GASTRIC MUCOSAL PROTECTION: FROM THE PERIPHERY TO THE CENTRAL NERVOUS SYSTEM
INTRODUCTION
Gastric mucosal integrity can be influenced both by peripheral and central mechanisms. In the last decades the peripheral mechanisms of gastric mucosal defense have been intensively studied, and many details have been clarified. Some of these results have served as a basis for the development of new strategies, therapeutic targets in the treatment of gastric mucosal lesions.
On the other hand, in the last 20 years, central regulation of gastric mucosal protection has been intensively studied as well, and convincing evidence was obtained on the role of central nervous system (CNS) in the regulation of gastric mucosal integrity. However, several mechanisms of the centrally induced gastric mucosal protection have not been clarified yet, and further studies are needed to determine the role of CNS under physiological and pathophysiological conditions in gastric mucosal homeostasis as well as to reveal how the central regulatory mechanisms can be utilized in human therapy.
GASTRIC MUCOSAL PROTECTION
Peripheral mechanisms of gastric mucosal protection: mediators, receptors
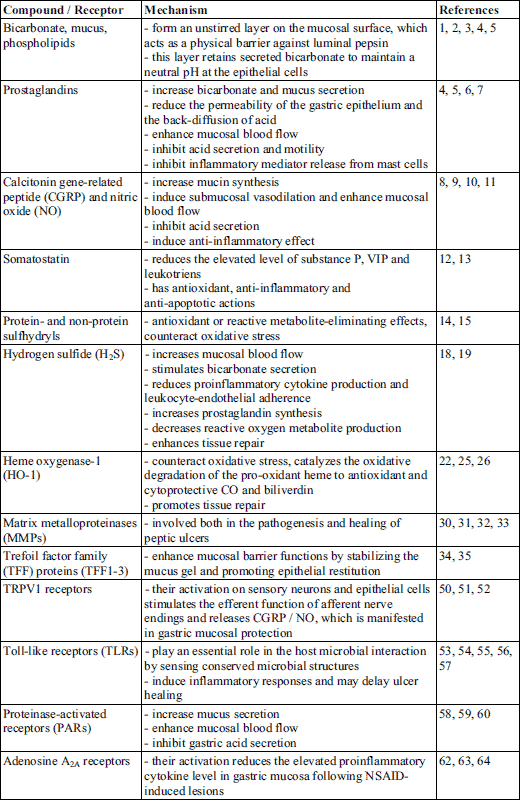
Gastric mucosal barrier to acid consists of several layers: the pre-epithelial mucus bicarbonate layer, an epithelial layer, and a post-epithelial layer which involves blood vessels, non-epithelial cells and enteric nerves. The latter two have basic role in generation of several substances which play a role in gastric mucosal integrity and gastric mucosal defense, e.g. bicarbonate, mucus, phospholipids, trefoil peptides and prostaglandins (PGs) (1-5). Prostaglandins of the E and I series are potent vasodilators, producing this effect in the stomach through EP2/EP4 and IP receptors (6, 7). Moreover, they reduce the permeability of the gastric epithelium (directly, or via enhancement of the effectiveness of surface-active phospholipids) (1, 6), thereby reducing acid back-diffusion. In addition, primary afferent sensory neurons innervate gastric mucosal and submucosal vessels, form a dense plexus at the mucosal base and regulate mucosal blood flow. Their stimulation results in the release of calcitonin gene-related peptide (CGRP) and substance (SP) CGRP partly directly, partly indirectly through the release of nitric oxide (NO) induces submucosal vasodilation (8-10). Namely, NO has a basic role in gastric mucosal defense by increasing gastric mucosal blood flow and microcirculation (8-10).
Somatostatin is also likely to be involved in gastric mucosal defense (11), it reduces the elevated level of SP, vasoactive intestinal polypeptide (VIP) and leukotriens in ethanol-induced gastric lesions (12), and also reduces stress-induced mucosal injury by inducing antioxidant, anti-inflammatory and anti-apoptotic actions (13). Our recent finding showed that its mucosal level dramatically decreased following intragastric administration of absolute ethanol parallel with the development of the gastric mucosal lesions in the rat, while gastroprotective agents, such as endomorphin reversed the reduced level of somatostatin (3).
Furthermore, protein- and non-protein sulfhydryls (such as reduced glutathione, GSH) were also shown as endogenous protective compounds (14), and it was suggested that the maintenance of a critical level of non-protein sulfhydryls in the gastric mucosa besides nitric oxide is necessary for the gastroprotective action (14, 15).
Recently the potential role of hydrogen sulfide in gastric mucosal defense was raised: H2S, similarly to NO, is an important mediator of gastric mucosal protection (16) and inhibition of endogenous H2S synthesis increases the susceptibility of the mucosa to damage induced by non-steroidal anti-inflammatory drugs (17). On the other hand, exogenous H2S donors can increase the resistance of the mucosa to injury (18). Several mechanisms are involved in the gastroprotective effect of H2S, such as maintenance and/or elevation of gastric mucosal blood flow, stimulation of bicarbonate secretion, reduced proinflammatory cytokine expression/release, increased prostaglandin synthesis, reduced leukocyte-endothelial adherence, decreased reactive oxygen metabolite production and enhanced tissue repair (18, 19).
In addition, several other factors, e.g. antioxidant enzymes, heme oxygenase-1, matrix metalloproteinases and trefoil factor family (TFF) proteins take part in the complex mucosal protective system (20).
Antioxidant enzymes, such as the above mentioned GSH, or superoxide dismutase (SOD) and catalase are able to counteract oxidative stress caused by excessive production and/or decreased elimination of reactive oxygen species (ROS). A decrease of SOD activity and GSH concentration significantly contributes to cell damage (21). ROS can induce tissue damage by promoting lipid peroxidation and increasing the production of inflammatory mediators and proinflammatory cytokines (22-24). Antioxidant enzymes are able to neutralize ROS, for instance SOD converts superoxide radical anion (O2●–) into hydrogen peroxide (H2O2), which is thereafter converted to water and oxygen by catalase.
