EFFECT OF ADIPONECTIN ON THE STEROIDOGENIC ACUTE REGULATORY PROTEIN, P450 SIDE CHAIN CLEAVAGE ENZYME AND 3β-HYDROXYSTEROID DEHYDROGENASE GENE EXPRESSION, PROGESTERONE AND ANDROSTENEDIONE PRODUCTION BY THE PORCINE UTERUS DURING EARLY PREGNANCY
INTRODUCTION
Adiponectin is an adipocyte-derived hormone that acts as a major regulator of energy metabolism and insulin sensitivity (1, 2). In plasma, adiponectin occurs in different forms: the trimeric low molecular weight (LMW), the hexameric middle molecular weight (MMW) and the 12-18meric high molecular weight (HMW) complexes (3). Adiponectin can be found in a smaller globular form, which is produced through proteolytic cleavage (4). It circulates at high levels in the bloodstream, and its serum concentration is decreased in obesity and insulin resistance (5, 6). In an obese subject, reduction in body weight leads to an increase in plasma adiponectin levels (7). In diabetic mice, administration of recombinant adiponectin improves insulin resistance (1). Disturbed serum levels of adiponectin have also been demonstrated in patients with pancreatic cancer (8) and in rats with post-myocardial infarction heart failure (9). Adiponectin exerts its action by binding to specific seven-transmembrane adiponectin receptors 1 and 2 (AdipoR1, AdipoR2). AdipoR1 was identified as a high-affinity receptor for adiponectin in the form of a trimer and is predominantly expressed in skeletal muscles, whereas AdipoR2 has a higher affinity for MMW and HMW adiponectin and is found mainly in the liver (10). The effects of adiponectin are mediated through the activation of the adenosine monophosphate activated protein kinase (AMPK), p38 mitogen-activated protein kinase (MAPK) and the peroxisome proliferators-activated receptor-α (PPARα) (11).
It is well known that reproduction can be affected by nutritional status. Adiponectin may be a part of a common endocrine system that controls metabolism and the reproductive system. It is believed to play a key role regarding reproduction by regulating the hypothalamic-pituitary-gonadal (HPG) axis (12, 13). The adiponectin system (adiponectin, AdipoR1 and AdipoR2) was recently found in porcine, rat and human hypothalami (14, 15), pituitary glands (16, 17) and ovaries (18-21). The secretion of gonadotropin-releasing hormone (GnRH) from GT1-7 hypothalamic cells was inhibited by adiponectin (22). Adiponectin decreased basal and GnRH-stimulated luteinizing hormone (LH) secretion by the isolated pituitary cells of rats (16). At the level of the mammalian ovaries, in vitro studies have shown that adiponectin is able to affect steroidogenic gene expression and steroidogenesis. This effect depends on differences among species, ovarian cell type and concentration of the hormone. Our group reported the inhibition of progesterone (P4) release by adiponectin (1, 10 µg/ml) in the culture of porcine luteal cells on days 10 to 12 of the oestrous cycle. In the culture of porcine granulosa cells, adiponectin at a higher dose (10 µg/ml) increased basal secretion of oestradiol (E2) (21). The addition of adiponectin to porcine granulosa cells increased the expression of steroidogenic acute regulatory protein (StAR) gene (23). However, no significant effect of both adiponectin doses was observed on basal production of P4 by porcine granulosa cells (21). It was previously suggested that adiponectin interacts with insulin and insulin-like growth factor I (IGF-I). In our previous study, adiponectin at a higher dose (10 µg/ml) in combination with insulin enhanced P4 secretion by porcine granulosa cells (21). In human granulosa cells, adiponectin also increased the IGF-I-induced secretion of P4 and E2 (19). The above mentioned data clearly demonstrates that adiponectin alters ovarian steroidogenic genes expression and the production of steroid hormones. This action may be modulated by insulin and IGF-I.
Little is known about the role of adiponectin in the uterus. The expression of adiponectin and its receptors has been found in human, mouse and porcine uteri (18, 24, 25). Our recent studies have demonstrated that the expression of adiponectin system in porcine endometrium and myometrium depends on the phase of the oestrous cycle and pregnancy (26, 27). It was reported earlier that the porcine uterus is a steroidogenic organ. In vitro studies have shown endometrial production of P4 and oestrone (E1) on days 30, 60 and 90 of gestation (28). In vitro secretion of P4, androstenedione (A4), testosterone (T), E1 and E2 by the porcine endometrium and the myometrium during early pregnancy and the oestrous cycle was also demonstrated (29-31). The expression of StAR, which facilitates the entry of cytosolic cholesterol into the mitochondrion, mRNA content of P450 side chain cleavage enzyme (CYP11A1), which catalyses conversion of cholesterol to pregnenolone, and expression of 3β-hydroxysteroid dehydrogenase (HSD3B1) gene, which is responsible for P4 and A4 production, was identified in the human endometrium (32, 33). In the porcine endometrium, StAR gene expression was found (34). It was determined that the uterus of pigs also possesses active 3β-hydroxysteroid dehydrogenase (31). The authors of the above studies have suggested that uterine steroids may be an alternative signal for recognition of pregnancy and maintenance, and initiation of implantation. To our knowledge, there is an absence of published studies describing the expression of CYP11A1 in the porcine uterus. We hypothesized that adiponectin is involved in the control of the steroidogenic activity of the porcine uterus and can interact with insulin in regulating this process. To accept or reject our hypothesis, we explored the influence of adiponectin on basal and insulin-induced: 1) expression of StAR, CYP11A1 and HSD3B1 genes; and 2) secretion of P4 and A4 by the endometrial and myometrial tissue explants harvested from pregnant pigs on days 10 to 11 of gestation (migration of the embryos to and within the uterus), days 12 to 13 (the maternal recognition of pregnancy), days 15 to 16 (implantation) and days 27 to 28 (the end of implantation) and cyclic pigs on days 10 to 11 of the cycle (the mid-luteal phase connected with period of fully active corpora lutea corresponding to the activity of corpora lutea during pregnancy).
