NEW FACTS AND THE CONCEPT OF PHYSIOLOGICAL REGULATION OF THE DOPAMINERGIC SYSTEM FUNCTION AND ITS DISORDERS
INTRODUCTION
The dopaminergic (DArgic) system and the neurotransmitter dopamine (DA) are responsible for many basic body functions, such as motivational and emotional behavior of humans and animals, control of involuntary as well as rapid motor function, and neurosecretion associated with the rhythm of light, biological clock, and reproduction (1-5). DA is also involved in cognitive processes, such as functioning of a working memory (6), and dopamine transporter (DAT) contributes to homeostatic sleep-wake regulation in humans (7).
Great interest in the DArgic system in humans primarily results from the fact that dysfunctions of the system, i.e., hypo- or hyperfunction, result in serious neurological disorders, such as: ADHD, which is most common in young people; depression, present in people of all ages; schizophrenia in adults; and Parkinson’s disease in older people (8-11). The genesis of these diseases is still poorly understood, but a relationship between activity of the DArgic system and function of the cavernous sinus has never been considered. We know today that, in the cavernous sinus, DA (among many other neurotransmitters) is counter-current, retrograde transferred from the brain venous effluent into the arterial blood supplying the brain (12-15). It has been established that DA, just like other DAT substrates, down-regulates the expression of DAT (16-18). If the retrograde transfer of DA in the cavernous sinus occurs under physiological condition over the whole lifetime, the uptake and transport of DA to the capillaries of the cortical and limbic structures must have an important regulatory function.
Therefore, it is surprising that in the 21st century, the era of molecular study and great progress in research on the physiology of humans and animals, a vascular complex located in a very privileged position - inside the skull but outside the dura mater, lying just beneath and around the pituitary gland, specifically innervated by the sympathetic plexuses, surrounded by a connective capsule, and through which the trunks of 10 cranial nerves pass - is recognized as a structure of incredibly low physiological significance. According to Simoens et al. (19), in antiquity Herophilus and Gallen described an unusual convolution of veins and arteries located at the base of the brain of animals, now called the miraculous network (rete mirabile), and Leonardo da Vinci was delighted with the vascular plexus. The scientific description of the cavernous sinus in humans was presented for the first time in the 18th century by the neuroanatomist J.B. Winslow (20). The vascular plexus is called nowadays by physiologists as the perihypophyseal vascular complex (14). We present the opinion that in both humans and animals the retrograde transfer of DA in the perihypophyseal vascular complex, and more precisely in the cavernous sinus, is involved in the regulation of the DArgic system activity.
DOPAMINE TRANSPORTER AND ITS ROLE IN REGULATION OF THE DOPAMINERGIC SYSTEM
There is a consensus that the activity of DArgic neurons is primarily determined by the action of DAT, located mainly in the membrane of the presynaptic DArgic neuron and effectively acting in the DArgic synapse (21-26). DAT belongs to the family of neurotransporter proteins. It transports DA through the cell membrane with energy from the electrochemical gradient of Na+/Cl– (27). Recently, a growing number of proteins have been shown to interact with DAT, and it is suggested that these interactions may be important in the regulation of transporter function (3). Phosphorylation and oligopolymerization have a significant effect on the activity of DAT molecules (3, 28-30).
Major concentrations of DArgic cell groups lay in the midbrain structures, such as the substantia nigra (A9), the interpeduncular nuclei, and the ventral tegmental area (A10). Two important DArgic cell groups (A12, A15) lie in the hypothalamus. Axons of the DArgic neurons located in the midbrain project to the DArgic forebrain nuclei, and terminate in the striatum, the amygdala, the olfactory bulb, and the hypothalamus (24). The nigrostriatal, mesolimbic, and mesocortical DArgic pathways are formed (5). Gonadal steroids modulate the mesocortical DArgic system (31). DA released in the DArgic neurons of the hypothalamus acts on neurons synthesizing hypothalamic neurohormones. In addition, it penetrates the portal vessels, reaches the adenohypophysis, and from there is transported via the venous blood gets to the cavernous sinus. Pioneering research on DA transfer from venous blood of the cavernous sinus to the arterial blood supplying the brain was performed on sheep (12, 13) and recently on rabbits (15).
DA released into the synaptic cleft acts on the postsynaptic neurons via specific DArgic receptors, which belong to the G-protein-coupled membrane receptor family. DAT removes the extracellular DA from the synaptic cleft back into the synaptic flask, which not only reduces DArgic stimulation, but also reduces the synthesis and storage of the DA in the synaptic flask vesicles; this is the reuptake and retrograde transfer of DA (23-25). DAT function can also be regulated by presynaptic receptor ligands (17). Removal of DAT dramatically prolongs the lifetime of extracellular DA. In mice with a genetically determined absence of DAT, the time of DA residence in the synaptic cleft is extended by 300-fold (1, 32). Extracellular DA levels result from a dynamic equilibrium between its release and reuptake by DArgic terminals (25, 26). Psychostimulants, such as cocaine, increase extracellular DA concentration by inhibiting the reuptake of DA (33-35). The presence of DAT was also shown in the membrane of dendrites and axons of DArgic neurons in the midbrain (34). Excessive concentrations of DA in the nigrostriatal neurons lead to formation of toxic hydroxyl radicals, and in the next stage of alpha-synuclein protein. Alpha-synuclein is a 140-amino-acid protein that forms a stable complex with DAT, which causes formation of the Lewy’s bodies and is typical for Parkinson’s disease degeneration of neurons (8, 25, 36).
PERIHYPOPHYSEAL CAVERNOUS SINUS AND DOPAMINE RETROGRADE TRANSFER
Structural features of the cavernous sinus
The cavernous sinus is an irregular cuboidal structure that is localized around the pituitary gland, lateral to the sella turcica. In humans and all animals, the left and right cavernous sinuses are connected together by the intracavernous sinus. In humans, rabbits, rats, mice, and many carnivores the left and right internal carotid artery, which provides the main supply of the arterial blood to the brain, passes through the left and right cavernous sinuses. In humans, this segment of the internal carotid artery was identified as a segment of T6 (cavernous segment of the internal carotid artery) (37). The left and right cavernous segments of the internal carotid artery are connected by anterior and posterior bends of the intercavernous internal carotid artery (38) (Fig. 1). In many species of ungulates (ruminants, pigs), the cavernous sinus is filled with a veno-venous network intertwined with an arterio-arterial network of the internal carotid artery or the network of the maxillary artery (carotid or maxillary rete mirabile) (Fig. 2).