Heme oxygenase-1 (HO-1 or Hsp32), the inducible form of heme oxygenase, also exerts cytoprotective effect. The expression of this enzyme may be induced by oxidative stress, inflammatory cytokines or heavy metals (24). HO-1 catalyzes the oxidative degradation of the pro-oxidant heme to antioxidant and cytoprotective carbon monoxide and biliverdin (which is then converted to bilirubin by biliverdin reductase) (22, 25). The activity of HO-1 is also increased during the healing of gastric ulcers, which indicates its involvement in the mucosal repair processes (26).
Matrix metalloproteinases (MMPs) play a role both in the pathogenesis and in healing of peptic ulcers. MMPs are zinc-dependent endopeptidases that degrade extracellular matrix proteins and are essential for extracellular matrix remodeling and wound healing (27). They are synthesized and secreted by various gastric cells (fibroblasts, epithelial and inflammatory cells) (28, 29), and several animal studies have demonstrated that NSAIDs or ethanol increased the activity of MMP-1, MMP-3, MMP-9 and MMP-13, while decreased the expression of MMP-2 (30-32). Also a recent human study showed that the expression of MMP-9 correlates with the severity and recurrence of gastric ulcers (33).
Trefoil factor family (TFF) proteins (TFF1-3) are also able to enhance mucosal barrier functions by stabilizing the mucus gel and promoting epithelial restitution (34, 35). Although the protective role of these small protease-resistant proteins has been demonstrated in various ulcer models (36, 37), the exact molecular mechanism is still not clear. Recent reports indicate that activation of the C-X-C chemokine receptor type 4 (CXCR4) and the apical Na+/H+ exchanger-2 (NHE2) is required for TFF-induced mucosal repair (38, 39).
Activation of immune cells can also affect gastric mucosal integrity. Mast cells and macrophages resident within the lamina propria act as “alarm cells.” Sensing the presence of foreign substances, these cells are capable of liberating an array of inflammatory mediators and cytokines that can alter mucosal blood flow and enhance the recruitment of granulocytes into the affected region. For example mast cells can be activated by several factors (ischemia, bacteria, antigens, bile acids, etc.). As a result, they release histamine and platelet-activating factor, which can increase the epithelial and vascular endothelial permeability. In addition, stimulation of the expression of adhesion molecules and release of tumor necrosis factor α (TNF-α) from mast cells can also be observed. TNF-α further stimulates leukocyte-endothelial adhesive interactions. In the contrary, prostaglandins and nitric oxide can suppress the reactivity of mast cells, consequently, can counteract many of these effects (40 - 42).
Under chronic inflammatory conditions (e.g. Helicobacter pylori infection) inflammatory cells in lamina propria do not produce only proinflammatory cytokines, but also anti-inflammatory cytokines, such as interleukin 10 (IL-10), which thereafter suppresses the production of various proinflammatory molecules, e.g. IL-1, IL-2, IL-8, TNF-α and IFN-γ (43). Consequently, IL-10 has a counter-regulatory effect in mucosal inflammatory processes, which may reduce tissue damage caused by inflammation, but may also hamper the elimination of harmful stimulus by suppression of the immune response (43).
Several peripheral receptors have been described to be involved in gastric mucosal defense/injury.
A specific ionotropic receptor is the transient receptor potential vanilloid-1 (TRPV1) (44). In the gastrointestinal tract, TRPV1 can be identified in intrinsic enteric neurons, extrinsic sensory neurons, epithelial and endocrine cells (45, 46). TRPV1 receptor is activated by capsaicin (47-49). Capsaicin given orally was found to inhibit gastric mucosal lesions in different experimental ulcer models (50, 51) and in humans (52) by stimulating the nerve endings and efferent function of primary afferents, resulting in the release of CGRP.
Toll-like receptors (TLRs) play an essential role in the host microbial interaction by sensing conserved microbial structures (pathogen-associated molecular patterns, PAMPs). In humans 10 family members (TLR1-10) have been identified thus far, which recognize different bacterial or viral components, like peptidoglycan (TLR2), lipopolysaccharide (TLR4) or flagellin (TLR5), but some of them (e.g. TLR2 and TLR4) are also capable of responding to different endogenous molecules, released during inflammation or tissue damages (53, 54). Gastric epithelial cells express various TLRs (TLR2, 4, 5 and TLR9), whose activation (e.g. by H. pylori) induces inflammatory responses and may delay ulcer healing (55-57). Therefore, antagonists of TLRs may serve as novel therapeutic approaches for gastrointestinal ulcers.
Proteinase-activated receptors (PARs), particularly PAR1 and PAR2, are also important regulators of GI functions. These unique G-protein-coupled receptors are distributed throughout the GI tract, and their activation has been reported to increase gastric mucus secretion and mucosal blood flow, to reduce gastric acid secretion and to induce cytoprotection (58-60). Interestingly, capsaicin-sensitive sensory neurons are involved in PAR2-, but not in PAR1-induced gastroprotection - the latter one seems to be mainly mediated by PGs (59, 60).
Adenosine has a basic role in signaling processes and induces numerous physiological responses in all mammalian tissues. Four adenosine receptors have been identified, namely A1, A2A, A2B and A3. Activation of A2A receptors elicits anti-inflammatory effects (61) and the selective α2A receptor agonist ATL-146e has been shown to reduce gastric mucosal lesions induced by water-immersion stress, aspirin- and indomethacin (62-64).
Are peripheral opioid receptors and α2-adrenoceptors involved in gastric mucosal protection?
The presence of µ- and δ-opioid receptors were demonstrated in gastric fundus, antrum and corpus, primarily located in the submucosal plexus, deep muscular plexus, and mucosa (65). We wondered if activation of these receptors can affect gastric mucosal defense. It was found that δ-opioid receptor selective peptides such as [D-Ala2,D-Leu5]-enkephalin (DADLE), [D-Pen2,D-Pen5]-enkephalin (DPDPE) and deltorphin II injected subcutaneously exerted a dose-dependent inhibition on the development of mucosal lesions induced by acidified ethanol, their ID50 values were 0.037 (0.02 – 0.057), 1.8 (1.3 – 2.52) and 3.5 (2.12 – 5.7) µmol/kg, respectively. Since opioid peptides cannot (or poorly) pass the blood-brain barrier, their mucosal protective effect is likely to be due to activation of peripheral opioid receptors. Because naltrindole, the selective δ-opioid receptor antagonist inhibited the gastroprotective effect of all above mentioned peptides, it was concluded that activation of δ-opioid receptors may mediate gastric mucosal protection (66). The mechanism of gastroprotective effect may be at least partly mediated by endogenous nitric oxide, as it was suggested also by previous findings (67).