MATERIALS AND METHODS
Experimental animals and tissue collection
The experiments were carried out in accordance with the ethical standards of the Animal Ethics Committee at the University of Warmia and Mazury in Olsztyn (the number of ethical approval 91/2011/DTN).
Twenty five mature gilts (Large White x Polish Landrace; 7 to 8 months of age, body weight of 120 to 130 kg) descended from private breeding farm were used in the study. The gilts were assigned to one of five experimental groups (n = 5 per group) as follows: days 10 to 11, 12 to 13, 15 to 16 and 27 to 28 of pregnancy, days 10 to 11 of the oestrous cycle. Females were monitored daily for oestrus behavior in the presence of an intact boar. The day of onset of the second oestrus was designated as day 0 of the oestrous cycle. The stage of the oestrous cycle was also confirmed on the basis of the morphology of the ovaries (35). Insemination was performed on days 1 to 2 of the oestrous cycle. Uteri collected after slaughter from cyclic and early-pregnant gilts were immediately placed in ice-cold PBS supplemented with 100 IU/ml penicillin and 100 µg/ml streptomycin and transported to the laboratory on ice within
1 hour for in vitro explant tissue culture. Pregnancy was confirmed by the presence of conceptuses. On days 10 to 11 and 12 to 13 of pregnancy, the uterine horns were flushed with 20 ml of sterile phosphate-buffered saline (PBS) to recover conceptuses. On days 15 to 16 and 27 to 28 of pregnancy, conceptus/throphoblast was dissected from the endometrium. All slices of the uteri on days 15 to 16 and 27 to 28 of pregnancy were collected at the implantation sites.
Endometrial and myometrial explant culture
Uteri collected from gilts during the studied periods of pregnancy and on days 10 to 11 of the cycle were washed three times in sterile PBS. Endometrial and myometrial explants cultures were performed based on a modification of the technique of Franczak et al. (29, 30). Endometrial and myometrial tissues from the uterine horns were dissected and cut into small slices (100 mg) and then washed three times in medium M199 (Sigma-Aldrich Co., USA). Individual endometrial and myometrial slices were placed into culture glass vials with 2 ml medium M199 containing 0.1% BSA (MP Biomedicals, USA), 5% dextran/charcoal-stripped newborn calf serum (Sigma-Aldrich Co.), penicillin (100 IU/ml) and streptomycin (100 µg/ml). The tissue cultures were preincubated in a shaking water bath for 2 h at 37°C in an atmosphere of 95% O2 and 5% CO2. After preincubation, the slices were treated for 24 h with control medium and recombinant human adiponectin (1, 10 µg/ml, BioVendor, USA). To test interaction between adiponectin and insulin, explants were treated with combination of adiponectin and insulin (10 ng/ml; Sigma-Aldrich Co.) for 24 hours. The doses of adiponectin and insulin were established based on Ledoux et al. (23) and Maleszka et al. (20, 21). Control slices were incubated without any treatment. All cultures were performed in duplicates in five independent experiments. After culture, the media were collected and stored at –20°C until P4 and A4 concentration were measured by radioimmunoassay (RIA). Furthermore, endometrial and myometrial tissue explants were snap-frozen in liquid nitrogen (for RNA extraction) and stored at –80°C until further analysis. The viability of tissue explants was monitored by measuring lactate dehydrogenase (LDH) activity in medium at 2 hour of preincubation as well as at the end of the treatment period. The release of LDH was performed using a Liquick Cor-LDH kit (Cormay, Poland) following the manufacturer’s instructions. The activity of LDH during the culture of tissue explants was compared to its activity in medium obtained after destruction of endometrial and myometrial cells by homogenization (positive control for causing cell death and the maximal release of LDH). Mean activity of LDH in cultured slices after treatment period was 55.1 ± 4.5 U/L for endometrium (1.8% of maximal release of LDH after total endometrial cells destruction) and 34.1 ± 4.9 U/L for myometrium (1.7% of maximal release of LDH after myometrial cells destruction).
Quantitative real-time PCR
Total RNA was extracted from all the tissue samples using peqGOLD TriFast isolation system (Peqlab, Germany). RNA concentration and quality were checked spectrophotometrically (Infinite M200 Pro, Tecan, Switzerland). One microgram of RNA was reversely transcribed into cDNA in a total volume of 20 µl with 0.5 µg oligo(dT)15 primer (Roche, Germany) using the Omniscript RT Kit (Qiagen, USA) at 37°C for 1 hour. The process was terminated by incubation at 93°C for 5 min. Specific primer pairs used to amplify parts of StAR, CYP11A1, HSD3B1, cyclophilin A (PPIA) and β-actin (ACTB) genes are detailed in Table 1. Quantitative real-time PCR analysis was carried out using a PCR System 7300 (Applied Biosystems, USA), as described previously (26). The PCR reaction included 10 ng cDNA, the appropriate forward and reverse primer at various concentrations (Table 1), 12.5 µl Power SYBR Green PCR Master Mix (Applied Biosystems, USA), and RNase free water in a final volume of 25 µl. The constitutively expressed genes, PPIA and ACTB, were used as the internal control to verify the quantitative real-time PCR. During the preliminary experiments it was found that expression of PPIA and ACTB was very similar in the endometrium and myometrium and was stable during the oestrous cycle and pregnancy and with treatments. Real-time PCR cycling conditions for StAR, CYP11A1, HSD3B1 and ACTB were as follows: enzyme activation and initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 61°C for 1 min and elongation at 72°C for 1 min. For PPIA reaction conditions were as follows: 50°C for 2 min, then enzyme activation and initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 1 min. Negative controls were performed in which cDNA was substituted by water, or reverse transcription was not performed before PCR. All samples were amplified in duplicates. The specificity of amplification was tested at the end of the PCR by melting-curve analysis. Product purity was confirmed by agarose gel electrophoresis. The real-time PCR amplification efficiencies of target and reference genes was 99 – 101%. Calculation of the relative expression level of StAR, CYP11A1 and HSD3B1 genes was conducted based on the comparative cycle threshold method (ΔΔCT) and normalized using the geometrical means of reference gene expression levels: PPIA and ACTB.