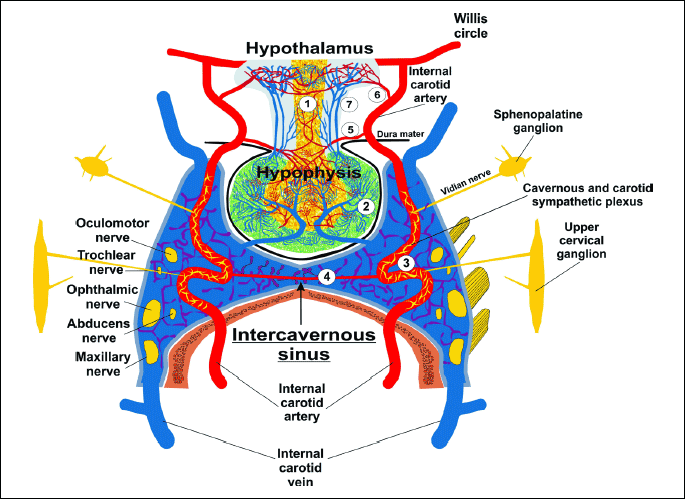
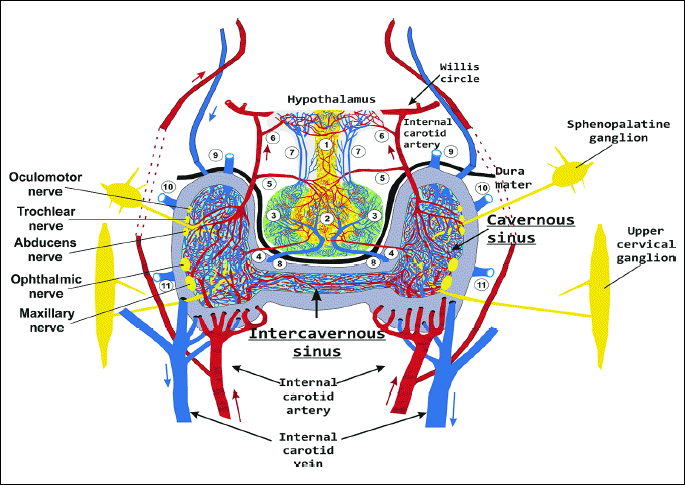
(1) infundibulum; (2) neurohypophysis; (3) adenohypophysis; (4) inferior hypophyseal artery; (5) middle hypophyseal artery; (6) superior hypophyseal artery; (7) long portal veins; (8) inferior hypophyseal vein; (9-11) venous connections to the cerebral sinuses.
The cavernous sinus is filled with venous blood outflowing from the brain and pituitary, and in part from the nose and eye. In the cavernous sinus of humans, rabbits, mice, rats, and many other species, the walls of the cavernous sinus veins are transformed into a fibrous trabecule allowing for free, though slowed down, flow of the venous blood. From the vein’s wall layers, only the endothelium remains, which directly covers the wall of the cavernous segment of the internal carotid artery, and anterior and posterior bends of the intercavernous interna carotid artery (38). In humans, the cavernous segment of the internal carotid artery (T-6) loses the lamina elastica externa and lamina elastica interna layers (39). In rabbits, mice and rats, age-related changes in the size and number of pores in the lamina elastica interna, affecting the permeation of different molecules through the walls of vessels, were shown (40-42). The above data indicate that in humans and laboratory animals, only four vascular layers separate the venous blood and arterial blood. In many species of ungulates (ruminants, pigs), the tunica adventitia of veins and arteries in the cavernous sinus combines in a common tunica adventitia (43) and the inner muscular layer of arterioles is reduced to 3–5 layers of muscle cells (44). Venous blood is separated from the arterial blood by five or six relatively thin vascular layers.
Innervation of the cavernous sinus and its meaning
The unique feature of the cavernous segment of the internal carotid artery (T6) is its wall, which has a dense network of sympathetic fibers that form a sympathetic carotid plexus. A similar sympathetic cavernous plexus is found on the wall of the cavernous sinus. Both plexuses originate from the sympathetic upper the cervical ganglion and the spheno-palatine ganglion (Vidian nerve) (38, 45) (Fig. 1 and Fig. 2). Moreover, ten trunks of the cranial nerve pass along the cavernous sinus. On each side the cavernous sinus pass: the oculomotor nerve (cranial nerve III), the trochlear nerve (n. IV), the maxillary nerve and the ophthalmic nerve, (which are branches of the trigeminal nerve: ns V1 and V2), and the abducens nerve (n. VI) (Fig. 1 and Fig. 2). A functional link between the nerves and the cavernous sinus has not been identified. However, it should be emphasized that Johnston et al. (46) demonstrated that chemicals could move from the cerebrospinal fluid into the cavernous sinus along the cranial nerves. When microfil (silastic material) was infused into the subarachnoid space (cisterna magna) in sheep postmortem, it was found in the space surrounding the venous network of the cavernous sinus and within epineural and endoneural spaces of the trigeminal nerve, as well as in lymphatic vessels emerging from epineurium of the trigeminal nerve (46) (Fig. 3). We present the opinion that cranial nerves III, IV, V1, V2, and VI - which originate from midbrain neurons and exit the midbrain and pass along the cavernous sinus - may participate in the transmission of DA from the midbrain to the venous blood of the cavernous sinus. The penetration of the DA along the cranial nerves from the midbrain into the cavernous sinus is strongly suggested by the following data:
- extracellular DA volume fraction is greater by 40%, and DA uptake rate is 200-fold lower in substantia nigra (midbrain) vs. striatum (34);
- our recent pilot study showed that when radiolabeled DA was infused for 20 minutes into the ventral tegmental area in the bled rabbit, the radioactivity was found in the cavernous sinus 30 minutes after the end of the infusion (unpublished data).