Moreover, based on the well-known interaction between opioid receptors and α2-adrenoceptors, the question was raised whether activation of α2-adrenergic receptors can elicit gastric mucosal protection as well. We found that α2-adrenoceptor stimulants, clonidine and rilmenidine injected either orally or subcutaneously (s.c.) exerted gastroprotective effect against ethanol-induced gastric lesions in a dose dependent manner, their ED50 values were 32 (12 – 84) and 25 (10 – 62.5) nmol/kg for clonidine; 25 (10 – 62.5) and 3.1 (0.5 – 20) nmol/kg for rilmenidine, following oral or s.c. administration, respectively (68). Pharmacological analysis with selective antagonists of the α2A/D and α2B/C-adrenoceptor subtypes suggested that the α2B/2C-adrenoceptor subtypes are likely to mediate this mucosal protective effect, while the α2A-one has no important role in it. We wondered if the distribution of α2-adrenoceptor subtypes in gastric mucosa confirms the concept on the peculiar role of α2B/2C-adrenoceptor subtypes in gastric mucosal protection. However, though expression of all the three subtypes could be detected in gastric mucosa of the rat, the dominant subtype was the α2A-one (68). Consequently, the findings on distribution of the adrenoceptor subtypes in gastric mucosa does not support the conclusion of pharmacological analysis, that the α2B/2C-adrenoceptor subtypes have a prominent role in mucosal defense (68). Moreover, ST 91 (2-[2,6-diethylphenylamino]-2-imidazoline), an α2B/2C-adrenoceptor subtype preferring, peripherally acting adrenoceptor stimulant exerted only a slight, non-significant inhibition of gastric mucosal lesions in the rat (68). In addition, the gastroprotective effect of rilmenidine given s.c. was antagonised by the intracerebroventricularly (i.c.v.) injected α2-adrenoceptor antagonist yohimbine (68). These findings suggest that the site of gastroprotective action of α2-adrenoceptor stimulants is not likely to be in the periphery, but rather in the CNS.
Centrally induced gastroprotection
1. Central opioid receptors and α2-adrenoceptors in gastric mucosal protection
The above results prompted us to analyze the role of central α2-adrenoceptors in gastroprotection. It was found that both clonidine and rilmenidine exerted gastroprotective effect following i.c.v. administration, their ED50 values are 200 (90 – 400) and 10 (1 – 10) pmol, respectively. In addition, ST 91, the above mentioned α2B/2C-adrenoceptor subtype preferring agonist, which passes poorly the blood-brain barrier and failed to significantly affect the gastric mucosal lesions following peripheral administration, proved to be effective following i.c.v. administration. The centrally initiated gastroprotective effect of clonidine (470 pmol), rilmenidine (45 pmol) and ST-91 (33 nmol) was antagonized by the non-selective α2-adrenoceptor antagonist yohimbine, as well as by the α2B/2C adrenoceptor preferring antagonists prazosin and ARC 239, indicating that α2B/2C-like adrenoceptor subtypes may mediate the action (68). The same conclusion could be drawn from the results of our subsequent study carried out in genetically engineered mice (69). In addition, naloxone also reversed the mucosal protective effect of clonidine, rilmenidine and ST-91 suggesting an opioid component in their action (68, 70). Therefore, we examined the effect of opioid peptides injected i.c.v. and intracisternally (i.c.) on ethanol-induced experimental ulcer formation. The results showed that DADLE, DPDPE and deltorphin II (selective d-opioid receptor agonists), DAGO ([D-Ala2,Phe4,Gly5-ol]-enkephalin, a selective µ-opioid receptor agonist) and β-endorphin (ligand of both receptor types) produced a dose-dependent inhibition of acidified ethanol-induced gastric mucosal damage. The ED50 values for β-endorphin, DAGO, DADLE, deltorphin II, and DPDPE were 3.5 (1.6 – 7.35), 6.8 (2.26 – 20.4), 75 (36 – 144), 120 (40 – 360), and 1100 (458 – 26409) pmol/rat, respectively, following i.c.v. administration, and 0.8 (0.62 – 1.024), 9.0 (2.4 – 33), 45 (16 – 126), 0.25 (0.08 – 0.775) and 7 (1.66 – 29.4) pmol/rat following i.c. injection (71).
The above results confirmed the pivotal role of CNS in regulation of gastric mucosal integrity. In the last two decades increasing number of evidence suggest that central administration of different neuropeptides, neurotransmitters and neuromodulators (either i.c.v, i.c., or directly into specific brain nuclei, e.g. the dorsal motor nucleus of vagus /DMNV) results in gastric mucosal protection (3, 10, 72, 73).
Different brain areas have been suggested to be involved in the centrally induced gastroprotection. Among them, the hypothalamus and particularly the dorsal vagal complex (DVC) (including DMNV, nucleus of the solitary tract /NTS/ and area postrema) seem to have a prominent role. Vagal dependent mechanism of gastroprotection was demonstrated e.g. for thyreotropine-releasing hormone (TRH), adrenomedullin, peptide YY (74-77), clonidine (78), opioid peptides (71), nociceptin, nocistatin (79), and angiotensin II (Ang II) (80).
2. Potential role of excitatory amino acids and nitric oxide in centrally-induced gastroprotection
Signals from sensory receptors in the gastrointestinal tract via primary afferents terminate in the NTS where they are integrated and transmitted to parasympathetic preganglionic neurons of the DMNV. Principally glutamate, GABA and norepinephrine are involved in synaptic connections between NTS and DMNV neurons and convey sensory signals to vagal efferent impulses (81). While less is known about catecholaminergic transmission between the NTS and DMNV, many studies have demonstrated that electrical stimulation of various NTS subnuclei elicits glutamatergic excitatory and GABAergic inhibitory currents in DMNV neurons (82-85).
