MELATONIN METABOLITE, N1-ACETYL-N1-FORMYL-5-METHOXYKYNURAMINE (AFMK), ATTENUATES ACUTE PANCREATITIS IN THE RAT:
IN VIVO AND IN VITRO STUDIES
INTRODUCTION
Acute pancreatitis is a nonbacterial disease of the pancreas, classified as mild, moderate or severe inflammation; this condition often leads to the systemic complications and high patient mortality which ranges from 25 – 40% (1-3). Improving the resistance of pancreas to acute inflammation and preventing the severe form of this disease have been the subjects of many experimental and clinical studies. To date, the pathogenesis of acute pancreatitis remains unexplained, and the treatment of this disease is nonspecific (4).
Melatonin was discovered as the pineal product, but subsequently it was also identified in many other tissues (5, 6). This indoleamine is well known as a multifunctional antioxidant and protector of tissue integrity against oxidative stress (7-15). Previous studies have shown that melatonin, and its amino acid precursor, L-tryptophan, protect the pancreas against the damage induced by acute inflammation (11, 13). Both melatonin and L-tryptophan function as direct scavengers of toxic reactive oxygen and nitrogen species (ROS and RNS) and activators of antioxidant enzymes, such as superoxide dysmutase (SOD), catalase (CAT), glutathione peroxidase (GPx), or glutathione reductase (GR) (10, 13, 16). Melatonin reduces the production of proinflammatory cytokines such as tumor necrosis factor a (TNF-α), interleukin 1β (IL-1β), interleukin 6 (IL-6), interleukin 22 (IL-22) and modulates the processes of apoptosis and necrosis (10, 11, 17-21). TNF-α activates the intracellular proinflammatory mechanisms, leading to the promotion of acute inflammation, whereas apoptosis is involved in the pancreatic protection against acute pancreatitis (22-24). Metabolites of melatonin and L-tryptophan are an additional class of biogenic amines, known as kynuramines (25). Among the best recognized kynuramines two melatonin derivatives: N1-acetyl-N1-formyl-5-metoxykynuramine (AFMK), and N1-acetyl-5-methoxy-kynuramine (AMK) received particular attention (25-27). Kynuramines were considered to be inactive, metabolic products of melatonin and L-tryptophan, but further studies revealed that these substances reduced lipid peroxidation and protected DNA from the damage (25, 27). AFMK is produced from melatonin via kynuric pathway initiated by key enzyme: indole-amine 2,3-dioxygenase (IDO) in many cell types, but formation of AFMK can also be triggered by a variety of different factors such as myelo- or hemoperoxidases, ROS, or ultraviolet radiation (28). AFMK has been identified in many organisms including plants, small aquatic species, and in mammals (in retina, central nervous system, and skin cells) (26, 29). In each of these locations, AFMK works as an effective cell protector against oxidative stress (25, 26, 30). Despite the known antioxidative effect of AFMK, other physiological roles of this melatonin metabolite remain to be defined. Although melatonin has been reported to play an important role in protecting pancreatic tissue against inflammatory damage (7, 8, 11, 13, 18, 31, 32) the effect of melatonin's metabolites on acute pancreatitis has yet to be examined. This study investigated the effects of AFMK on pancreatic inflammation in vivo and in vitro and compared these effects with those of melatonin.
MATERIAL AND METHODS
Materials
Following items were purchased: caerulein and melatonin from Sigma Co. (St Louis, MO, USA), AFMK from Cayman Europe, (Tallin, Estonia), amylase reagent from Dialab Diagnostic Ges. MBH, (Wien, Austria), LPO-586 commercial kit for determination of malondialdehyde and 4-hydroxynonenal (MDA + 4-HNE) and GPx commercial kit from OXIS Research (Portland, OR, USA) and Vetbutal from Biowet (Pulawy, Poland). The following materials were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA): protein A-Agarose, polyclonal primary anti-TNF-α and GPx antibodies and secondary rabbit anti-goat antibodies linked to HRP (horseradish peroxidase-conjugated). SuperSignal West Pico Chemiluminescent Substrate melatonin was purchased from Sigma Co (St. Louis, MO, USA).
The in vivo study
1. Animals and experimental protocol
All procedures were approved by the Jagiellonian University Ethics Committee and performed in accordance with the policies on humane care and use of laboratory animals. The experiments were carried using male Wistar rats (weight 220 – 250 g). Animals were fed commercial pellet chow, housed in cages under standard conditions, in temperature-controlled environment with a 12/12 light/dark cycle, with free access to food and water. Prior to the experiments, rats were fasted for 24 hours, but access to water was not limited. All experiments were carried out in the morning. During the experiments, rats were placed in individual Bollman cages. Acute pancreatitis (AP) was induced by subcutaneous (s.c.) infusion of caerulein at dose of 5 µg/kg/h for 5 hours. Caerulein was diluted in saline and infused at a rate of 1 mL/h. Control groups received 0.5 mL of vehicle saline injected intraperitoneally (i.p.), followed 30 min later by s.c. infusion of 0.9% saline for 5 hours (33). AFMK (5, 10 or 20 mg/kg) was dissolved in physiological saline with addition of absolute ethanol. Melatonin (20 mg/kg) was dissolved in absolute ethanol and diluted in physiological saline to appropriate concentrations. The above tested substances were given in a volume of 0.5 mL to the rats as a bolus i.p. injection 30 min prior to the start of caerulein administration.