Radioimmunoassay of steroid hormones
Concentrations of P4 were analysed by RIA according to the method described by Ciereszko et al. (36, 37) and concentrations of A4 were determined according to the method of Dziadkowiec et al. (38). Cross-reactivities of the antiserum against P4 and A4 have been published previously (38, 39). The no-extraction assay was used to monitor P4 levels. Efficiency of extraction for the A4 assay was 85.3 ± 0.3%. The sensitivities of the assays for P4 and A4 were 1 pg/ml and 2.2 pg/ml, respectively. The standard curve range was from 1 pg/ml to 1500 pg/ml for P4, and from 1 pg/ml to 500 pg/ml for A4. The intra- and interassay coefficients of variation were 1% and 7.36% for P4, and 2.1% and 5.1% for A4, respectively.
Statistical analysis
Statistical analysis was performed using the Statistica program (StatSoft Inc., USA). All data are reported as the mean ± standard error of the mean from five independent observations. One-way analysis of variance and the Fisher’s least significant difference post hoc test was used to compare the effect of treatments on gene expression and steroid secretion. The model included the effect of adiponectin in two different concentrations, the effect of adiponectin in two different concentrations with insulin and the effect of insulin alone. One-way analysis of variance and the Fisher’s least significant difference post hoc test were also applied to analyse the effect of the oestrous cycle phase and pregnancy period on basal content of CYP11A1 mRNA. Values for P < 0.05 were considered statistically significant.
RESULTS
The effect of adiponectin on steroidogenic acute regulatory protein (StAR) gene expression in the endometrial and myometrial tissue explants
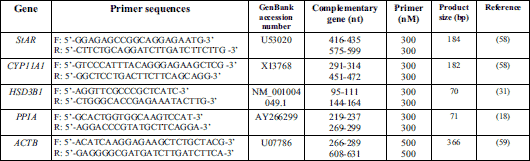
StAR gene expression was found in the endometrium and myometrium during the early stage of pregnancy from day 10 to day 28 and on days 10 to 11 of the oestrous cycle (Fig. 1A and 1B).
In the endometrial tissue explants, the stimulatory effect of adiponectin at a dose of 1 µg/ml and at both doses (1, 10 µg/ml) on StAR mRNA content was observed on days 12 to 13 and 15 to 16 of pregnancy, respectively. The inhibitory effect of adiponectin at a dose of 10 µg/ml on StAR mRNA expression was determined on days 10 to 11 and 27 to 28 of pregnancy. On days 10 to 11 of the oestrous cycle, the expression of StAR gene was inhibited by adiponectin at both doses (1, 10 µg/ml) (P < 0.05; Fig. 1A).
Insulin alone in relation to the control reduced StAR gene expression in the endometrium on days 10 to 11 of the oestrous cycle (P < 0.05). During all studied days of pregnancy, insulin had no effect on StAR mRNA content (Fig. 1A).
In the endometrial tissue, adiponectin (10 µg/ml) in combination with insulin stimulated StAR mRNA expression compared to insulin alone on days 10 to 11 of pregnancy. Similarly, adiponectin at a dose of 1 µg/ml with addition of insulin enhanced the gene expression on days 12 to 13 and 27 to 28 of pregnancy when compared with the insulin treatment (P < 0.05; Fig. 1A).
In the myometrial tissue explants, on days 10 to 11 and 27 to 28 of pregnancy, StAR gene expression was increased in response to adiponectin (1, 10 µg/ml). On days 12 to 13 of pregnancy and on days 10 to 11 of the cycle, adiponectin (10 µg/ml) decreased expression of StAR gene. Similarly, on days 15 to 16 of pregnancy, adiponectin at both doses (1, 10 µg/ml) suppressed StAR mRNA expression (P < 0.05; Fig 1B).
Insulin alone compared to the control diminished StAR mRNA content in the myometrium on days 12 to 13 of pregnancy (P < 0.05). In contrast, on days 27 to 28 of pregnancy, insulin enhanced the gene expression. On days 10 to 11 and 15 to 16 of pregnancy, and on days 10 to 11 of the cycle, insulin did not affect StAR gene expression (Fig 1B).
In the myometrium, adiponectin at a lower dose (1 µg/ml) in combination with insulin provoked an increase in StAR mRNA expression in comparison with insulin alone on days 10 to 11 and 27 to 28 of pregnancy, and on days 10 to 11 of the cycle. Similarly, on days 12 to 13 of pregnancy, adiponectin at a higher dose (10 µg/ml) with insulin significantly increased StAR mRNA content. The expression of StAR gene was inhibited by adiponectin (1, 10 µg/ml) in combination with insulin compared to the insulin treatment on days 15 to 16 of pregnancy (P < 0.05; Fig. 1B).