The vagal afferent-vagal efferent reflex (vagovagal reflex) plays a crucial role in upper gastrointestinal reflexes, and in regulation of gastric motor activity (81). Activation of both non-NMDA (kainate) and NMDA receptors in the DMNV in vivo increases gastric contractility, and this effect was blocked by the appropriate antagonists (86).
Activation of vagal efferent cholinergic nerves may affect gastric mucosal integrity e.g. through stimulation of gastric motor activity (87, 88). On the other hand, cholinergic activation was shown to stimulate the release of gastric mucosal PGs and NO, as well as the effector function of capsaicin-sensitive afferent fibers containing CGRP resulting in enhanced mucosal microcirculation, and consequently gastric mucosal protection (51, 89-91).
Based on these data the question was raised if glutamatergic/GABAergic system may be involved in centrally initiated gastroprotection. As described above, central δ-opioid receptors may mediate gastric mucosal protection (71). The site of action is most probable in the brainstem, since δ-opioid receptor agonists proved to be more effective and potent given i.c. than i.c.v. injection. Since δ-opioid receptors were identified in the NTS, but not in the DMNV (92), (while µ-opioid receptor was shown in both DMNV and NTS (93)), NTS was supposed to be the site of action of δ-opioid receptor stimulants. We wondered if glutamatergic pathway would play a role in conveying the opioid-receptor induced action to the DMNV. As Figs. 1 and 2 show, AP-7 (DL-2-amino-7-phosphonoheptanoic acid), a competitive antagonist of the NMDA receptors blocked the gastroprotective effect of the δ-opioid receptor selective ligand deltorphin II and that of β-endorphin given both i.c.v. and i.c. These findings suggest that glutamate through NMDA receptors might play a role in the δ-opioid receptor-induced gastroprotective action. If NMDA receptors are involved indeed in mediation of the effect of opioid peptides, NMDA itself should induce mucosal protective effect as well. Accordingly, as Fig. 3 shows, NMDA injected i.c.v. exerted mucosal protective action in the doses of 5 and 10 pmol.
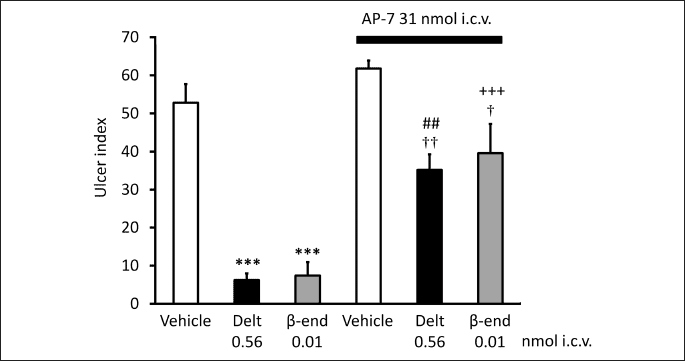
Each column represents mean ± S.E.M., n = 5; ***P < 0.001 compared with vehicle-treated group (column 1); ##P < 0.01 compared with deltorphin II-treated group (column 2); +++P < 0.001 compared with β-endorphin-treated group (column 3); †P < 0.05, ††P < 0.01 compared with AP-7 + vehicle-treated group (column 4) (ANOVA, Newman-Keuls post hoc test).
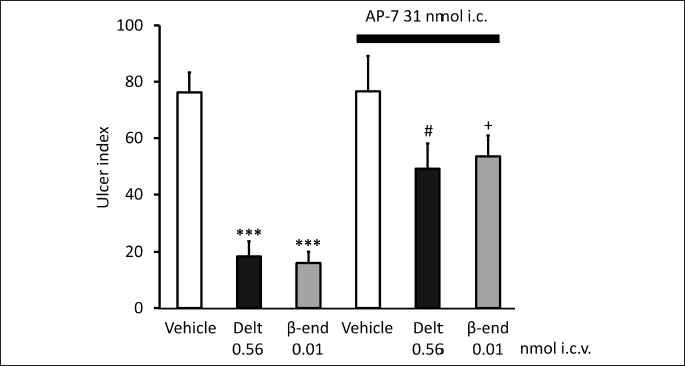
Each column represents mean ± S.E.M., n = 5; ***P < 0.001 compared with vehicle-treated group (column 1); #P < 0.05 compared with deltorphin II-treated group (column 2); +P < 0.05 compared with β-endorphin-treated group (column 3) (ANOVA, Newman-Keuls post hoc test).
In accordance with this finding, L-glutamate injected into the lateral hypothalamus was shown to increase defensive mechanisms (e.g. mucosal blood flow) (94). Moreover, the injection of kainate (a specific agonist for the kainate receptor, that mimics the effect of glutamate) into the raphe pallidus exerted gastroprotective action as well (95).
Furthermore, co-localization of neuronal nitric oxide synthase and NMDA receptor subunit 1 in NTS was described, which provides anatomical support for the hypothesis that NMDA receptor activation can affect NTS-controlled functions via actions on neurons that synthesize nitric oxide (NO) (96). The NO production after NMDA receptor activation in the NTS was markedly reduced by prior i.c. injection of L-NAME (NG-nitro-L-arginine methyl ester), an NO synthase inhibitor, suggesting that the increase in NO level after NMDA receptor activation is caused by activation of NO synthase in the NTS (97). Further studies confirmed that NO has prominent role in NMDA-mediated actions (97, 98).
Based on these data we wondered if NO may also be involved in the centrally-induced effect of opioid peptides. As Figs. 3 and 4 demonstrate, the NO synthase inhibitor NG-nitro-L-arginine (LNNA, 670 nmol i.c.v.) blocked the gastroprotective effect of NMDA, as well as that of deltorphin II and β-endorphin, and the effect was reversed by L-arginine, but not by D-arginine (not shown) indicating that NO is likely to mediate the gastroprotective effect of both NMDA and opioid peptides.