Animals were allocated to 10 groups, each group consisting of 6 – 8 rats:
1) Control group - animals that received 0.5 mL of vehicle i.p. followed by s.c. infusion of 0.9% saline for 5 h;
2) Mel group - rats given 20 mg/kg of melatonin before saline infusion;
3) AFMK5 group - rats given 5 mg/kg of AFMK followed by infusion of 0.9% saline;
4) AFMK10 group - rats given 10 mg of AFMK followed by infusion of 0.9% saline;
5) AFMK20 group - rats given 20 mg/kg of AFMK followed by infusion of 0.9% saline;
6) AP (acute pancreatitis) group - rats that received a s.c. infusion of caerulein;
7) AP + AFMK5 group - rats given 5 mg/kg of AFMK before caerulein administration;
8) AP + AFMK10 group - rats given 10 mg/kg of AFMK before caerulein administration;
9) AP + AFMK20 group - rats given 20 mg /kg of AFMK before caerulein administration;
10) AP + Mel group - rats given 20 mg/kg of melatonin before caerulein administration.
2. Biochemical parameters
After 6 hours of experiment rats were subjected to pentobarbital anesthesia and blood samples were collected from the vena cava to measure serum amylase activity and TNF-α levels. Amylase activity in each sample was measured by enzymatic method as previously reported (34). Serum concentration of TNF-α was measured using an ELISA commercial kit (R&D systems, Minneapolis MN, USA) (33). The blood samples were left for 2 hours at room temperature for coagulation and then centrifuged (at 3500 rpm for 10 min). Serum samples were frozen and kept at –80°C until assayed.
3. Pancreatic weight and histological examination
The pancreata were carefully dissected, rinsed and weighted. Representative samples of pancreatic tissue were collected and processed for histopathological assessment. Histological studies were carried out on pancreatic samples fixed in 10% formalin and stained with hematoxylin and eosin. Specimens were examined by a professional histologist without knowledge of the treatment given. The histological grading of edema, neutrophil infiltration and hemorrhage changes were assessed using the 0 - 3 range as described previously (for edema: 0 = no edema, 1 = interlobular edema, 2 = interlobular edema and moderate interlobular edema, 3 = interlobular edema and severe interlobular edema; for neutrophil infiltration, or vacuolization of acinar cells, from 0 = no infiltration, or vacuolization, to 3 = maximal alternations) (34).
4. Determination of MDA + 4-HNE and glutathione peroxidase (GPx)
Samples of pancreatic tissue were taken to determine lipid peroxidation products: malonylodialdehyde and 4-hydroxynonenal (MDA + 4-HNE) and the activity of glutathione peroxidase (GPx). Specimens were homogenized and tissue homogenates were measured using Bioxytech LPO-586 kit (Oxis International, Inc., Portland, USA) according to the manufacturer's protocol. In short, samples of pancreatic tissue weighting about 300 mg were homogenized in the phosphate buffer (20 mmol/L, pH 7.4), using homogenizer Ultra-Turrax T25, Janke & Kunkel IKA - Labortechnik (sample volume 0.5 mL, temp 4°C, 2400/min for 15 s). Then, 10 µL of 0.5 M butylated hydroxytoluene in acetonitrile was added to each sample to prevent tissue oxidation. Samples were centrifuged and the pellets were immediately frozen at –70°C until the assay. MDA + 4-HNE was measured in duplicate and expressed as nM/g of tissue (7).
The activity of GPx was measured in the pancreatic samples perfused with 0.9% NaCl, homogenized and centrifuged, as described previously (8). The colorimetric assay for assessment of GPx activity (Bioxytech, Oxis, Portland, USA) was used. GPx activity was measured in duplicate and expressed as pg/mL.
The in vitro study
Pancreatic AR42J cells have been purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). Cells were subcultured weekly in the RPMI 1640 with Glutamax-I (Gibco BRL, Gaithersburg, MD, USA) and 10% fetal bovine surovice (FBS, heat-inactivated; Gibco BRL) with addition of 100 U/ml Penicyline and 100 µg/ml Streptomycine (Sigma, St. Louis, MO, USA). Cell culture was grown in standard conditions (37°C and 5% CO2). For the experiment 1 × 106 AR42J cells were seeded on a 10 mm dish in RPMI 1640 with addition of 2% fetal bovine serum with supplementation of antibiotics. For stimulation melatonin or AFMK (both used at concentrations of 10–12, 10–10, or 10–8M), were added, without or with addition of caerulein (10–8M). AR42J cell incubated in presence of caerulein (10–8M) alone were used as AP control. Basal control cells were incubated without any investigated substances. After 48 hours of incubation cells were harvested by scraping with rubber 'police man' in ice cold PBS (phosphate buffered saline) and collected by short spin centrifugation at 4°C. Then, cell pellets were resuspended in 400 µL of extraction buffer containing 10 mmol/L Hepes, 10 mmol/L KCl, 2 mmol/L MgCl2, 1 mmol/L EDTA (ethylenediamine tetraacetic acid), 1 mmol/L DTT (dithiothreitol), 0.1 mmol/L PMSF (phenylmethylsulphone fluoride) pH 7.4 and kept on ice for 15 min. Subsequently, samples were added with 25 µL 10% NP-40, mixed vigorously and centrifuged at 14000 × g for 15 s at 4°C. The supernatant containing cytosolic fraction of proteins was removed and stored at –80°C until further analysis. Protein signals for TNF-α, GPx, proapoptotic BAX, antiapoptotic Bcl-2, or caspase-3 were determined by Western blot.
Western blot
The extracts of total cellular proteins were prepared as described previously (35). After electrophoresis and transfer of the samples, the PVDF membrane (BioRad, USA) was blocked with 5% non-fat dried milk in PBS for 2 hours at room temperature. Blocking procedure was followed with 1- hour exposure to primary antibody diluted 1:1000 and secondary antibody goat anti-mouse IgG-HRP, rabbit anti-goat IgG-HRP diluted 1:5000. After each antibody probing membrane was washed three times for 15 min in TBST buffer (0.1 M Tris pH 8.0; 1.5 M NaCl; 0.5% TritonX-100). All antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Detection of membrane bound proteins was performed using BM Chemiluminescence Blotting Substance (Boehringer, Mannheim, Germany). GAPDH analysis was performed to document equal protein loading. All presented results were obtained in 4 consecutive experiments and are representative for the observed phenomenon.