The effect of adiponectin on CYP11A1 gene expression in the endometrial and myometrial tissue explants
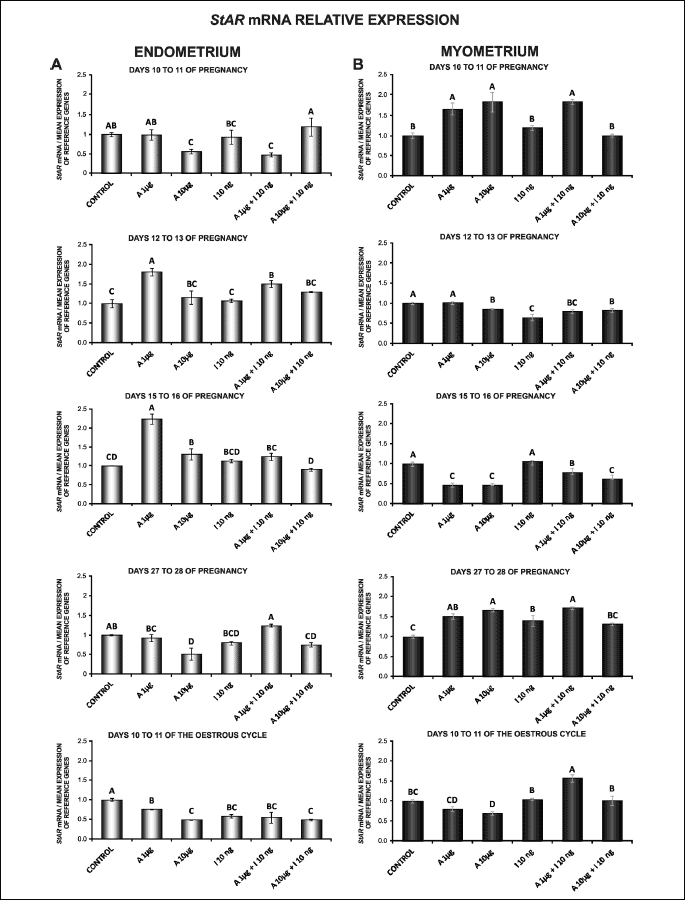
CYP11A1 mRNA content was reported in the endometrium and myometrium during all studied days of pregnancy and on days 10 to 11 of the oestrous cycle (Fig. 2A and 2B). In endometrial tissue explants, adiponectin at both doses (1, 10 µg/ml) caused a decrease in CYP11A1 mRNA content on days 10 to 11 and 15 to 16 of pregnancy, and on days 10 to 11 of the cycle. On days 12 to 13 of pregnancy, basal expression of CYP11A1 gene was also reduced by adiponectin (1 µg/ml) (P < 0.05; Fig. 2A).
Insulin alone in relation to the control enhanced CYP11A1 gene expression in the endometrium on days 12 to 13 and 27 to 28 of pregnancy. In contrast, on days 10 to 11 and 15 to 16 of pregnancy, and on days 10 to 11 of the cycle, insulin diminished the gene expression (P < 0.05; Fig. 2A).
In the endometrial tissue, adiponectin treatment at a higher dose (10 µg/ml) with insulin increased CYP11A1 mRNA content in relation to insulin alone on days 15 to 16 of pregnancy. On days 12 to 13 and 27 to 28 of pregnancy, under the influence of 1 and 10 µg/ml of adiponectin a significant decrease in the gene expression was observed in insulin-induced cultures compared to insulin alone (P < 0.05; Fig. 2A).
Treatment of the myometrial explants on days 10 to 11 of pregnancy and on days 10 to 11 of the cycle with adiponectin (10 µg/ml) resulted in significant increase in CYP11A1 mRNA content. On days 15 to 16 of pregnancy, adiponectin at a lower dose (1 µg/ml) provoked an increase in CYP11A1 gene expression. On days 12 to 13 of pregnancy, expression of CYP11A1 mRNA content was attenuated by adiponectin (10 µg/ml) (P < 0.05; Fig. 2B).
Insulin alone compared to the control stimulated CYP11A1 mRNA content in the myometrium on days 15 to 16 and 27 to 28 of pregnancy. On days 12 to 13 of pregnancy, insulin diminished the expression of CYP11A1 gene (P < 0.05). On days 10 to 11 of pregnancy and on days 10 to 11 of the cycle, the administration of insulin did not affect CYP11A1 mRNA expression (Fig. 2B).
In the myometrial explants, adiponectin (1, 10 µg/ml) in combination with insulin increased CYP11A1 gene expression in comparison with insulin alone on days 10 to 11 of pregnancy and on days 10 to 11 of the cycle. In contrast, on days 27 to 28 of pregnancy, the expression of CYP11A1 mRNA content was suppressed by adiponectin (1, 10 µg/ml) with insulin compared to insulin alone. A similar effect was caused by adiponectin (1 µg/ml) in combination with insulin on days 15 to 16 of pregnancy (P < 0.05; Fig. 2B).
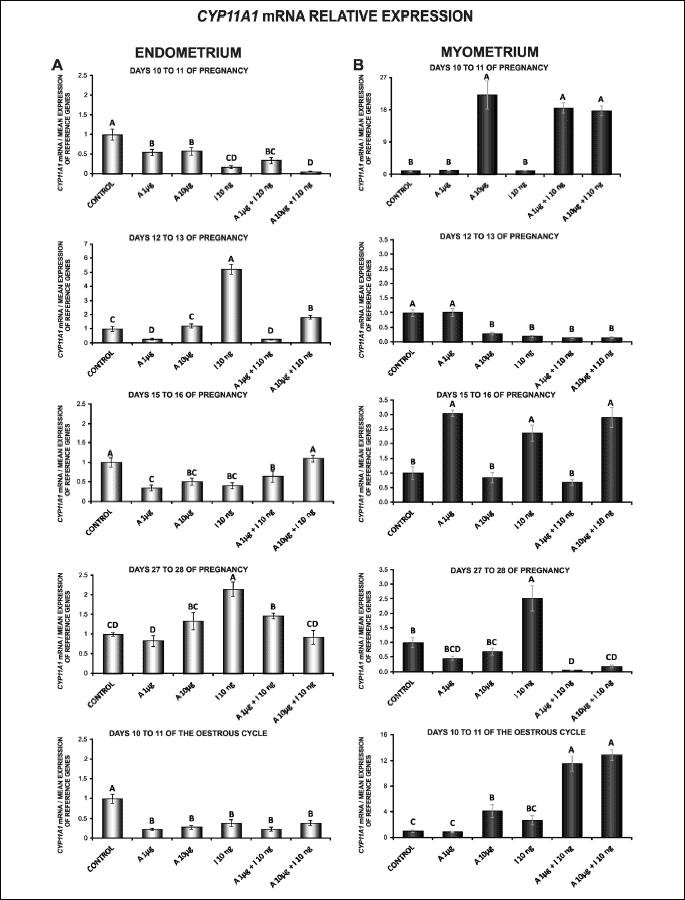
Basal expression of CYP11A1 gene in the endometrial and myometrial tissue explants on days 10 to 11 of the cycle and during pregnancy
In the endometrium, the expression of CYP11A1 gene was lower during all studied stages of pregnancy relative to days 10 to 11 of the porcine oestrous cycle. During pregnancy, CYP11A1 mRNA content was higher on days 10 to 11 of gestation compared to days 12 to 13, 15 to 16 and 27 to 28 of gestation (P < 0.05; Fig. 3A).