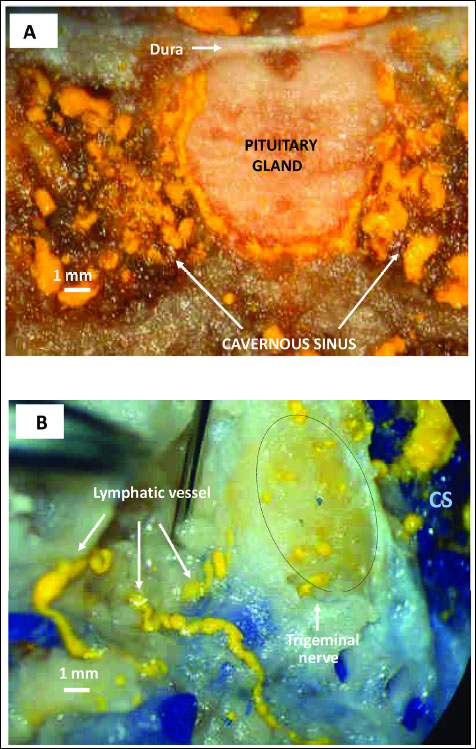 |
Fig. 3. Transfer of yellow Microfil into the cavernous sinus after its injection into the cistern magna (reproduced with permission of authors from Johnston et al. Cerebrospinal Fluid Res, 2007). (A) Coronal section illustrating yellow Microfil distributed around the pituitary gland and within the cavernous sinus. (B) Cut surface of the trigeminal nerve (dashed circle) adjacent to the cavernous sinus (CS) showing yellow Microfil distribution within the nerve. Lymphatic vessels (white arrows) containing yellow Microfil can be seen emerging from the epineurium of the nerve. |
Physiological functions of the cavernous sinus
Physiological studies on the function of the cavernous sinus have been undertaken in the 1970s, mainly in the works of Hayward and Baker (47, 48). It was established that arterial blood flowing to the brain is cooled by the cold venous blood flowing from the nose to the cavernous sinus. The proposed countercurrent exchange of heat between the arterial rete mirabile and venous blood of the cavernous sinus was accepted by physiologists and widely publicized. However, in recent decades, previously used methods for the temperature measurement have been criticized, and the new findings with use of telemetric methods showed that under physiological conditions countercurrent heat exchange in the cavernous sinus has no special significance in protecting the brain from overheating (49-52).
A completely new view on the function and physiological significance of the cavernous sinus was caused the discovery that neuropeptides (neurotransmitters), as well as other physiological regulators, may permeate the perihypophyseal vascular complex from the venous blood of the cavernous sinus (venous outflow from the brain and pituitary) to the arterial blood supplying the brain. Studies on isolated pig heads supplied with their own blood through the carotid arteries showed that after infusion of radiolabeled gonadotropin releasing hormone (GnRH), beta-endorphin or progesterone to the cavernous sinus (through the angular oculi vein), these hormones were found in the arterial blood supplying the brain and pituitary (53). To determine whether a mixing of the venous and arterial blood can occur in the cavernous sinus, autologous red blood cells labeled with radioactive chromium (51Cr) and suspended in saline were infused into the cavernous sinus through the angular oculi vein. In none of the experiments was 51Cr radioactivity found in arterial blood supplying the brain (53).
In further studies on the isolated animal heads perfused with autologous blood, or in vivo on anesthetized animals, it was demonstrated in swine and sheep that after infusion into the cavernous sinus, radioactive neurotransmitters such as GnRH (54, 55), oxytocin (56) DA (12, 13), and beta-endorphin (57), these radioactive neurotransmitters were found in arterial blood supplying the brain and pituitary. When progesterone, estradiol, testosterone, and male pheromone androstenol were injected into the nasal cavity, they reach the cavernous sinus via the angular oculi vein and were found in arterial blood supplying the brain and pituitary, as well as in many brain structures (58-61). However, prolactin (PRL) and luteinizing hormone (LH) were not retrograde transferred from venous blood of the cavernous sinus into arterial blood supplying the brain (62).
The discovery of local retrograde and destination transfer of regulatory factors pointed to a previously unknown function of the cavernous sinus in the humoral physiological regulation (14). It demonstrated the possibility of the local retrograde transfer of neurotransmitters from the venous brain outflow to the arterial blood supplying the brain, as well as showing that the cavernous sinus played a significant role in the local destination transfer of priming pheromones that permeated from the venous blood outflowing from the nose into the arterial blood supplying the brain (14).
In 1996, D.A. Oren presented the hypothesis of phototransduction via the humoral pathway with participation of the cavernous sinus. According to this concept, the energy of visible light stimulates production in the retina (from the hem) of a neurogenic regulator, carbon monoxide (CO), which in the cavernous sinus permeates into arterial blood, and after reaching the brain causes several changes in its activity (63). The study performed by Koziorowski and co-workers (64) showed that CO production in the eye depends on the intensity of natural light reaching the retina, and its concentration in the venous blood outflow from the eye varies depending on the time of day and the season, and thus confirmed the Oren’s hypothesis. Moreover, in experiments performed on animals, it has been shown that CO introduced into the cavernous sinus reached the suprachiasmatic nuclei and altered the expression of the main biological clock genes per and cry, which regulate circadian and seasonal cycles (65). Therefore, it was proved for the first time that the CO, a physiological regulator, was transmitted from the venous blood of the cavernous sinus to the arterial blood and influenced the processes taking place in the specific brain structures. This led to the conclusion that retrograde and destination transfer of hormones and other physiologically active substances may be an universal physiological regulatory mechanism, operating with only minor modifications in various species of animals and in humans (14).