Western blots were exposed to X-ray membranes. The intensity of protein signals was measured using system G-Box EF (Synoptics, GB) and the results have been presented as a ratio of measured absorbance from each blot versus measurement assessed for the housekeeping gene GAPDH.
Statistical analysis
Results are expressed as means ± S.E.M. Comparison of the differences between the mean values of various groups of experiments was made by an analysis of variance or the Student's t test for unpaired data and Wilcoxon test for paired data. Differences with a P value of < 0.05 were considered statistically significant.
RESULTS
The in vivo study
1. The effects of AFMK on pancreatic weight, serum amylase activity and serum TNF-α concentration
In the control rats mean pancreatic weight was 780 ± 70 mg, whereas serum amylase level was 1690 ± 125 IU/L and TNF-α concentration was 4.11 ± 0.9 pg/mL. AFMK (5, 10, or 20 mg/kg) or melatonin (20 mg/kg) given alone to the experimental animals failed to affect significantly the pancreatic weight, pancreatic morphology, serum amylase activity or TNF-α concentration (Fig. 1).
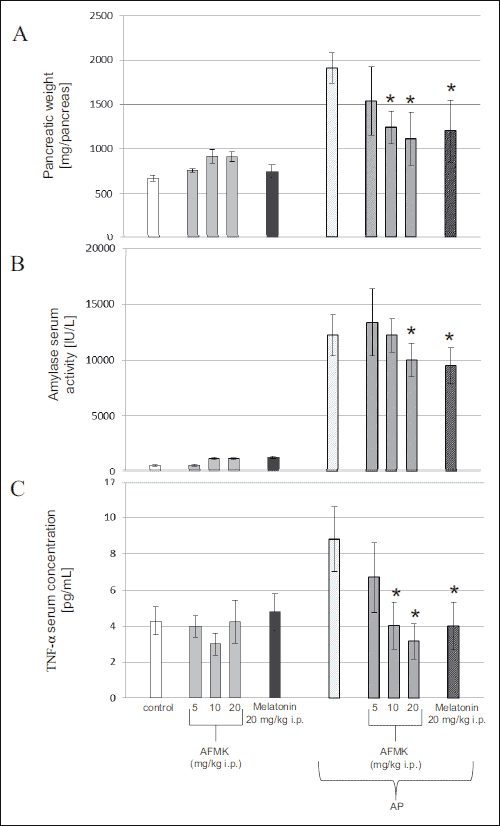 |
Fig. 1. Effects of AFMK (5, 10 or 20 mg/kg) or melatonin (20 mg/kg) on pancreatic weight (A), amylase serum activity (B) and TNF-α serum concentration (C) in the rats subjected to caerulein-induced pancreatitis (AP). Control = intact animals. Means ± S.E.M. from the separate experiments, each performed on 6 – 8 rats. Asterisks indicate significant (P < 0.05) decrease below the values detected in the rats with AP alone. |
Subcutaneous infusion of caerulein to the rats produced acute pancreatitis (AP) in all animals tested. Pancreatic weight and amylase activity were significantly increased to 1880 ± 170 mg and 12260 ± 1840 IU/L, respectively, whereas TNF-α concentration rose to 8.8 ± 1.8 pg/mL (Fig. 1A, 1B and 1C). In the AP rats, typical pancreatic lesions were observed: excess peritoneal fluid accumulated and the pancreas was grossly swollen. Edema was accompanied by perivascular infiltration of leukocytes and typical vacuolization of the acinar cells (Fig. 2, Table 1). In the rats pretreated with AFMK prior to the induction of AP the dose-dependent reduction of pancreatic edema, serum amylase activity, serum TNF-α concentration and morphological signs of inflammation have been found (Figs. 1, 2 and Table 1). Application of AFMK at highest dose used (20 mg/kg) to the AP rats resulted in a significant reduction of pancreatic weight, serum amylase activity and serum TNF-α concentration (to 1110 ± 300 mg/kg, 10000 ± 1220 IU/L and 3.10 ± 0.9 pg/mL respectively). These reductions were comparable to the protective effect of melatonin given at a dose of 20 mg/kg (1200 ± 350 mg/pancreas, 9600 ± 1270 IU/L and 4.0±1.2 pg/mL, respectively) (Fig 1A, 1B, and 1C).
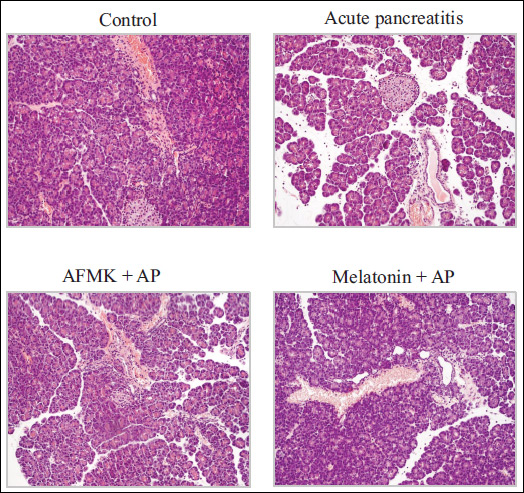 |
Fig. 2. Histological pictures of pancreas of rats exposed to caerulein-induced pancreatitis (AP), AP rats pretreated with AFMK at dose of 20 mg/kg (AP + AFMK), and AP rats pretreated with melatonin at dose of 20 mg/kg (AP + melatonin). Control = intact animals. |

2. The effect of AFMK on MDA + 4-HNE and GPx concentrations in the pancreatic tissue
The concentration of lipid peroxidation products (MDA + 4-HNE) in the pancreatic tissue of control rats achieved 5.9 ± 1.4 nM/g tissue, whereas GPx was 44.1 ± 7.0 pg/mL (Fig. 3A and 3B). In control rats, application of AFMK (5, 10 or 20 mg/kg), or melatonin (20 mg/kg) did not influence MDA + 4-HNE levels or GPx activity in the pancreas.