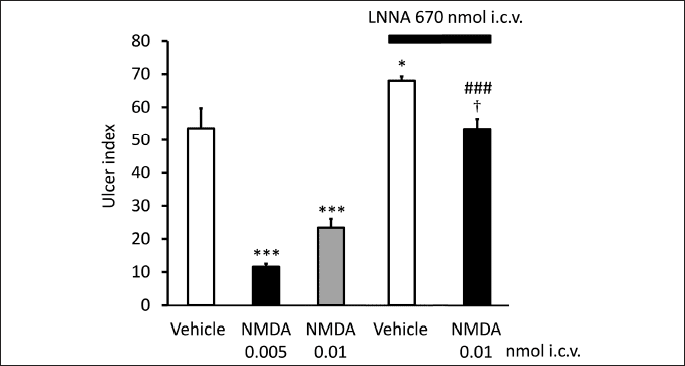
Each column represents mean ± S.E.M., n = 5; *P < 0.05, ***P < 0.001 compared with vehicle-treated group (column 1); ###P < 0.001 compared with NMDA (0.01 nmol)-treated group (column 3); †P < 0.05 compared with LNNA + vehicle-treated group (column 4) (ANOVA, Newman-Keuls post hoc test).
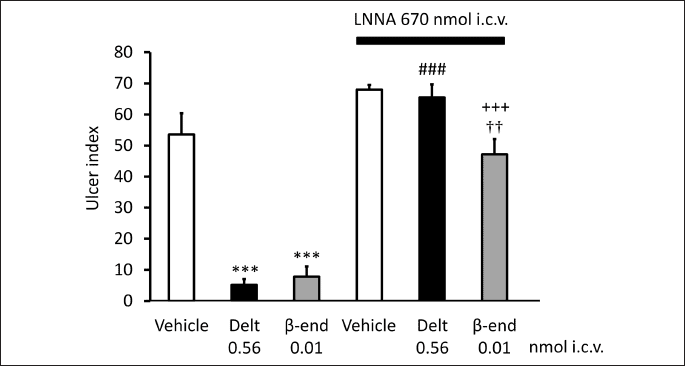
Each column represents mean ± S.E.M., n = 5; ***P < 0.001 compared with vehicle-treated group (column 1); ###P < 0.001 compared with deltorphin II-treated group (column 2); +++P < 0.001 compared with β-endorphin-treated group (column 3); ††P < 0.01 compared with LNNA + vehicle-treated group (column 4) (ANOVA, Newman-Keuls post hoc test).
On the basis of these findings it might be speculated that opioid peptide-induced gastroprotecive effect is mediated, at least partly by an NMDA-NO pathway. To answer the question, whether this chain of events - according to our original hypothesis - occurs within the DVC, and activation of δ- (µ)-opioid receptor results in activation of DMNV through NMDA-NO pathway, further studies are needed.
3. Gastroprotective effect of endogenous substances: neuropeptides and non-neuropeptides
The role of CNS in mucosal injury/protection has been raised already in the 19th century. However, systematic analysis of the mechanism of centrally initiated gastric mucosal protection started only about 20 years ago.
As depicted above, DVC has a prominent role in the regulation of gastrointestinal functions. Different receptor populations have been identified in the DVC, such as µ- and δ-opioid receptors (92, 93), α2-adrenoceptors (99, 100), cannabinoid CB1- and CB2 receptors (101-104), angiotensin II AT1 receptor (105, 106), nociceptin NOP receptor (107) and tachykinin NK1, NK2 and NK3-receptors (108-111) (Table 2).
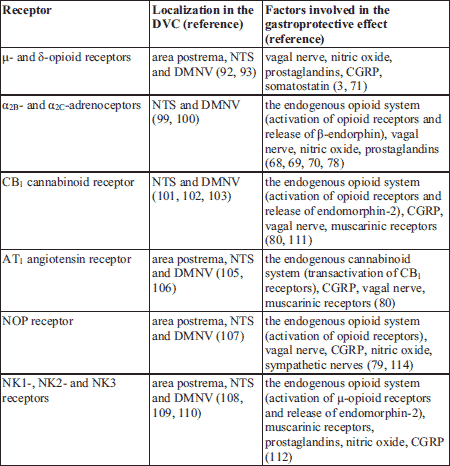
Our research in the last decade focused on the mechanism of centrally initiated gastric mucosal protection. As a first step we examined which of the receptors localized in the DVC may have a role in gastric mucosal defense/injury. Our results showed that activation of opioid-, (10, 71) α2-, (68-70, 78) nociceptin- (79) cannabinoid CB1 (111), neurokinin NK1-, NK2-, NK3-receptors (112) and angiotensin II AT1-receptors (80) initiated a chain of events resulting in stimulation of mucosal protective processes. Further studies are needed to clarify whether gastroprotection can be induced also by elevation of the endogenous level of neuropeptides, e.g. via increase of the endogenous opioid levels by opiorphin or its synthetic analogue, inhibitors of enkephalin-inactivating peptidases (113).
The mechanism of centrally initiated mucosal protection is under an intensive analysis. Our results suggest a vagal dependent mechanism of centrally induced gastroprotective action for α2 stimulants (78), opioid peptides (71), angiotensin II (80) as well as nociceptin and nocistatin (79), in accordance with the results of Polidori et al. (114).
However, sympathetic nervous system may also be involved in the gastroprotective effect of opioid peptides and α2-adrenoceptor agonists, since their effect markedly decreased following i.c.v. administration of the catecholaminergic neurotoxine, 6-hydroxydopamine, that reduced the noradrenaline concentration in a significant manner in the NTS (73). In the periphery, the decreased gastric mucosal level of CGRP and partly somatostatin due to ethanol administration was restored by i.c.v. administration of endomorphins (3), substance P (112) and cannabinoids (80).
In our further experiments we aim to analyse the interactions between different neuropeptides and other neurotransmitters/neuromodulators in gastroprotection. Some data of the literature suggest interaction of neuropeptides in gastric mucosal defense. For example TRH-enkephalin interaction was observed in the amygdaloid complex during gastric stress ulcer formation in rats (115), or stress induced the release of CRF, that stimulated the release of β-endorphin and somatostatin, and reduced that of TRH (116, 117). Our results suggest that endogenous opioids seem to be involved in the gastroprotective process of α2-adrenoceptor agonists (68, 78), nociceptin, nocistatin (79) and cannabinoids (111). The production of the endocannabinoid 2-arachydonoylglycerol (2-AG) and transactivation of the CB1 receptors may also contribute to the gastric mucosal protective mechanism of Ang II (80).