In the myometrium, the expression of CYP11A1 gene was lower on days 10 to 11 of oestrous cycle than during all studied stages of pregnancy (except for days 10 to 11). During pregnancy, the highest CYP11A1 mRNA expression was noted on days 27 to 28 of gestation, and the lowest on days 10 to 11 of gestation (P < 0.05; Fig. 3B).
The effect of adiponectin on 3β-hydroxysteroid dehydrogenase (HSD3B1) gene expression in the endometrial and myometrial tissue explants
HSD3B1 mRNA expression was observed in the endometrial and myometrial tissue explants during the early stage of pregnancy from day 10 to day 28 and on days 10 to 11 of the oestrous cycle (Fig. 4A and 4B).
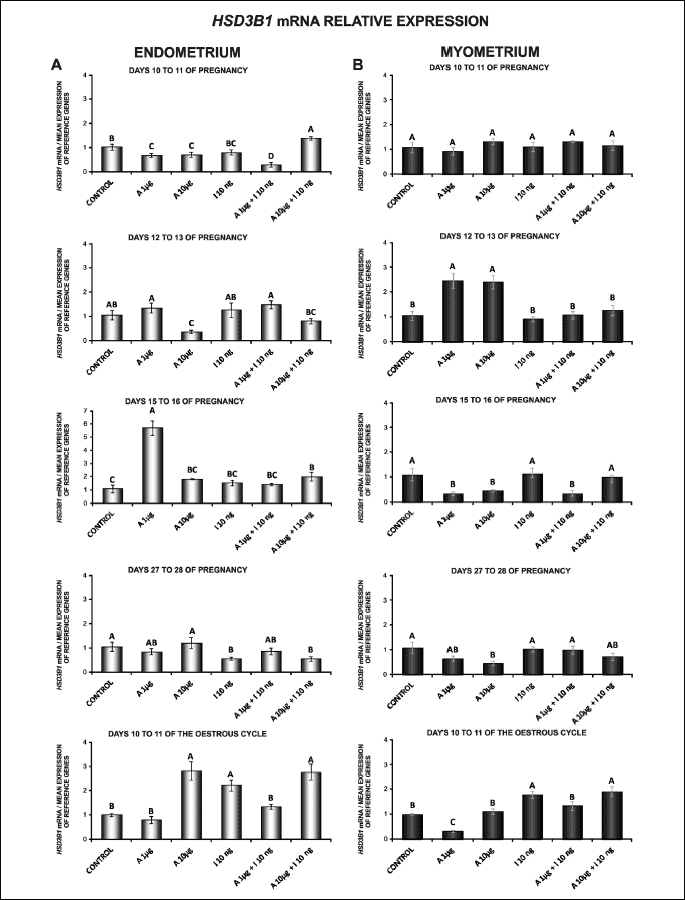
In the endometrium, on days 15 to 16 of pregnancy, HSD3B1 gene expression was enhanced by adiponectin at the concentration of 1 µg/ml. A similar effect was caused by adiponectin (10 µg/ml) on days 10 to 11 of the cycle. On days 10 to 11 of pregnancy, adiponectin at doses of 1 and 10 µg/ml reduced HSD3B1 mRNA expression. Adiponectin at a higher dose (10 µg/ml) decreased HSD3B1 mRNA content on days 12 to 13 of pregnancy (P < 0.05; Fig. 4A).
Insulin alone compared to the control increased HSD3B1 mRNA content in the endometrium on days 10 to 11 of the cycle. On days 27 to 28 of pregnancy, insulin decreased the gene expression (P < 0.05). There was no effect of insulin on the content of HSD3B1 mRNA in these explants on days 10 to 11, 12 to 13 and 15 to 16 of pregnancy (Fig. 4A).
In the endometrium, on days 10 to 11 of pregnancy, adiponectin at a higher dose (10 µg/ml) caused an increase in insulin-stimulated HSD3B1 gene expression in relation to insulin alone, while administration of adiponectin at a lower dose (1 µg/ml) with insulin resulted in significant decrease in the expression. On days 10 to 11 of the cycle, adiponectin (1 µg/ml) with insulin diminished HSD3B1 mRNA expression (P < 0.05; Fig. 4A).
In the myometrial explants, adiponectin at both doses (1, 10 µg/ml) increased HSD3B1 mRNA content on days 12 to 13 of pregnancy. On days 15 to 16 of pregnancy, under the influence of 1 and 10 µg/ml of adiponectin a significant decrease in basal HSD3B1 mRNA expression was observed. Adiponectin at a higher dose (10 µg/ml) on days 27 to 28 of pregnancy, and adiponectin at a lower dose (1 µg/ml) on days 10 to 11 of the cycle decreased HSD3B1 gene expression (P < 0.05; Fig. 4B).
Insulin alone in relation to the control increased HSD3B1 mRNA expression in the myometrium on days 10 to 11 of the cycle (P < 0.05). During all studied days of pregnancy, insulin had no effect on the gene expression (Fig. 4B).
In the myometrial tissue explants, on days 15 to 16 of pregnancy and on days 10 to 11 of the cycle, adiponectin at a dose of 1 µg/ml with insulin attenuated HSD3B1 mRNA expression compared to insulin alone (P < 0.05; Fig. 4B).