RELATIONSHIPS BETWEEN DOPAMINE RETROGRADE TRANSFER IN THE CAVERNOUS SINUS AND FUNCTION OF THE DOPAMINERGIC SYSTEM
Retrograde transfer of dopamine in the cavernous sinus
Pioneering research on the effect of DA retransfer in the cavernous sinus on the activity of DArgic nuclei A15 of the hypothalamus was performed in 2001–2004 (12, 13). It has been shown that a few minutes after the introduction of radiolabeled DA to the cavernous sinus (via the angular oculi vein), DA was present in the arterial blood supplying the brain. Moreover, the influence of the season (spring, winter) on the intensity of the transfer of DA from the venous blood of the cavernous sinus into the arterial blood supplying the brain was demonstrated (12). Using an isolated sheep head perfused with autologous blood and radiolabeled or cold DA infusion, the transfer of DA from venous blood of the cavernous sinus to the arterial blood supplying the brain was studied (13). Transfer of DA by a countercurrent mechanism in the ewe changes with seasons and endocrine stage. Data compiled from Skipor et al. (13, 14) are presented in Fig. 4.
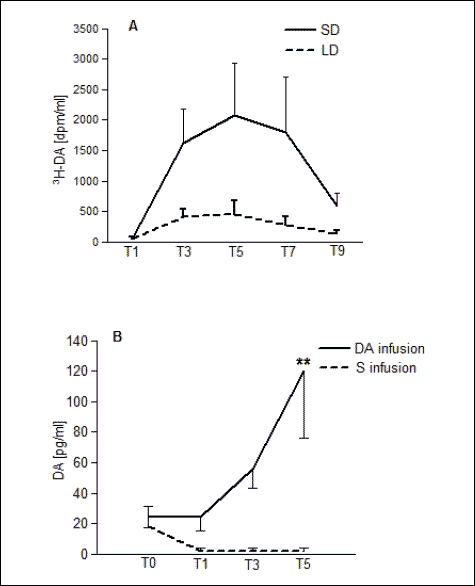 |
Fig. 4. DA retrograde transfer in sheep (compiled with permission on the basis of Skipor et al. 2001 and 2004). (A) The dynamic transfer of 3H-DA in ovariectomized female sheep in spring (LD, long days), and in autumn (SD, short days). (B) The dopamine concentration in blood plasma of the arterial blood flowing through the brain, following infusion of 1 mg of DA or saline into cavernous sinus. (DA-dopamine infusion, S-saline infusion). |
The studies performed by Skipor et al. (13, 14) were focused only on the impact of DA infusion into the cavernous sinus on the secretory function of the hypothalamic neurons associated with reproduction in sheep. So far, no studies have been carried out on the influence of the retrograde retransfer of DA in the cavernous sinus on hypo- and hyperfunction of striatal DArgic neurons. The morphological structure of the cavernous sinus in sheep (Artiodactyles) differs significantly from the structure of the cavernous sinus in humans. However, a very close similarity in morphology of the cavernous sinus in humans and rabbits directed an interest toward the transfer of DA in the rabbit. Recent studies on DA transfer in the rabbit have shown that DA could penetrate the cavernous sinus from the venous blood of the cavernous sinus to the arterial blood supplying the brain, and DA and its metabolites reached many structures in various areas of the brain, including the cortical and subcortical regions (15) (Fig. 5 and Fig. 6).
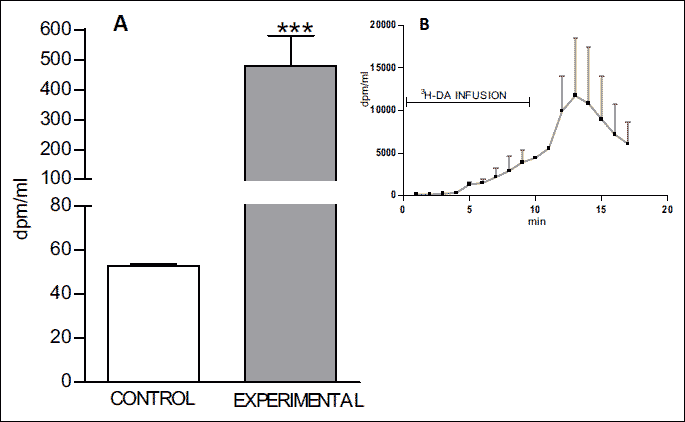
(A) Mean (± S.E.) concentration of radioactivity in blood samples collected from rabbit’s brain basal artery during and after infusion of 3H-DA. (B) The dynamic presentation of radioactivity in blood samples collected from rabbit’s brain basal artery during and after infusion of 3H-DA.
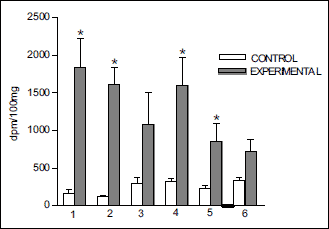 |
Fig. 6. Accumulation (mean ± S.E.) of radioactivity in some brain structures after infusion of 3H-DA into right cavernous sinus of the rabbit isolated head perfused with Hanseleit-Krebs buffer solution mixed with homologous blood in a 3:1 ratio: (1) pia mater; (2) pons; (3) ventral tegmental area; (4) mammilary body; (5) hipocampus; (6) corpus striatum. (Muszak et al. J. Physiol Pharmacol 2014, with permission). |
If DA is retrogradely transferred from the brain venous effluent to the arterial blood supplying the brain in the rabbit, in which the cavernous sinus morphology is very similar to that in humans, we can suggest that the DA retrograde transfer may also occur in humans.
The mechanism of neurotransmitter transfer in the cavernous sinus
The mechanism of transfer of DA, and other neurotransmitters from venous blood of the cavernous sinus to arterial blood of the internal carotid artery has not been elucidated. It is hard to believe that it is done exclusively on the basis of the concentration gradient. A blocking of Na+ K+ ATP-ase with oubain administered into the cavernous sinus reduces beta-endorphin countercurrent transfer from venous blood of the cavernous sinus to the arterial blood supplying the brain (55). The presence of LH/hCG receptor mRNA transcripts in the walls of both arterial and venous compartments of the cavernous sinus-carotid rete complex was demonstrated. It suggested that LH could modulate GnRH transfer acting directly on the vascular smooth muscle (55).
