J Appl Physiol 1989; 66: 1620-1625.
- Majerczak J, Nieckarz Z, Karasinski J, Zoladz JA. Myosin heavy chain composition in the vastus lateralis muscle in relation to oxygen uptake and heart rate during cycling in humans. J Physiol Pharmacol 2014; 65: 217-227.
- Pradet-Balade B, Boulme F, Beug H, Mullner EW, Garcia-Sanz JA. Translation control: bridging the gap between genomics and proteomics. Trends Biochem Sci 2001; 26: 225-229.
A c c e p t e d : May 16, 2014