The effect of adiponectin on P4 secretion by the endometrial and myometrial tissues explants
In endometrial explants, on days 10 to 11 and 27 to 28 of pregnancy, the secretion of P4 was enhanced in response to 10 µg/ml of adiponectin. On days 12 to 13 of pregnancy, the addition of this treatment at a dose of 1 µg/ml caused an increase in P4 production. On days 10 to 11 of the cycle, adiponectin at a higher dose (10 µg/ml) decreased P4 release (P < 0.05; Fig. 5A).
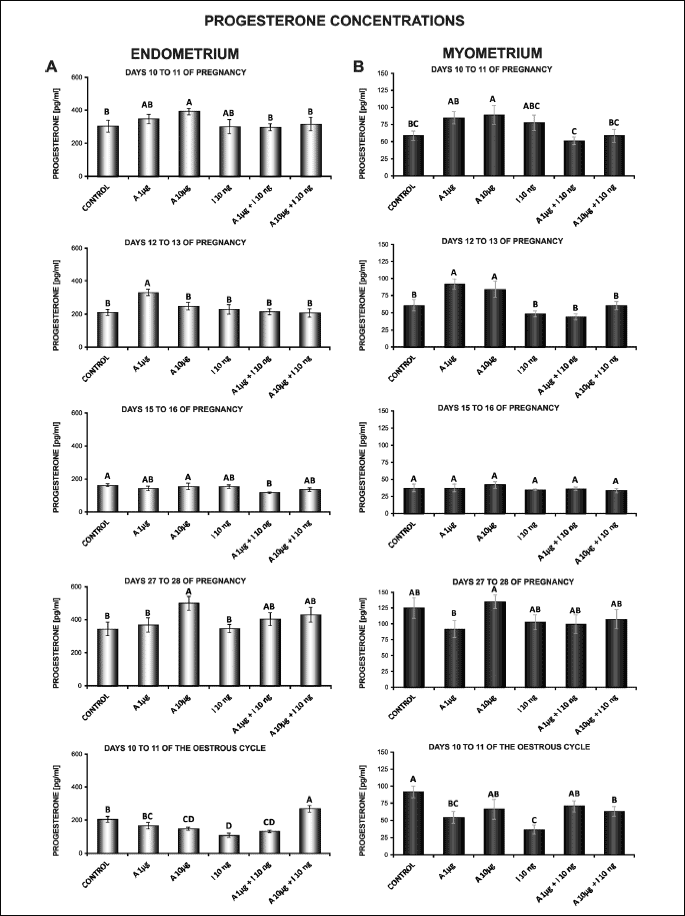
Insulin alone compared to the control decreased P4 release in the endometrium on days 10 to 11 of the cycle (P < 0.05). During all studied days of pregnancy, insulin did not affect P4 output (Fig. 5A).
In the endometrial tissue explants, on days 10 to 11 of the cycle, the secretion of P4 was stimulated by adiponectin (10 µg/ml) in combination with insulin compared to insulin alone (P < 0.05). During all studied days of pregnancy, the effect of adiponectin at both doses (1, 10 µg/ml) on insulin-stimulated P4 production was non-significant (Fig. 5A).
In the myometrium, adiponectin at a higher dose (10 µg/ml) on days 10 to 11 of pregnancy and at both doses (1, 10 µg/ml) on days 12 to 13 of pregnancy provoked an increase in P4 secretion. On days 10 to 11 of the cycle, adiponectin at a lower dose (1 µg/ml) reduced P4 production (P < 0.05; Fig. 5B).
Insulin alone in relation to the control decreased P4 production in the myometrium on days 10 to 11 of the cycle (P < 0.05). During all studied days of pregnancy, insulin did not affect the release (Fig. 5B).
In the myometrial tissue explants, on days 10 to 11 of the cycle, adiponectin at both doses (1, 10 µg/ml) with insulin enhanced the secretion of steroid compared to insulin alone (P < 0.05). During pregnancy, the release of P4 was not changed in response to adiponectin (1, 10 µg/ml µg/ml) with insulin (Fig. 5B).
The effect of adiponectin on A4 secretion by the endometrial and myometrial tissue explants
In the endometrium, adiponectin at both concentrations (1, 10 µg/ml) decreased basal secretion of A4 on days 10 to 11 of pregnancy and on days 10 to 11 of the cycle (P < 0.05; Fig. 6A).
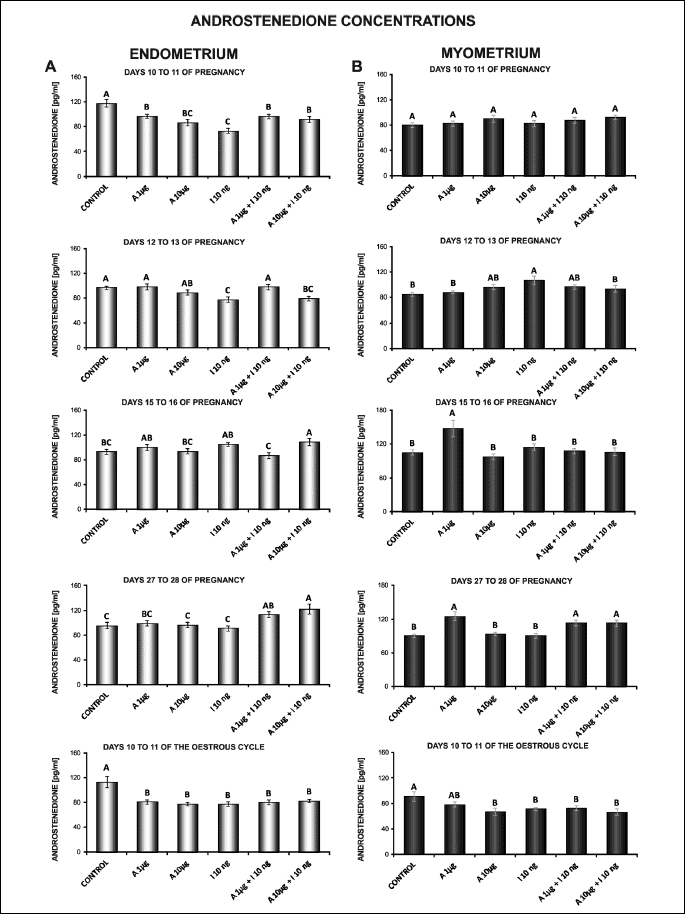
Insulin alone compared to the control diminished A4 production in the endometrium on days 10 to 11 and 12 to 13 of pregnancy, and on days 10 to 11 of the cycle (P < 0.05). On days 15 to 16 and 27 to 28 of pregnancy, A4 release was not changed under insulin influence (Fig. 6A).