Lack of LH (30 kDa) and PRL (23 kDa) permeation, together with earlier findings regarding the permeation of DA (0.19 kDa), testosterone (0.19 kDa), progesterone (0.32 kDa), oxytocin (1.0 kDa), GnRH (1.2 kDa) and beta-endorphin (3.4 kDa), suggest that the molecular mass of the hormone may be a major factor determining the transfer of hormones in the cavernous sinus (62).
Pharmacological studies on chromium (III) chloride effect on monoaminergic system in mice indicated that the antidepressant-like activity of chromium was dependent on the noradrenergic as well as dopaminergic and serotonin systems (66). Extremely rich adrenergic innervation of the internal carotid artery cavernous segment (44, 45) suggests the presence of significant expression of the extracellular norepinephrine transporter (NET) in the arterial wall. The transporter, which belongs to the family of monoamine transporters, can transport both norepinephrine and DA (67). Therefore, the question arises whether NET is involved in the transport of DA through the arterial wall of the cavernous fragment of the internal carotid artery, and whether it is possible that this influences the retrograde transfer of DA? The clarification of this question could be of great practical importance, since some pharmacological agents that locally regulate DA permeation and retrograde transfer might exist, and in this way, the activity of the DArgic system in the subcortical and cortical areas of brain might be regulated.
Dopamine influence on the activity of the dopamine transporter
DA enters the cavernous sinus with venous effluent from the hypothalamus and pituitary. However, a large part (about 65%) of dopamine permeating in the rabbit cavernous sinus to the arterial blood reaches the brain with arterial blood already in the form of metabolites (15). Dopamine metabolism has been extensively studied for many years (25, 26, 34, 68-72). In the striatal astrocytes, DA may be converted to metabolites, such as dihydroxyphenylacetic acid, methoxytyramine and homovanillic acid by enzymes: monoamine oxydase (MAO) and catechol-O-methyltransferase (COMT) (blood-brain barrier action) (68-70). COMT operates in astrocytes but there is no its activity in DArgic nigrostriatal neurons (68-69). Age-dependent estrogen concentration may influence the activity of COMT (70).
DA uptake in vascular epithelial cells is the first step of the blood-brain barrier system that protects neurons from entry by DA. When radioactive DA (3H-DA) is infused into the rabbit cavernous sinus, the largest uptake of 3H-DA (3H-DA/mg) is in the vasculature of the pia mater, in comparison to other brain structures (Fig. 6). Reception of DA by astrocytes acts as the second step of the blood-brain barrier. Striatal astrocytes can take up DA as well as L-DOPA which is therapeutically efficacious in patients with Parkinson’s disease and other hypofunctionings of the DArgic system (70). Primary cultured striatal astrocytes (expressed aromatic amino acid decarboxylase) convert L-DOPA to DA (71). In striatal astrocytes, DA is metabolized quickly while L-DOPA relatively more slowly (69). However, despite DA metabolization, the striatal astrocytes (continuously supplied by DA retrograde transfer from cavernous sinus) contain in their cytoplasm free particles of DA for a period of time. The striatal astrocyte cell membrane contains receptors, channels and transporters for neurotransmitters involved in astrocyte-neuron transmission (72-74). We think that the increased concentration of DA in the cytoplasm of astrocytes, even for a short time, facilitates the permeation of DA molecules (molecular mass 0.19 kD) through the astrocyte cell membrane and DA diffusion towards DArgic synapse, according to gradient concentration. This results in increased DA concentration in the area of the DArgic synaptic cleft and binding of the DA with receptors and DAT. An effective DA radius after quantal release is 2 µm for the activation of low affinity DA receptors and 7 – 8 µm for high affinity receptors (34). We conclude that DArgic activity is regulated by the extracellular concentration of DA resulting from a dynamic equilibrium between its release and uptake in DArgic terminals (25) as well as from its constant supply from striatal astrocytes into the area around DArgic synapses. A number of experiments support the idea that expression of DAT activity is regulated directly by interaction with their substrates. Brief repeated periods of DA exposure or DA and amphetamine treatments down-regulate activity of DAT. Experiments performed in an in vitro model and in vivo documented that prolonged exposure to DA, amphetamine or tyramine, greatly reduced DAT function. Frequent (every 2 min) applications of DA in rats changed the parameters in the clearance of DA, which was accompanied by a profound down-regulation of DAT function (16). In another study, the function of DAT was reduced by DA and amphetamine, and was enhanced by cocaine, when they were used at appropriate concentrations or doses (17). Down-regulation of DAT function was also demonstrated after DArgic neuron lesion (18). Based on these results and data from our earlier studies, we suggest that under physiological conditions, a continuous supply of DArgic terminal region by retrogradely transferred DA may inhibit the expression of DAT by a down-regulation mechanism. It would be a control of the DArgic system based on feedback, which is the primary physiological regulatory mechanism. It is also likely, but it should be proven in further studies, that DA metabolite or active substances inhibiting the expression of DAT (produced in astrocytes under the influence of DA metabolites), may be released in perivascular striatal astrocytes.