The role of CNS in gastric mucosal homeostasis has been well documented in the last 15 – 20 years. Analysis of the central regulation of gastric functions, identification of endogenous substances and their receptors that may influence the central and peripheral mechanisms of gastric mucosal defense, clarification of the interaction of neuropeptides (brain-gut peptides) with each other and with other endogenous substances, all may serve as a basis for better understanding of the complex mechanism of the maintenance of gastric mucosal integrity as well for the development of new strategies to enhance gastric mucosal resistance against injury.
Conflict of interests: None declared.
REFERENCE
- Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev 2008; 88: 1547-1565.
- Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 2008; 135: 41-60.
- Gyires K, Nemeth J, Zadori ZS. Gastric mucosal protection and central nervous system. Curr Pharm Des 2013; 19: 34-39.
- deFoneska A, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol 2010; 26: 604-610.
- Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 2005; 288: 1-19.
- Takeuchi K. Gastric cytoprotection by prostaglandin E2 and prostacyclin: relationship to EP1 and IP receptors. J Physiol Pharmacol 2014; 65: 3-14.
- Kotani T, Kobata A, Nakamura E, Amagase K, Takeuchi K. Roles of cyclooxygenase-2 and prostacyclin/IP receptors in mucosal defense against ischemia/reperfusion injury in mouse stomach. J Pharmacol Exp Ther 2006; 316: 547-555.
- Li DS, Raybould HE, Quintero E, Guth PH. Calcitonin gene-related peptide mediates the gastric hyperemic response to acid back-diffusion. Gastroenterology 1992; 102: 1124-1128.
- Holzer P. Neural emergency system in the stomach. Gastroenterology 1998; 114: 823-839.
- Gyires K. Neuropeptides and gastric mucosal homeostasis. Curr Top Med Chem 2004; 4: 63-73.
- Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol 2007; 7: 563-569.
- Karmeli F, Eliakim R, Okon E, Rachmilewitz D. Somatostatin effectively prevents ethanol- and NSAID-induced gastric mucosal damage in rats. Dig Dis Sci 1994; 39: 617-625.
- Nassar NN, Schaalan MF, Zaki HF, Abdallah DM. Octreotide ameliorates gastric lesions in chronically mild stressed rats. World J Gastroenterol 2011; 17: 1135-1142.
- Szabo S, Nagy L, Plebani M. Glutathione, protein sulfhydryls and cysteine proteases in gastric mucosal injury and protection. Clin Chim Acta 1992; 206: 95-105.
- Ali AT. The role of nitric oxide and sulphydryls in gastric mucosal protection induced by sodium cromoglycate in rats. J Pharm Pharmacol 1995; 47: 739-743.
- Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal 2010; 12: 1125-1133.
- Fiorucci S, Antonelli E, Distrutti E, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 2005; 129: 1210-1224.
- Wallace JL, Caliendo G, Santagada V, Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br J Pharmacol 2010; 159: 1236-1246.
- Wallace JL. Hydrogen sulfide: a rescue molecule for mucosal defence and repair. Dig Dis Sci 2012; 57: 1432-1434.
- Al Jiboury H, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol 2012; 28: 594-601.
- Kwiecien S, Jasnos K, Magierowski M, et al. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress - induced gastric injury. J Physiol Pharmacol 2014; 65: 613-622.
- Bindu S, Mazumder S, Dey S, et al. Nonsteroidal anti-inflammatory drug induces proinflammatory damage in gastric mucosa through NF-kappaB activation and neutrophil infiltration: anti-inflammatory role of heme oxygenase-1 against nonsteroidal anti-inflammatory drug. Free Radic Biol Med 2013; 65: 456-467.
- Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut 1992; 33: 1467-1472.
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 1997; 37: 517-554.
- Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta 1994; 1223: 9-14.
- Guo JS, Cho CH, Wang WP, Shen XZ, Cheng CL, Koo MW. Expression and activities of three inducible enzymes in the healing of gastric ulcers in rats. World J Gastroenterol 2003; 9: 1767-1771.
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827-839.
- Shahin M, Konturek JW, Pohle T, Schuppan D, Herbst H, Domschke W. Remodeling of extracellular matrix in gastric ulceration. Microsc Res Tech 2001; 53: 396-408.
- Park SH, Hong H, Han YM, et al. Nonsteroidal anti-inflammatory drugs (NSAID) sparing effects of glucosamine hydrochloride through N-glycosylation inhibition; strategy to rescue stomach from NSAID damage. J Physiol Pharmacol 2013; 64: 157-165.
- Pradeepkumar Singh L, Vivek Sharma A, Swarnakar S. Upregulation of collagenase-1 and -3 in indomethacin-induced gastric ulcer in diabetic rats: role of melatonin. J Pineal Res 2011; 51: 61-74.
- Kim SJ, Park YS, Paik HD, Chang HI. Effect of anthocyanins on expression of matrix metalloproteinase-2 in naproxen-induced gastric ulcers. Br J Nutr 2011; 106: 1792-1801.
- Ganguly K, Swarnakar S. Induction of matrix metalloproteinase-9 and -3 in nonsteroidal anti-inflammatory drug-induced acute gastric ulcers in mice: regulation by melatonin. J Pineal Res 2009; 47: 43-55.
- Li SL, Zhao JR, Ren XY, Xie JP, Ma QZ, Rong QH. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J Gastroenterol 2013; 19: 4590-4595.
- Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 2005; 62: 2932-2938.
- Hoffmann W. Trefoil factor family (TFF) peptides: regulators of mucosal regeneration and repair, and more. Peptides 2004; 25: 727-730.
- Konturek PC, Brzozowski T, Konturek SJ, et al. Role of spasmolytic polypeptide in healing of stress-induced gastric lesions in rats. Regul Pept 1997; 68: 71-79.
- Farrell JJ, Taupin D, Koh TJ, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest 2002; 109: 193-204.
- Xue L, Aihara E, Wang TC, Montrose MH. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem 2011; 286: 38375-38382.
- Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem 2009; 284: 3650-3662.
- Kubes P, Kanwar S, Niu XF, Gaboury JP. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J 1993; 7: 1293-1299.
- Kanwar S, Wallace JL, Befus D, Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol 1994; 266: 222-229.
- Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J 1996; 10: 731-740.
- Bodger K, Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut 1997; 40: 739-744.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389: 816-824.