Treatment of the endometrial explants on days 10 to 11 and 27 to 28 of pregnancy with adiponectin (1, 10 µg/ml) and insulin in relation to insulin alone caused an increase in A4 secretion. Adiponectin at a lower dose (1 µg/ml) in combination with insulin increased A4 secretion on days 12 to 13 of pregnancy, and decreased the steroid production on days 15 to 16 of pregnancy (P < 0.05; Fig. 6A).
In the myometrial tissue explants, on days 15 to 16 and 27 to 28 of pregnancy, basal secretion of A4 was enhanced by a lower dose of adiponectin (1 µg/ml). On days 10 to 11 of the cycle, adiponectin at a higher dose (10 µg/ml) decreased A4 production (P < 0.05; Fig. 6B).
Insulin alone compared to the control increased A4 secretion on days 12 to 13 of pregnancy and decreased the production of A4 on days 10 to 11 of the cycle (P < 0.05). On days 10 to 11, 15 to 16 and 27 to 28 of pregnancy, insulin had no effect on A4 release (Fig. 6B).
In the myometrium, insulin-induced secretion of A4 was enhanced by both doses of adiponectin (1, 10 µg/ml) in relation to insulin alone on days 27 to 28 of pregnancy. On days 12 to 13 of pregnancy, adiponectin (10 µg/ml) with insulin diminished A4 production (P < 0.05; Fig. 6B).
DISCUSSION
To our knowledge, this is the first report of the influence of adiponectin and insulin on uterine steroidogenesis. Our results demonstrate that adiponectin modulates basal and insulin-induced expression of StAR, CYP11A1 and HSD3B1 genes and secretion of P4 and A4 by endometrial and myometrial tissue explants harvested from pregnant pigs on days 10 to 11, 12 to 13, 15 to 16 and 27 to 28 of gestation, and from cyclic pigs on days 10 to 11 of the cycle. In the present study, we have shown, for the first time, the expression of CYP11A1 mRNA in the porcine uterus during the early stage of pregnancy and the mid-luteal phase of the oestrous cycle. In the endometrium, the expression of CYP11A1 gene was higher on days 10 to 11 of the cycle compared to all tested days of pregnancy. In contrast, in the myometrium, CYP11A1 mRNA content was higher during pregnancy than on days 10 to 11 of the cycle. The expression of HSD3B1 gene and P4 secretion in endometrial tissue explants was more pronounced on days 10 to 11 of pregnancy than on days 10 to 11 of the oestrous cycle. During pregnancy, the highest StAR, CYP11A1 and HSD3B1 mRNA expression and P4 and A4 release was detected on days 10 to 11 of gestation. In the myometrium, the highest StAR gene expression was found on days 15 to 16 of pregnancy, CYP11A1 mRNA content on days 27 to 28 and HSD3B1 gene expression on days 15 to 16 and 27 to 28 of pregnancy. The production of P4 was the highest on days 27 to 28 of gestation, whereas the release of A4 on days 15 to 16 of pregnancy (StAR, HSD3B1, P4 and A4 data not shown). Our results disagree with the studies of HSD3B1 gene expression, P4 and A4 production levels obtained previously by Wojciechowicz et al. (31). Disagreement can be result of methodological differences. Their results were obtained on endometrial and myometrial slices weighing 200 – 210 mg and the incubation time was 12 hours.
The expression of key steroidogenic enzymes was found earlier in the uterus. Indeed, the presence of StAR, CYP11A1 and HSD3B1 transcripts was reported in the human endometrium (32, 33). In mice, Ben-Zimra et al. (40) reported the gene expression of CYP11A1 and HSD3B1 in the endometrium during pregnancy. In pigs, the expression of StAR gene was found in the endometrium on days 12 and 16 of the oestrous cycle and pregnancy (34). It has been documented that in pigs, the expression of HSD3B1 gene and the activity of 3β-hydroxysteroid dehydrogenase were different in the endometrium and myometrium during early pregnancy and the oestrous cycle (31). Here we demonstrated, the expression of StAR gene, which facilitates the entry of cytosolic cholesterol into the mitochondrion, mRNA content of CYP11A1, which catalyses conversion of cholesterol to pregnenolone, and HSD3B1 gene expression, converting dehydroepiandrosterone into A4 and pregnenolone into P4, in the porcine endometrium and myometrium during early pregnancy and the mid-luteal phase of the cycle. In the present study, we observed that the influence of adiponectin on expression of studied steroidogenic genes depends on the type of tissue, stage of pregnancy and presence of insulin.
Steroid hormones are essential for the establishment of normal endometrial receptivity, the recognition of pregnancy and the successful establishment of implantation. Adiponectin is considered to be important modulator of these processes. As indicated in our previous study, the higher porcine plasma concentrations of adiponectin were observed on days 2 to 3, 10 to 12 and 14 to 16 of the oestrous cycle relative to the follicular phase (20). Our unpublished studies have shown the highest level of adiponectin in porcine plasma on days 15 to 16 and 27 to 28 of pregnancy compared to days 10 to 11 of the cycle and days 10 to 11, 12 to 13 and 30 to 32 of pregnancy. Our recent studies have shown that the adiponectin system (genes and proteins) is present in the porcine endometrium and myometrium during the oestrous cycle and early pregnancy (26, 27). In the endometrium, adiponectin protein concentration was higher on days 12 to 13 of gestation (which is when recognition of pregnancy occurs in pigs) than on days 10 to 11, 15 to 16 and 27 to 28 of gestation. The highest AdipoR2 protein content was found during implantation (days 15 to 16). The high expression of AdipoR1 and AdipoR2 mRNAs in the porcine endometrium was also reported by Lord et al. (18).