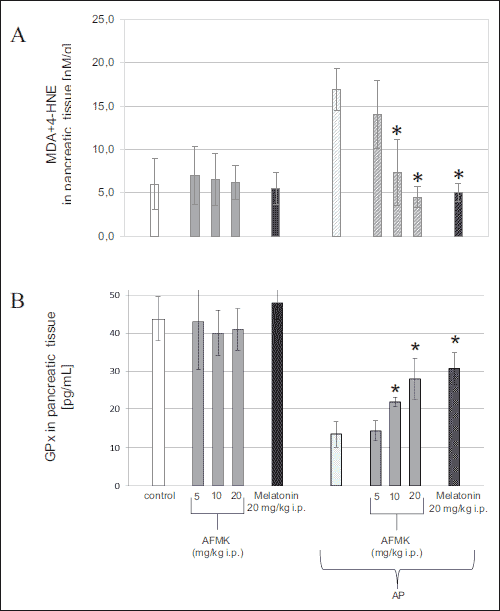 |
Fig. 3. MDA + 4-HNE (A) and GPx (B) in pancreatic tissue in AP rats subjected to AFMK (5, 10 or 20 mg/kg) or melatonin (20 mg/kg). Control = intact animals. Means ± S.E.M. from the separate experiments, each performed on 6 – 8 rats. Asterisks indicate significant (P < 0.05) decrease below the values detected in the rats with AP alone. |
Acute pancreatitis resulted in significant rises of MDA + 4-HNE in the pancreas to 16.7 ± 2.4 nM/g of tissue, whereas GPx was reduced to 13.1 ± 1.8 pg/mL (Figs 3A and 3B). AFMK given to the rats prior to caerulein infusion caused significant decline in MDA + 4-HNE content in the pancreas and was accompanied by marked augmentation of GPx. With dose of 20 mg/kg AFMK, lipid peroxidation products were reduced to 4.7 ± 0.7 nM/g tissue, and GPx increased to 28.2 ± 6.7 pg/mL in the rats suffering from AP. These values were similar to those caused by melatonin given at a dose of 20 mg/kg, which resulted in the reduction of MDA + 4-HNE to 5.1 ± 0.5 nM/g tissue and rise of GPx to 30.7 ± 5.5 pg/ml (Fig. 3A and 3B).
The in vitro study
1. The effect of AFMK and melatonin on GPx and TNF-α signals in pancreatic AR42J cells
GPx and TNF-α protein signals were detected in all samples tested. In the control samples, the GPx/GAPDH ratio was 82 ± 2.0, whereas the ratio of TNF-α/GAPDH was 9.8 ± 0.3 (Fig. 4A and 4B). The addition of melatonin or AFMK (both at doses of 10–8 - 10–12M) failed to affect these signals significantly (data not shown).
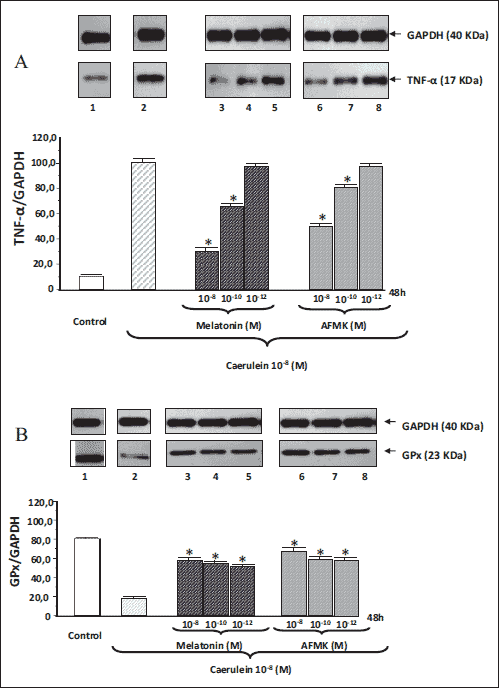 |
Fig. 4. Western blot analysis of TNF-α (A) and GPx (B) protein levels in the AR42J pancreatic cell line under basal conditions (line 1), following stimulation of these cells with 10-8M of caerulein alone (line 2), or combination of caerulein with melatonin (lines 3 - 5), or caerulein with AFMK (lines 6 - 8). Line 1: basal conditions; line 2: caerulein 10-8M; line 3: caerulein10–8M + melatonin 10–8M; line 4: caerulein 10–8M + melatonin 10–10M; line 5: caerulein10–8M + melatonin 10–12M; line 6: caerulein10–8M + AFMK 10–8M; line 7: caerulein10–8M + AFMK 10–10M; line 8: caerulein10–8M + AFMK 10–12M. The blots were probed with GAPDH to document equal protein loading. Asterisks indicate significant (P < 0.05) decrease below the value obtained in cells treated with caerulein 10–8M alone. All results shown were obtained in 4 consecutive experiments and are representative of observed phenomenon. |
Stimulation of AR42J cells with caerulein (10–8M) resulted in the significant reduction of GPx/GAPDH ratio to 18.9 ± 2.0, whereas TNF-α/GAPDH ratio achieved 100 ± 5.0. Both investigated substances; melatonin or AFMK, when added to the pancreatic AR42J cells stimulated with caerulein, significantly increased the GPx signal. The high concentrations of AFMK diminished the signal of TNF-α. The highest level of GPx protein was observed with the highest used dose of melatonin or AFMK, (10–8M) and reached 56.5 ± 4.0 and 65.7 ± 6.2, respectively. In contrast to GPx, this dose of melatonin or AFMK diminish the TNF-α signal to 30.2 ± 6.6 and 49.2 ± 4.9, respectively (Fig. 4A and 4B).