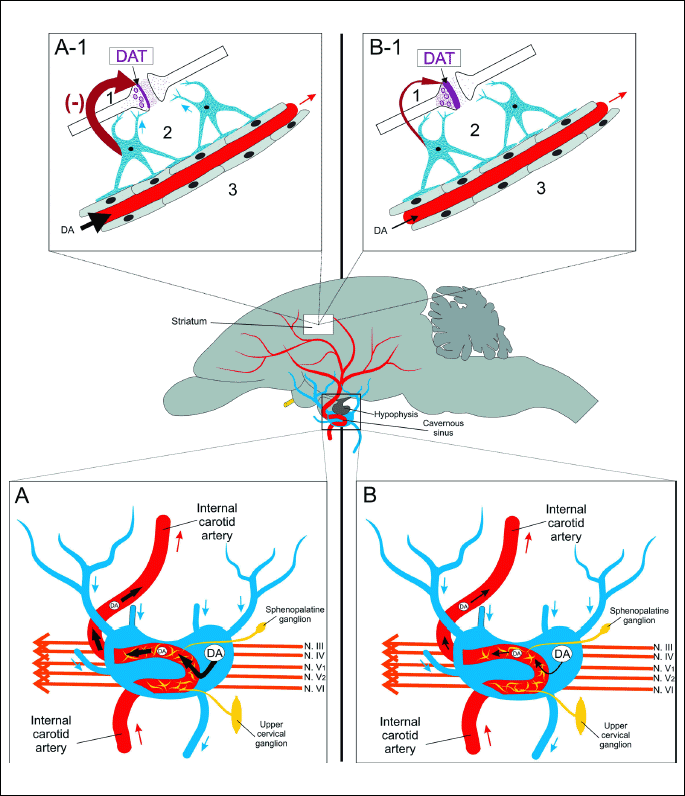
Left side of the figure: normal physiological condition: (A) Magnification of the cavernous sinus with marked course of the cavernous segment (segment T6) of the internal carotid artery, sympathetic innervation, and cranial nerves which take their origin in the midbrain and pass throughout the cavernous sinus. High concentration of DA in arterial blood, due to its retrograde transfer in the cavernous sinus, is transported to the brain (thick black arrows). (A-1) Magnification of portion of the striatum: - continuous inhibition of DAT expression by DA (supplied from the cavernous sinus) by the down regulation mechanism. DArgic synapse with limited expression of DAT in membrane of the nigrostratial neuron: (1) nigrostratial neuron; (2) astrocytes; (3) endothelium of capillary vessel. The supply of DA regulating expression of DAT is marked with thick dark red arrow.
Right side of the figure: hypofunction of dopaminergic system (in Parkinson’s disease or other hypofunction) (B) Lack or limited retrograde transfer of DA in the cavernous sinus results in low concentration of DA transported with arterial blood to the brain (thin black arrows). (B-1) Lack or limited inhibition of DAT expression by DA by down regulation mechanism (thin dark red arrow). A high expression of DAT in the nigrostratial neuron.
Because the inhibition or increase of DAT activity by retrogradely transferred DA is a chronic process, we suggest that short-term therapy by administration of L-DOPA will not effectively remove disturbances in dopaminergic system activity. Therefore, the mechanism of physiological permeation of DA from venous to arterial blood is a very important step in the regulation of DArgic system function and it should first be understood. We suppose that in the future, this first basic stage of the mechanism for DA permeation in the cavernous sinus may be easily pharmacologically regulated.
We conclude that DA is continuously retrogradely transferred in the cavernous sinus by a countercurrent mechanism from the venous blood of the cavernous sinus into the arterial blood supplying the brain.
We present the view that in animals and humans, DArgic activity may be regulated depending on the intensity of DA retrograde transfer from the cavernous sinus over the whole lifetime and can have an impact on the occurrence of its hypo- or hyperfunction. We suggest that under physiological conditions, DA - continuously retrogradely transferred, and carried by the arterial blood from the cavernous sinus to endothelial cells and perivascular striatal astrocytes - inhibit the expression of striatal DAT by a down-regulation mechanism.
Many data indicate that DA reaches the cavernous sinus with venous brain effluent, and suggest that it also moves to the cavernous sinus along the endoneurium and perineurium of 10 trunks of five cranial nerves (III, IV, V1, V2, VI). These nerves originate from the region of the midbrain in which the concentration of DAergic neurons and the concentration of extracellular DA is largest. The changes in the retrograde transfer activity in the cavernous sinus - age-dependent and regulated by the hormones may be the primary cause of dysfunction of the DArgic system. A new concept of DArgic function and genesis of its dysfunction with involvement of DA retrograde transfer in the cavernous sinus is presented in Fig. 7.
SUGGESTIONS FOR FUTURE STUDIES
The data presented above suggest that research aimed to explain the genesis (including the age) of the hypo- or hyperfunction of the DArgic system, and DArgic system dysfunction causing Parkinson’s disease, ADHD, schizophrenia and many other psychiatric disorders, should consider two areas:
- the cavernous sinus, where DA is taken-up, and transferred from the venous blood of the cavernous sinus to the arterial blood supplying the brain. To regulate this process pharmacologically, understanding the mechanism and explanation of what determines its course is necessary.
- brain DArgic structures, whose activity is regulated primarily by the action of DAT. I t is essential to clarify whether the expression of the DAT is regulated exclusively by extracellular DA or by DA metabolites reaching the presynaptic membrane, and any factor secreted by striatal astrocytes under the influence of DA metabolites.
Acknowledgments: Authors are grateful to Professor Miles Johnston for kind permission to publish photographs of his paper (Johnston et al. 2007) and thank Dr. P. Gilun for computer preparation of illustrations (Figs 1, 2 and 7). Publication is financed by the statutory funds of the Polish Ministry of Science and Higher Education in 2014.
Conflict of interests: None declared.
REFERENCES
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 1998; 95: 4029-4034.
- Greengard P. The neurobiology of slow synaptic transmission. Science 2001; 294:1024-1030.
- Torres GE. The dopamine transporter proteome. J Neurochem 2006; 97 (Suppl.1): 3-10.
- Gowrishankar R, Hahn MK, Blakely RD. Good riddance to dopamine: roles for dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochem Int 2014; 73: 42-48.
- Money KM, Stenwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci 2013; 7: 260-277.
- Vollbrecht PJ, Simmler LD, Blakely RD, Deutch AY. Dopamine denervation of the prefrontal cortex increases expression of the astrocytic glutamate transporter GLT-1. J Neurochem 2014; 130: 109-114.
- Holst SC, Bersagliere A, Bachmann Y, Berger W, Achermann P, Landolt HP. Dopaminergic role in regulating neurophysiological markers of sleep homeostasis in humans. J Neurosci 2014; 34: 566-573.
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord 2013; 28: 715-724.
- Amsterdam JD, Newberg AB, Soeller I, Shults I. Greater striatal dopamine transporter density may be associated with major depressive episode. J Affect Disord 2012; 141: 425-431.