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol 2003; 465: 121-135.
- Zhao H, Simasko SM. Role of transient receptor potential channels in cholecystokinin-induced activation of cultured vagal afferent neurons. Endocrinology 2010; 151: 5237-5246.
- Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004; 38: 377-384.
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 1999; 51: 159-212.
- Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther 2011; 131: 142-170.
- Szolcsanyi J, Bartho L. Capsaicin-sensitive afferents and their role in gastroprotection: an update. J Physiol (Paris) 2001; 95: 181-188.
- Holzer P, Lippe IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience 1988; 27: 981-987.
- Mozsik G, Szolcsanyi J, Domotor A. Capsaicin research as a new tool to approach of the human gastrointestinal physiology, pathology and pharmacology. Inflammopharmacology 2007; 15: 232-245.
- Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 2007; 19: 3-10.
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 2011; 30: 16-34.
- Smith MF, Jr., Mitchell A, Li G, et al. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem 2003; 278: 32552-32560.
- Nadatani Y, Watanabe T, Tanigawa T, et al. High-mobility group Box 1 inhibits gastric ulcer healing through Toll-like receptor 4 and receptor for advanced glycation end products. PLoS One 2013; 8: e80130.
- Lagunes-Servin H, Torres J, Maldonado-Bernal C, et al. Toll-like receptors and cytokines are upregulated during Helicobacter pylori infection in children. Helicobacter 2013; 18: 423-432.
- Nishikawa H, Kawai K, Nishimura S, et al. Suppression by protease-activated receptor-2 activation of gastric acid secretion in rats. Eur J Pharmacol 2002; 447: 87-90.
- Kawabata A, Nishikawa H, Saitoh H, et al. A protective role of protease-activated receptor 1 in rat gastric mucosa. Gastroenterology 2004; 126: 208-219.
- Kawabata A, Kinoshita M, Nishikawa H, et al. The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J Clin Invest 2001; 107: 1443-1450.
- Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol 1994; 76: 5-13.
- Odashima M, Otaka M, Jin M, et al. Selective adenosine A receptor agonist, ATL-146e, attenuates stress-induced gastric lesions in rats. J Gastroenterol Hepatol 2005; 20: 275-280.
- Odashima M, Otaka M, Jin M, et al. Attenuation of gastric mucosal inflammation induced by aspirin through activation of α2A adenosine receptor in rats. World J Gastroenterol 2006; 12: 568-573.
- Koizumi S, Odashima M, Otaka M, et al. Attenuation of gastric mucosal inflammation induced by indomethacin through activation of the α2A adenosine receptor in rats. J Gastroenterol 2009; 44: 419-425.
- Nishimura E, Buchan AM, McIntosh CH. Autoradiographic localization of opioid receptors in the rat stomach. Neurosci Lett 1984; 50: 73-78.
- Gyires K, Ronai AZ, Toth G, Darula Z, Furst S. Analysis of the role of delta opioid receptors in gastroprotection in the rat. Life Sci 1997; 60: 1337-1347.
- Gyires K. The role of endogenous nitric oxide in the gastroprotective action of morphine. Eur J Pharmacol 1994; 255: 33-37.
- Gyires K, Zadori ZS, Shujaa N, Minorics R, Falkay G, Matyus P. Analysis of the role of central and peripheral alphα2-adrenoceptor subtypes in gastric mucosal defense in the rat. Neurochem Int 2007; 51: 289-296.
- Zadori ZS, Shujaa N, Brancati SB, Hein L, Gyires K. Both alphα2B- and alphα2C-adrenoceptor subtypes are involved in the mediation of centrally induced gastroprotection in mice. Eur J Pharmacol 2011; 669: 115-120.
- Fulop K, Zadori Z, Ronai AZ, Gyires K. Characterisation of alphα2-adrenoceptor subtypes involved in gastric emptying, gastric motility and gastric mucosal defence. Eur J Pharmacol 2005; 528: 150-157.
- Gyires K, Ronai AZ. Supraspinal delta- and mu-opioid receptors mediate gastric mucosal protection in the rat. J Pharmacol Exp Ther 2001; 297: 1010-1015.
- Tache Y, Adelson D, Yang H. TRH/TRH-R1 receptor signaling in the brain medulla as a pathway of vagally mediated gut responses during the cephalic phase. Curr Pharm Des 2014; 20: 2725-2730.
- Gyires K. Analysis of the Effect of Different Neuropeptides in Gastric Mucosal Defense Initiated Centrally. In: Cell/Tissue Injury and Cytoprotection/Organoprotection in the Gastrointestinal Tract: Mechanisms, Prevention and Treatment, Filaretova LP, Takeuchi K (eds). Basel, Karger, 2012, vol 30, pp. 161-169.
- Yang H, Kawakubo K, Tache Y. Intracisternal PYY increases gastric mucosal resistance: role of cholinergic, CGRP, and NO pathways. Am J Physiol 1999; 277: G555-G562.
- Tache Y, Yoneda M. Central action of TRH to induce vagally mediated gastric cytoprotection and ulcer formation in rats. J Clin Gastroenterol 1993; 17: 58-63.
- Tache Y. Brainstem neuropeptides and vagal protection of the gastric mucosal against injury: role of prostaglandins, nitric oxide and calcitonin-gene related peptide in capsaicin afferents. Curr Med Chem 2012; 19: 35-42.
- Kaneko H, Mitsuma T, Nagai H, et al. Central action of adrenomedullin to prevent ethanol-induced gastric injury through vagal pathways in rats. Am J Physiol 1998; 274: 1783-1788.
- Gyires K, Ronai AZ, Mullner K, Furst S. Intracerebroventricular injection of clonidine releases beta-endorphin to induce mucosal protection in the rat. Neuropharmacology 2000; 39: 961-968.
- Zadori ZS, Shujaa N, Koles L, Kiraly KP, Tekes K, Gyires K. Nocistatin and nociceptin given centrally induce opioid-mediated gastric mucosal protection. Peptides 2008; 29: 2257-2265.
- Gyires K, Ronai AZ, Zadori ZS, et al. Angiotensin II-induced activation of central AT receptors exerts endocannabinoid-mediated gastroprotective effect in rats. Mol Cell Endocrinol 2014; 382: 971-978.
- Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci 2006; 126-127: 2-8.
- Willis A, Mihalevich M, Neff RA, Mendelowitz D. Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol 1996; 271: 1614-1619.
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol 1991; 260: G531-G536.
- Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 2004; 1017: 208-217.
- Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol 2003; 549: 775-785.
- Sivarao DV, Krowicki ZK, Abrahams TP, Hornby PJ. Vagally-regulated gastric motor activity: evidence for kainate and NMDA receptor mediation. Eur J Pharmacol 1999; 368: 173-182.
- Takeuchi K, Nishiwaki H, Okabe S. Effects of dopamine on gastric mucosal lesions induced by ethanol in rats. Possible involvement of antigastric motor activity mediated with alpha 2-adrenoceptors. Dig Dis Sci 1988; 33: 1560-1568.
- Takeuchi K, Niida H, Matsumoto J, Ueshima K, Okabe S. Gastric motility changes in capsaicin-induced cytoprotection in the rat stomach. Jpn J Pharmacol 1991; 55: 147-155.
- Kiraly A, Suto G, Tache Y. Role of nitric oxide in the gastric cytoprotection induced by central vagal stimulation. Eur J Pharmacol 1993; 240: 299-301.
- Kiraly A, Suto G, Livingston EH, Guth PH, St Pierre S, Tache Y. Central vagal activation by TRH induces gastric hyperemia: role of CGRP in capsaicin-sensitive afferents in rats. Am J Physiol 1994; 267: G1041-G1049.
- Kato K, Matsuno Y, Matsuo Y, et al. Role of mucosal prostaglandins in vagally-mediated adaptive cytoprotection in the rat. Gastroenterol Jpn 1992; 27: 1-8.
- Mansour A, Fox CA, Burke S, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 1994; 350: 412-438.
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat 1995; 8: 283-305.
- Namiki T, Egawa M, Tominaga S, Inoue S, Takamura Y. Effects of GABA and L-glutamate on the gastric acid secretion and gastric defensive mechanisms in rat lateral hypothalamus. J Auton Nerv Syst 1993; 44: 217-223.
- Kaneko H, Kaunitz J, Tache Y. Vagal mechanisms underlying gastric protection induced by chemical activation of raphe pallidus in rats. Am J Physiol 1998; 275: 1056-1062.
- Lin LH, Talman WT. N-methyl-D-aspartate receptors on neurons that synthesize nitric oxide in rat nucleus tractus solitarii. Neuroscience 2000; 100: 581-588.
- Matsuo I, Hirooka Y, Hironaga K, et al. Glutamate release via NO production evoked by NMDA in the NTS enhances hypotension and bradycardia in vivo. Am J Physiol Regul Integr Comp Physiol 2001; 280: R1285-R1291.
- Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 2001; 24: 211-215.
- Tavares A, Handy DE, Bogdanova NN, Rosene DL, Gavras H. Localization of alpha 2A- and alpha 2B-adrenergic receptor subtypes in brain. Hypertension 1996; 27: 449-455.
- Rosin DL, Talley EM, Lee A, et al. Distribution of alpha 2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol 1996; 372: 135-165.
- Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005; 310: 329-332.
- Partosoedarso ER, Abrahams TP, Scullion RT, Moerschbaecher JM, Hornby PJ. Cannabinoid 1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol 2003; 550: 149-158.
- Castelli MP, Piras AP, Melis T, et al. Cannabinoid CB1 receptors in the paraventricular nucleus and central control of penile erection: immunocytochemistry, autoradiography and behavioral studies. Neuroscience 2007; 147: 197-206.
- Accorsi-Mendonca D, Almado CE, Dagostin AL, Machado BH, Leao RM. Inhibition of spontaneous neurotransmission in the nucleus of solitary tract of the rat by the cannabinoid agonist WIN 55212-2 is not via CB1 or CB2 receptors. Brain Res 2008; 1200: 1-9.
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience 1998; 82: 827-841.
- Diz DI, Barnes KL, Ferrario CM. Hypotensive actions of microinjections of angiotensin II into the dorsal motor nucleus of the vagus. J Hypertens Suppl 1984; 2: 53-56.
- Neal CR, Jr., Mansour A, Reinscheid R, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 1999; 412: 563-605.
- Polidori C, Massi M, Guerrini R, Grandi D, Lupo D, Morini G. Peripheral mechanisms involved in gastric mucosal protection by intracerebroventricular and intraperitoneal nociceptin in rats. Endocrinology 2005; 146: 3861-3867.
- Mazzone SB, Geraghty DP. Characterization and regulation of tachykinin receptors in the nucleus tractus solitarius. Clin Exp Pharmacol Physiol 2000; 27: 939-942.
- Lewis MW, Travagli RA. Effects of substance P on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 2001; 281: G164-G172.
- Dixon MK, Nathan NA, Hornby PJ. Immunocytochemical distribution of neurokinin 1 receptor in rat dorsal vagal complex. Peptides 1998; 19: 913-923.
- Shujaa N, Zadori ZS, Ronai AZ, et al. Analysis of the effect of neuropeptides and cannabinoids in gastric mucosal defense initiated centrally in the rat. J Physiol Pharmacol 2009; 60 (Suppl.7): 93-100.
- Brancati SB, Zadori ZS, Nemeth J, Gyires K. Substance P induces gastric mucosal protection at supraspinal level via increasing the level of endomorphin-2 in rats. Brain Res Bull 2013; 91: 38-45.
- Benyhe Z, Toth G, Wollemann M, et al. Effects of synthetic analogues of human opiorphin on rat brain opioid receptors. J Physiol Pharmacol 2014; 65: 525-530.
- Ray A, Henke PG. TRH-enkephalin interactions in the amygdaloid complex during gastric stress ulcer formation in rats. Regul Pept 1991; 35: 11-17.
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro). Brain Res 1986; 399: 152-155.
- Mizoguchi H, Watanabe H, Hayashi T, et al. Possible involvement of dynorphin A-(1-17) release via mu1-opioid receptors in spinal antinociception by endomorphin-2. J Pharmacol Exp Ther 2006; 317: 362-368.
A c c e p t e d : February 5, 2015