2. The effect of AFMK and melatonin on Bcl-2, Bax and caspase 3 protein signals in pancreatic AR42J cells
The signals for Bcl-2, an antiapoptotic protein, and for Bax, a proapoptotic protein, were assessed in all samples. In unstimulated control samples under basal conditions, the ratio Bcl-2/GAPDH was 10.1 ± 0.2, whereas the Bax/GAPDH ratio was 21 ± 0.6 (Fig. 5A and 5B). An addition of melatonin or AFMK (both at doses of 10–8 - 10–12M) did not produce a significant change of these signals (data not shown).
Incubation of pancreatic acinar cell culture with caerulein (10–8M) markedly increased the signal for Bcl-2 protein to 32 ± 2.2, and reduced significantly that of Bax to 2.3 ± 0.1. The signal for antiapoptotic Bcl-2 was significantly reduced in the AR42J acinar cells incubated in the combination of caerulein (10–8M) and melatonin (10–8 - 10–12M) and a similar reduction was observed in the cells treated with caerulein (10–8M) and AFMK (10–8 - 10–12M). The most pronounced inhibition was detected in the samples incubated with the highest concentration of melatonin or AFMK (10–8M). In these samples the ratio Bcl-2/GAPDH was reduced to 4.0 ± 0.2 and 4.2 ± 0.3, respectively (Fig. 5A and 5B).
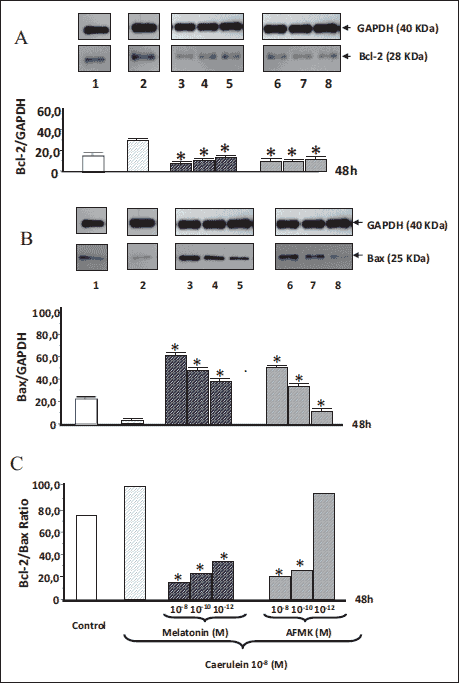 |
Fig. 5. Western blot analysis of antiapoptotic Bcl-2 (A) and proapoptotic (B) protein levels and their ratio (C) in the AR42J pancreatic cell line. Explanation as for Fig. 6. Cross indicates significant (P < 0.05) increase above the control value. Asterisks indicate significant (P < 0.05) decrease below the value obtained in cells incubated with caerulein 10–8M alone. |
In contrast, an addition of melatonin or AFMK to the pancreatic acinar cell culture incubated with caerulein significantly and dose-dependently increased the level of a proapoptotic protein Bax. The highest level of Bax protein was observed in samples subjected to incubation with dose of 10–8M of melatonin or AFMK, when the ratio of Bax/GAPDH reached 62.4 ± 2.3 and 50.0 ± 3.0, respectively (Fig. 5A and 5B).
Fig. 5C shows the Bcl-2/Bax ratio in AR42J cells. This ratio amounted 75,2 under basal conditions, whereas in cells subjected to caerulein hyperstimulation it was increased to 100. An addition of melatonin (10–8 - 10–12M) to cells treated with caerulein markedly and dose-dependently decreased the Bcl-2/Bax ratio. High doses of AFMK (10–8 and 10–10M) also resulted in a marked reduction of this ratio, indicating that both melatonin and AFMK (in high doses) activated proapoptotic signaling pathway in AR42J cells exposed to caerulein hyperstimulation (Fig. 5C).
The proapoptotic caspase-3 protein was not observed in the control samples, but treatment of these samples with melatonin or AFMK (10–8 - 10–12M) significantly elevated the level of this executor caspase. The most pronounced rise of the caspase-3/GAPDH ratio was detected at the dose of 10–8M of melatonin or AFMK and reached 58.4 ± 4.3 and 52 ± 5.5, respectively (Fig. 6A). Incubation of acinar cell culture with caerulein (10–8M) resulted in a significant upregulation of caspase-3 abundance, and increased the caspase-3/GAPDH ratio to 87 ± 6.3. When melatonin or AFMK were added to caerulein-treated cells a significant augmentation of caspase-3 protein over the level produced with caerulein alone was observed. The most abundant rise of caspase-3/GAPDH ratio was detected with the dose of 10–8M of melatonin or AFMK (182 ± 5.0 and 157 ± 4.9, respectively) (Fig. 6B).