- Krause KH, Dresel SH, Krause J, Fougere CI, Ackenheil M. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neuroscience Behav Rev 2003; 27: 605-613.
- Marcota M, Sin J. Pantazopoulos H, Jonoilionis R, Berretta S. Reduced dopamine transporter expression in the amygdale of subject diagnosed with schizophrenia. Schizophr Bull 2014; 40: 984-991.
- Skipor J, Wasowska B, Grzegorzewski W, Zezula-Szpyra A, Stefanczyk-Krzymowska S, Thiery JC. Transfer of dopamine by counter-current mechanism in the ewes changes with endocrine stage. Biogenic Amines 2001; 16: 431-445.
- Skipor J, Wasowska B, Picard S, Thiery JC. Dopamine access to the median eminence and brain throughout the vascular pathway in sheep. Reprod Biol 2004; 4: 91-106.
- Krzymowski T, Stefanczyk-Krzymowska S. Local retrograde and destination transfer of physiological regulators as an important regulatory system and its role. Facts and hypothesis. J Physiol Pharmacol 2012; 63: 3-16.
- Muszak J, Krzymowski T, Gilun P, Stefanczyk-Krzymowska S. Countercurrent transfer of dopamine from venous blood in the cavernous sinus to the arterial blood supplying the brain - the perfused rabbit head as an experimental model. J Physiol Pharmacol 2014; 65: 641-648.
- Gulley JM, Doolen S, Zahniser NR. Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem 2002; 83: 400-411.
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Europ J Pharmacol 2003; 479: 139-152.
- Afonso-Oramas D, Cruz-Muros I, Barroso-Chinea P, et al. The dopamine transporter is differentially regulated after dopaminergic lesion. Neurobiol Dis 2010; 40: 518-530.
- Simoens P, Lauwers H, De Geest JP, De Schaepdrijver L. Functional morphology of the cranial retia mirabilia in the domestic mammals. Schweiz Arch Tierheilkd 1987; 129: 295-307.
- Thakur, JD, Sonig A, Khan IS, Connor DE Jr, Pait TG, Nanda AA. Jacques Benigne Winslow (1669-1760) and the misnomer cavernous sinus. World Neurosurg 2014; 81: 191-197.
- Saunders C, Ferrer JV, Shi L, et al. Amphethamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Nat Acad Sci USA 2000; 97: 6850-6855.
- Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Ann Rev Pharmacol Toxicol 2003; 43: 261-284.
- Kahling KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol 2003; 470: 153-158.
- Bjorklund A, Dunnett B. Dopamine neuron systems in the brain: an up date. Trends Neurosci 2007; 30: 194-202.
- Zhank H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia 2012; 2: 5-13.
- Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states Trends Pharmacol Sci 2013; 34: 489-496.
- Nelson N. The family of Na+/Cl– neurotransmiter transporters. J. Neurochem 1998; 71: 1785-1803.
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters molecular function of important drug targets. Trends Pharmacol Sci 2006; 27: 375-383.
- Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of dopamine transporter by phosphorylation. Handb Exp Pharmacol 2006; 175: 197-214.
- Eriksen J, Jorgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem 2010; 113: 27-41.
- Sarvari M, Deli L, Kocsis P, Mark L, et al. Estradiol and isotype-selective estrogen receptor agonists modulate the mesocortical dopaminergic system in gonadectomized female rats. Brain Res 2014; 1583: 1-11.
- Jones SB, Gainetdinov RR, Wightman RM, Caron MC. Mechanism of amphetamine action revealed in mice lacking the dopamine transporter, J Neurosci 1998; 18: 1979-1986.
- Schmitt KC, Reith ME. Regulation of the dopamine transporter. Aspects relevant to psychostymulant drugs of abuse. Ann NY Acad Sci 2010; 1187: 316-340.
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in nigrostriatal pathway. Brain Res Rev 2008; 58: 303-313.
- Yorgason JT, Jones SR, Espana RA. Low and high affinity dopamine transporter inhibitors block dopamine uptake within 5 sec of intervenous injection. Neuroscience 2011; 182: 125-132.
- Swant J, Goodwin JS, North A, et al. Alfa-synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J Biol Chem 2011; 286: 43933-43943.
- Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery 1996; 38: 425-433.
- Dalgic A, Boyaci S, Aksoy K. Anatomical study of the cavernous sinus emphasizing operative approaches. Turk Neurosurg 2010; 20: 186-204.
- Masuoka T, Hayashi N, Hori E, Kuwayama N, Ohtani O, Endo S. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol Med Chir (Tokyo) 2010; 50: 179-182.
- Wong LC, Langille BL. Developmental remodeling of the internal elastic lamina of rabbit arteries. Circ Res 1996; 78: 799-805.
- Boumaza S, Arribas SM, Osborne-Pellegrin M, et al. Fenestrations of the carotid internal elastic lamina and structural adaptation in stroke-prone spontaneously hypertensive rats. Hypertension 2001; 37: 1101-1107.
- Lee K, Forudi F, Saidel GM, Penn MS. Alterations in internal elastic lamina permeability as a function of age and anatomical site precede lesion development in apolipoprotein E-null mice. Circ Res 2005; 97: 450-456.
- Khamas WA, Ghoshal NG, Bal HS. Histomorphologic structure of the carotid rete-cavernous sinus complex and its functional importance in sheep (ovis aries). Am J Vet Res 1984; 45: 156-158.
- Santamaria L, Dieguez G, Garcia-Villalon AL, Nava Hernandez E, Gomez B, Lluch S. Histomorphometry and innervations of the rete mirabile and brain vessels of Artiodactyla. In: Stroke and Microcirculation, J. Cervos-Navarra, R. Ferszt (eds). Raven Press, New York, 181-185.
- Marinello G, Annecchiarico H, Sardo L, Buonamassa S, de Divitiis B. Connection of sympathetic fibres inside the cavernous sinus: a microanatomical study. Clin Neurol Neurosurg 2000; 102: 1-5.
- Johnston M, Armstrong D, Koh L. Possible role of the cavernous sinus veins in cerebrospinal fluid absorption. Cerebrospinal Fluid Res 2007; 4: 3-12.