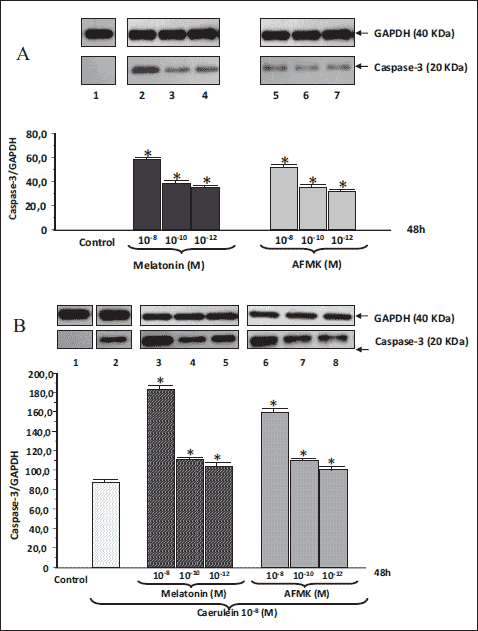 |
Fig. 6. (A) Effect of melatonin (10–8 - 10–12M) or AFMK (10–8 - 10–12M) on caspase 3 signal in the AR42J pancreatic cell line. Western blot analysis: line 1: basal control; line 2: melatonin 10–8M; line 3: melatonin 10–10M; line 4: melatonin 10–12M; line 5: AFMK 10–8M; line 6: AFMK 10–10M; line 7: AFMK 10–12M. Asterisks indicate significant (P < 0.05) increase above the control value. (B) Western blot analysis of caspase 3 protein in the AR42J pancreatic cell line. line 1: basal control; line 2: caerulein 10–8M; line 3: melatonin 10–8M; line 4: melatonin 10–10M; line 5: melatonin 10–12M; line 6: AFMK 10–8M; line 7: AFMK 10–10M; line 8: AFMK 10–12M. Asterisks indicate significant (P < 0.05) increase above the value obtained in cells incubated with caerulein 10–8M. The blots were probed with GAPDH to document equal protein loading. All results shown were obtained in 4 consecutive experiments and are representative of observed phenomenon. |
DISCUSSION
Melatonin is an evolutionarily conserved, highly effective tissue protector against oxidative stress and inflammatory damage (36, 37). This indoleamine is also involved in the modulation of reproductive cycle and release of pituitary hormones (38, 39). Its metabolite, AFMK, shares the antioxidative and protective properties of melatonin (27, 30, 40-43). In the experimental studies, AFMK was shown to prevent oxidative damage of cortical neurons and to increase the viability of hippocampal cells exposed to ROS (44).
Our present data shows, for the first time, that AFMK alleviated acute pancreatic inflammation and diminished pancreatic damage induced by acute pancreatitis. The favorable effects of AFMK were analogous to those of its precursor, melatonin. Protective effects of AFMK on acute pancreatitis were observed in vivo and was manifested by the reduction of morphometric signs of inflammation and by marked decreases of amylase serum activity and TNF-α serum concentration. These changes were accompanied by significant declines of pancreatic lipid peroxidation products, MDA + 4-HNE and a rise of an antioxidant enzyme, i.e., GPx, in the pancreas. This data shows that AFMK influences GPx activity and TNF-α as well as modulates apoptotic signal transduction pathway in the acinar pancreatic cell line AR42J.
AFMK is an efficient scavenger of ROS (45, 46). One molecule of AFMK has the capacity to donate from two to four electrons (25, 45, 47). Based on the previous reports, this melatonin derivative is an excellent deactivator of the hydroxyl (OH) and superoxide (.O2–) radical (27, 32). Conversely, free radicals lead to the generation of AFMK from melatonin; and this cascade reaction increases the effectiveness of melatonin as a tissue protector. Both melatonin and AFMK work together to limit the oxidation of lipid membranes and to protect the cell viability (27, 30, 43). Since AFMK is easily formed from melatonin by both enzymatic and nonenzymatic means, this metabolite is considered an important part of the antioxidant protection initiated by its parent molecule, melatonin (27).
In the previous studies, melatonin was documented as a powerful activator of antioxidant enzymes, including SOD, CAT, GPx and GR (8, 10, 13, 16, 32, 48). This indirect antioxidant action of melatonin is an essential mechanism of tissue protection afforded by this indoleamine. Herein, we show that AFMK increases the activity of an antioxidant enzyme, GPx, in the pancreatic tissue. This observation indicates that in addition to direct scavenging effects produced by melatonin and AFMK, both these substances also activate antioxidant enzyme to protect the tissue from the injury induced by oxidative stress and inflammation. Stimulation of enzymatic antioxidant defense by AFMK is supported by the data from the current in vitro study wherein AFMK improved the protein signal for GPx in the pancreatic cell line AR42J. To the best of our knowledge, this is the first demonstration presenting an improvement of enzymatic antioxidant defense caused by AFMK in the pancreas.
As observed herein, the application of AFMK to the rats with acute pancreatitis resulted in the marked reduction of TNF-α serum concentration. Moreover, we found that the addition of AFMK to the pancreatic acinar cell line AR42J stimulated by caerulein resulted in a significant decline of the TNF-α protein. Our data is in agreement with the previous study of Silva et al. (47), who found that AFMK inhibits TNF-α and interleukin 8 release from neutrophils. Thus, the pancreatoprotective effect of this melatonin metabolite could be also explained by the modulatory action of AFMK on the immune function and decreased production of proinflammatory cytokines. This effect is also consistent with the immunomodulatory influence of melatonin, which was reported to inhibit the production of several pro-inflammatory mediators including TNF-α, IL-1β, IL-6, IL-8, IL-22, VEGF or prostaglandin E2 (7, 8, 13, 17, 18, 49-53).
Another possible strategy of AFMK in pancreatic protection could involve modulation of apoptosis of pancreatic acinar cells. This programmed cell death is a positive mechanism that enhances pancreatic defense and reduces inflammation (23, 24, 54). In contrast to necrosis, apoptosis does not interrupt cell membrane and therefore the inflammatory mediators and lysosomal enzymes are not released from the acinar cells. This may produce the development of the inflammatory process in the surrounding tissues and protect them from damage (23). An apoptotic signaling pathway is modulated by a variety of factors, among them two proteins are vitally important, i.e., the proapototic Bax and the antiapoptotic Bcl-2. A proapoptotic signal transduction pathway activates caspase-3, the final executor of apoptosis (23).
The present data documents that both melatonin and AFMK significantly and dose-dependently increase proapoptotic Bax protein and reduce signals for antiapoptotic Bcl-2 in AR42J acinar cells subjected to high doses of caerulein. In these cells, AFMK and its precursor markedly enhanced active caspase-3, indicating that both substances effectively induce apoptosis in pancreatic acinar cells. Melatonin has been shown previously to stimulate apoptosis in pancreatic cancer cell line via modulating the Bax/Bcl-2 balance (19). Recent reports indicated that melatonin reduced Bcl-2 expression and improved signals for apoptosis-related caspase-3 and caspase-7 in the cancer cells (20, 21). The current study shows, for the first time, that AFMK stimulates proapoptotic enzyme caspase 3 induces apoptosis in the pancreatic acinar cell line AR42J.
As mentioned previously, the protective effect of AFMK on acute pancreatitis may be related to its interaction with cyclooxygenase (COX-2) and inducible nitric oxide (iNOS), which contribute to the pathogenesis of acute pancreatitis (49). Since AFMK inhibits COX-2 and iNOS activity in the macrophages and reduces generation of prostaglandins and nitric oxide (55), it is likely that these effects of AFMK may be responsible for the alleviation of pancreatitis; this requires further study. Another protective mechanism of AFMK was observed in pancreatic cancer cell line PANC-1. These cells are derived from pancreatic ductal cells, and represent a model of most popular pancreatic adenocarcinoma. It was observed that AFMK applied at high concentrations to these cells resulted in the stimulation of HSP proteins, and this mechanism could be responsible for increased defense of pancreatic adenocarcinoma cells (56).
AFMK is produced from melatonin by multiple processes, but this melatonin metabolite is not detected in serum under normal conditions (57). AFMK was identified in some tissues including the retina and skin keratinocytes and was significantly increased following exposure to UV B, which is consistent with photoprotective role of melatonin and its metabolites (40, 58). It was proposed that the elevated formation of AFMK, and other kynuramines from melatonin could be indicative for oxidative stress or activation of inflammatory cells (27). This suggestion was supported by detection of high level of AFMK in cerebrospinal fluid of patients with meningitis (59). AFMK is formed as the result of interaction of melatonin with ROS/RNS and therefore it functions in the continuing cascade by which melatonin detoxifies free radicals (25-27, 36).
Our present data presents the evidence that AFMK is an effective pancreatic protector against acute damage working as a neutralizer of free radicals, and activator of an antioxidant enzyme. It is very likely that AFMK is involved in the pancreatic tissue protection and is responsible for a considerable part of the beneficial effect of melatonin on the pancreas as part of its scavenging cascade.
Conflict of interests: None declared.
REFERENCES
- Nesvaderani M, Eslick GD, Vaag D, Faraj S, Cox MR. Epidemiology, aethiology and outcomes of acute pancreatitis: A retrospective cohort study. Int J Surg 2015; 23: 68-74.
- Feng YC, Wang M, Zhu F, Qiun RY. Study on acute recent stage pancreatitis. World J Gastroenterol 2014; 20: 16138-16145.
- Gluszek S, Koziel D. Prevalence and progression of acute pancreatitis in the swietokrzyskie voivodeship population. Pol Przegl Chir 2012; 84: 618-625.
- Lankish PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015; 386: 85-96.
- Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 2011; 17: 3888-3898.
- Acuna-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation and potential functions. Cell Mol Life Sci 2014; 71: 2997-3025.
- Jaworek J, Leja-Szpak A, Bonior J, et al. Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J Pineal Res 2003; 34: 40-52.
- Jaworek J, Szklarczyk J, Jaworek AK, et al. Protective effect of melatonin on acute pancreatitis. Int J Inflam 2012; 2012: 173675. doi: 10.1155/2012/173675.
- Fernandez A, Ordonez R, Reiter RJ, Gonzalez-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res 2015; 59: 292-307.
- Szabolcs A, Reiter RJ, Letoha T. Effect of melatonin on the severity of L-arginine-induced experimental pancreatitis in rats. World J.Gastroenterol 2006; 14; 12: 251-258.
- Jaworek, J, Nawrot-Porąbka K, Leja-Szpak, et al. Melatonin as modulator of pancreatic enzyme secretion and pancreatoprotector. J Physiol Pharmacol 2007; 58: 65-80.
- Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J 2010; 24: 3603-3624.
- Jaworek J, Leja-Szpak A, Kot M, et al. The role of melatonin in the pancreatic protection: could melatonin be used in the treatment of acute pancreatitis? Curr Pharm Des 2014; 20: 4834-4840.
- Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 2014; 57: 131-146.
- Galano A, Medina ME, Tan DX, Reiter RJ. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physiochemical analysis. J Pineal Res 2015; 58: 107-116.
- Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004; 36: 1-9.
- Huai JP, Sun XC, Chen MJ, et al. Melatonin attenuates acute pancreatitis-associated lung injury in rats by modulating interleukin 22. World J Gastroenterol 2012; 18: 5122-5128.
- Gulben K, Ozdemir H, Berberoglu U, et al. Melatonin modulates the severity of taurocholate-induced acute pancreatitis. Dig Dis Sci 2010; 55: 941-946.
- Xu C, Wu A, Zhu H, et al. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line via modulating of Bcl-2/Bax balance. Biomed Pharmacother 2013; 67: 133-139.
- Wei JY, Li MW, Zhou LL, Lu QN, He W. Melatonin induced apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. J Pineal Res 2015; 58: 429-438.
- Laothong U, Hiraku Y, Oikawa S, Intuyod K, Murata M, Pinlaor S. Melatonin induces apoptosis in cholangiocarcinomacell lines by activating the reactive oxygen species-mediated mitochondrial pathway. Oncol Rep 2015; 33: 1443-1449.
- Binker MG, Cosen-Binker LI. Acute pancreatitis: the stress factors. World J Gastroenterol 2014; 20: 5801-5807.
- Chen GY, Dai RW, Luo H, et al. Effect of percutaneous catheter drainage on pancreatic injury in rats with severe acute pancreatitis induced by sodium taurocholate. Pancreatology 2015; 15: 71-77.
- Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology 2011; 140: 2116-2125.
- Harderland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 2009; 47: 109-126.