- Hayward JN, Baker MA. A comparative study of the role of the cerebral arterial blood in the regulation of brain temperature in five mammals. Brain Res 1969; 16: 417-440.
- Baker MA, Hayward JN. The influence of the nasal mucosa and the carotid rete upon hypothalamic temperature in sheep. J Physiol 1968; 198: 561-579.
- Mitchell D, Maloney SK, Laburn HP, Knight MH, Kuhnen G, Jessen C. Activity blood temperature and brain temperature of free-ranging springbok. J Comp Physiol 1997; 167: 335-343.
- Fuller A, Moss DG, Skinner JD, Jessen PT, Mitchell G, Mitchell D. Brain, abdominal and arterial blood temperatures of free-ranging eland and their natural habitat. Pflugers Arch 1999; 438: 671-680.
- Fuller A, Maloney SK, Kamerman PR, Mitchell G, Mitchell D. Absence of selective brain cooling in free-ranging zebras in their natural habitat. Exp Physiol 2000; 85: 209-217.
- Fuller A, Hetem RS, Meyer LC, Maloney SK. Angularis oculi vein blood flow modulates the magnitude but not the control of selective brain cooling in sheep. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1409-R1417.
- Krzymowski T, Skipor J, Grzegorzewski W. Cavernous sinus and carotid rete of sheep and sows as a possible place for counter current exchange of some neuropeptides and steroid hormones. Anim Reprod Sci 1992; 29: 225-240.
- Grzegorzewski WJ, Skipor J, Wasowska B, Krzymowski T. Countercurrent transfer of 125I-LHRH in the perihypophyseal cavernous sinus-carotid rete vascular complex, demonstrated on isolated pig heads perfused with autologous blood. Domest Anim Endocrinol 1997; 14: 149-160.
- Skipor J, Bao S, Grzegorzewski W, Wasowska B, Rao CV. The inhibitory effect of hCG on counter current transfer of GnRH and the presence of LH/hCG receptors in the perihypophyseal cavernous sinus-carotid rete vascular complex of ewes. Theriogenology 1999; 51: 899-910.
- Grzegorzewski W, Skipor J, Wasowska B, Krzymowski T. Counter current transfer of oxytocin from the venous blond of the perihypophyseal cavernous sinus to the arterial blood of carotid rete supplying the hypophysis and brain depends on the phase of the estrous cycle in pigs. Biol Reprod 1995; 52: 139-144.
- Skipor J, Grzegorzewski W, Wasowska B, Krzymowski T. Counter current transfer of β-endorphin in the perihypophyseal cavernous sinus-carotid rete vascular complex of sheep. Exp Clin Endocrinol Diabetes 1997; 105: 308-313.
- Krzymowski T, Grzegorzewski W, Stefanczyk-Krzymowska S, Skipor J, Wasowska B. Humoral pathway for transfer of the boar pheromone, androstenol, from the nasal mucosa to the brain and hypophysis of gilts. Theriogenology 1999; 52: 1225-1240.
- Stefanczyk-Krzymowska S, Krzymowski T, Grzegorzewski W, Wasowska B, Skipor J. Humoral pathway for local transfer of the priming pheromone androstenol from the nasal cavity to the brain and hypophysis in anaesthetized gilts. Exp Physiol 2000; 85: 801-809.
- Skipor J, Grzegorzewski W, Krzymowski T, Einer-Jensen N. Local transport of testosterone from the nasal mucosa to the carotid blood and the brain in the pig. Pol J Vet Sci 2000; 3: 19-22.
- Skipor J, Grzegorzewski W, Einer-Jensen N, Wasowska B. Local vascular pathway for progesterone transfer to the brain after nasal administration in gilts. Reprod Biol 2003; 3: 143-159.
- Skipor J, Grzegorzewski W, Wasowska B, Krzymowski T. Luteinizing hormone and prolactin are not retrograde transferred in perihypophyseal vascular complex in ewes. Reprod Biol 2004; 4: 195-201.
- Oren DA. Humoral phototransduction: blood is a messenger. Neuroscientist 1996; 2: 207-210.
- Koziorowski M, Stefanczyk-Krzymowska S, Tabecka-Lonczynska A, Gilun P, Kaminski M. Gaseous messenger carbon monoxide is released from the eye into the ophthalmic venous blood depending on the intensity of sunlight. J Biol Regul Homeost Agents 2012; 26: 111-118.
- Gilun P, Stefanczyk-Krzymowska S, Romerowicz-Misielak M, Tabecka-Lonczynska A, Przekop F, Koziorowski M. Carbon monoxide-mediated humoral pathway for the transmission of light signal to the hypothalamus. J Physiol Pharmacol 2013; 64: 761-772.
- Piotrowska A, Siwek A, Wolak M, et al. Involvement of the monoaminergic system in the antidepressant-like activity of chromium chloride in the forced swim test. J Physiol Pharmacol 2013; 64: 493-498.
- Schwartz JW, Blakely RD, DeFelice LJ. Binding and transport in norepinephrine transporters. J Biol Chem 2003; 278: 9768-9777.
- Myohanen TT, Schendzielorz N, Mannisto PT. Distribution of catechol-0-methyltransferaze (COMT) proteins and enzymatic activities in wild-type and soluble COMT deficient mice. J Neurochem 2010; 11: 1632-1643.
- Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal 2013; 11: 34-59.
- Schendzielorz N, Rysa A, Reenila I, Raasmaja A, Mannistro P. Complex estrogenic regulation of catechol-o-methyltransferase (COMT) in rats. J Physiol Pharmacol 2011; 62: 483-490.
- Asanuma M, Miyazaki I, Murakami S, Diaz-Corrales FJ, Ogawa N. Striatal astrocytes acts as a reservoir for L-DOPA. PLoS One 2014; 9: e106362.
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationship: for better and for worse. Trends Neurosci 2010; 34: 76-87.
- Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorder. Neuroterapeutics 2010; 7: 494-506.
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neuroterapeutics 2010; 7: 338-353.
A c c e p t e d : February 13, 2015