- Reiter RJ, Tan DX, Galano A. Melatonin reduces lipid peroxidation and membrane fluidity. Front Physiol 2014; 5: 377.
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter JR. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species. J Pineal Res 2007; 42: 28-42.
- Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol 2013; 29: 146-152.
- Tan DX, Manchester LC, Dimascio P, Martinez GR, Prado FM, Reiter RJ. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J 2007; 21: 1724-1729.
- Galano A, Tan DX, Reiter JR. On the free radical scavenging activities of melatonin metabolites; AFMK and AMK. J Pineal Res 2013; 54: 245-257.
- Qi W, Tan DX, Reiter RJ, et al. Melatonin reduces lipid peroxidation and tissue edema in cerulein-induced acute pancreatitis in rats. Dig Dis Sci 1999; 44: 2257-2262.
- Carrasco C, Rodriguez AB, Pariente JA. Effect of melatonin on the oxidative damage and pancreatic antioxidant defenses in cerulein-induced acute pancreatitis in rats. Hepatobiliary Pancreat Dis Int 2014; 13: 442-446.
- Jaworek J, Bonior J, Pierzchalski P, et al. Leptin protects the pancreas from damage induced by caerulein overstimulation by modulating cytokine production. Pancreatology 2002; 2: 89-99.
- Szklarczyk J, Jaworek J, Czech U, Bonior, Kot M, Tomaszewska R. Bilateral vagotomy attenuates the severity of secretagogue-induced acute pancreatitis in the rat. Adv Med Sci 2014; 59: 172-177.
- Sambrook J, Fritsch EF, Maniatis T, Nolan C. Molecular Cloning: A Laboratory Manual. New York, Cold Spring Harbor Laboratory Press, 1989.
- Manchester LC, Coto-Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 2015; 59: 403-419.
- Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease-14 months follow up. J Physiol Pharmacol 2014: 65: 75-82.
- Kutzler MA. Alternative methods for feline fertility control: use of melatonin to suppress reproduction. J Feline Med Surg 2015; 17: 753-757.
- Juszczak M, Roszczyk M, Kowalczyk E, Stepniak B. The influence of melatonin receptor antagonists, luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT) on melatonin-dependent vasopressin and adrenocorticotropic hormone (ACTH) release from the rat hypothalamo-hypophyseal system. in vitro and in vivo studies. J Physiol Pharmacol 2014; 65: 777-784.
- Janjetovic Z, Nahmias ZP, Hanna S, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res 2014; 57: 90-102.
- Dragicevic N, Copes N, O'Neal-Moffitt G, et al. Melatonin treatment restores the mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res 2011; 51: 75-86.
- Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J 2013; 27: 2742-2755.
- Manda K, Ueno M, Anzai K. AFMK, a melatonin metabolite attenuates x-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res 2007; 42: 386-393.
- Manda K, Ueno M, Anzai K. Space radiation-induced inhibition of neurogenesis in the hippocampal dentate gyrus and memory impairment in mice: ameliorative potential of the melatonin metabolite, AFMK. J Pineal Res 2008; 45: 430-438.
- Tan DX, Manchester LC, Burkhardt S, et al. N1-acetyl-N1-formyl-5-methoxy-kynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J 2001; 15: 2294-2296.
- Schaefer M, Hardeland R. The melatonin metabolite N1-acetyl-N1-formyl-5-methoxy- kynuramine is a potent singlet oxygen scavenger. J Pineal Res 2009; 46: 49-52.
- Silva SO, Rodriquez MR, Ximenes VF, Bueno-da-Silva AE, Amarante-Mendes GP, Campa A. Neutrophils as a specific target for melatonin and kynuramines; effect on cytokine release. J Neuroimmunol 2004; 156: 146-152.
- Czechowska G, Celinski K, Korolczyk A, et al. Protective effect of melatonin against thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol 2015; 66: 567-579.
- Chen HM, Chen JC, Ng CJ, Chiu DF, Chen MF. Melatonin reduces pancreatic prostaglandins production and protects against caerulein-induced pancreatitis. J Pineal Res 2006; 40: 34-39.
- Ganguly K, Sharma AV, Reiter RJ, Swamakar S. Melatonin promotes angiogenesis during protection and healing of indomethacin-induced gastric ulcer: role of matrix metalloproteinase-2. J Pineal Res 2010; 49: 130-140.
- Jung KH, Hong SW, Zheng HM, et al. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res 2010; 48: 239-250.
- Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez-Gallego J. A review of the molecular aspects of melatonin anti-inflammatory actions: recent insights and new perspectives. J Pineal Res 2013; 54: 1-14.
- Borges LDA S, Dermargos A, DA Silva Junior EP, et al. Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J Pineal Res 2015; 58: 166-172.
- Tian H, Zhang X, Wu C, et al. Effects of Baicalin and Octreotide on the serum TNF-αlpha level and apoptosis in multiple organs of rats with severe acute pancreatitis. Inflammation 2009; 32: 191-201.
- Mayo JC, Sainz RM, Tan DX, et al. Antiinflammatory action of melatonin and its metabolites N1-acetyl-N1-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK) in macrophages. J Neuroimmunol 2005; 165: 139-149.
- Leja-Szpak A, Pierzchalski P, Goralska M, et al. Kynuramines induce overexpression of heat shock proteins and M1/M2 receptors J Physiol Pharmacol 2015; 66: 711-718.
- Harthe C, Claudy D, Dechaud H, Vivien-Roels B, Pevet P, Claustrat B. Radioimmunoassay of N1-acetyl-5-methoxykynuramine (AMK); a melatonin oxidative metabolite. Life Sci 2003; 73: 1587-1597.
- Silva SO, Ximenes VF, Livramento JA, Catalani LH, Campa A. High concentration of melatonin metabolite N1-acetyl-N1-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: a possible immunomodulatory mechanism. J Pineal Res 2005; 39: 302-306.
A c c e p t e d : June 6, 2016