Adiponectin and both receptors genes and proteins were detected in the uterus of pregnant rabbits and mice during the peri-implantation period. Before implantation, adiponectin protein was observed mainly in glandular and luminal epithelial cells, as well as in the stromal cells of rabbit endometrium and in the epithelial cells of mouse uterus. During implantation, the expression of adiponectin and its receptor in the endometrium of rabbits and adiponectin protein within the endometrium of mice was significantly higher in implantation sites (localized mainly in decidual cells) than in interimplantation sites (localized mainly in the epithelial cells) (25, 41). The termination of delayed implantation elevated the expression of adiponectin and both receptors in mice luminal epithelia, which suggests that adiponectin system expression is important for blastocyst activation and uterine receptivity. The stimulatory effect of decidualization on the expression of the adiponectin system in mice uterine tissue was confirmed by using artificial decidualization and an in vitro decidualization system (25). An increase of both adiponectin receptors in human endometrial cells (both stromal and epithelial) during the decidualization process was also demonstrated (42). The previously mentioned results suggest that adiponectin signalling by an auto/paracrine mechanism may play a significant role in embryo development and decidualization. During the implantation window, the expression of AdipoR1 and AdipoR2 was decreased in the endometrium of infertile women compared to the fertile control group. These results suggest that adiponectin plays a role in human uterine receptivity, and this observation may constitute a novel factor for prediction of implantation failure (43). The aforementioned results provided further evidence that adiponectin may play an essential role in pregnancy recognition and implantation.
The de novo synthesis of steroids in the uterus might be a crucial factor for effective implantation and maintenance of pregnancy. Available data indicate that uterine receptivity for conceptus implantation requires actions of P4 and/or oestrogens on the uterus to modulate locally produced growth factors, cytokines and prostaglandins through auto- and paracrine pathways (44). P4 concentration remained fairly unchanged throughout pregnancy; however, not statistically significant decrease was found in the mean P4 level in the porcine peripheral blood plasma from days 14 to days 28 of pregnancy (45, 46). A downward trend, although no significant, was also observed in the P4 concentration in peripheral blood measured in the jugular vein, in uterine arteries close to the ovary as well as close to the cervix in pigs from days 12 to 25 of pregnancy. Moreover, the level of P4 in the branch of uterine artery close to the ovary and close to the cervix was significantly greater than in the jugular vein on days 12 of pregnancy (47). It was reported that endometrial secretion of P4 (31) and the concentration of P4 in uterine washings (48) were higher in pregnant pigs than in cyclic ones on days 12 to 13, when maternal recognition of pregnancy in pigs occurs, which suggests that P4 found in uterine arteries may be derived not only from the ovaries, but also from uterine tissues. In our study, the expression of StAR gene and secretion of endometrial P4 were stimulated by adiponectin at a lower dose on days 12 to 13 of gestation. In the myometrium, during this stage of pregnancy, adiponectin at both doses increased HSD3B1 mRNA content and P4 release. On days 10 to 11 of pregnancy, adiponectin enhanced P4 production in the endometrium, and StAR, CYP11A1 gene expression and P4 release in the myometrium. In our previous study, in the myometrium, the highest concentration of AdipoR1 protein was found on days 12 to 13 of pregnancy, and the highest expression of AdipoR2 protein was detected on days 10 to 11 and 12 to 13 of gestation (27). Thus, our findings suggest that adiponectin may contribute to a higher level of P4 in the uterus during maternal recognition of pregnancy and to an elevated concentration of P4 in porcine peripheral blood plasma before day 14 of pregnancy.
A4 is converted to E1 by P450arom and is the major circulating androgen in pigs (49). It has been proposed that in pigs, uterine-derived A4 may serve as a substrate for oestrogens production mainly around days 12 to 13 of both pregnancy and the oestrous cycle and that porcine gravid uterus on days 15 to 16 is a higher source of A4 than the cyclic uterus (31). Concentration of A4 in porcine uterine fluid was also greater than respective plasma values of pregnant gilts between days 9 and 15 (48). Two phases of oestrogens secretion are essential for establishment of pregnancy. Pig conceptuses secrete oestrogens between days 10 to 12 of gestation providing the initial signal for maternal recognition of pregnancy in pig (50), and between days 15 and 25 to 30 of gestation (51). We observed that StAR mRNA expression and A4 production were enhanced by adiponectin with insulin compared to insulin alone in the endometrium on days 10 to 11 of pregnancy (10 µg/ml) and on days 12 to 13 (1 µg/ml) of pregnancy. During implantation, adiponectin (1 µg/ml) stimulated StAR and HSD3B1 genes expression in the endometrium, which correlates with the highest level of adiponectin in porcine plasma (our not published results) and the concentration of AdipoR2 protein in the endometrium presented in our previous studies (27). In the myometrium, adiponectin increased CYP11A1 mRNA content and A4 secretion. On days 27 to 28 of pregnancy, adiponectin and/or adiponectin with insulin enhanced StAR gene expression and A4 release in the uterus. We propose that adiponectin and/or adiponectin with insulin have a positive effect on the level of A4 , which could be a substrate for oestrogens in the uterus during early pregnancy. Based on the current study, we suggest that adiponectin modulates the local expression of steroidogenic enzymes and secretion of P4 and A4, which may be important for maternal recognition of pregnancy and implantation.
The above results are in agreement with previously published studies on other steroidogenic tissues such as adrenal cells and Leydig cells. Adiponectin has been shown to increase steroid production through increase in StAR expression (52, 53). However, in human Leydig cells, it has been also shown that a repressive action by adiponectin at a low dose on cAMP-dependent CYP11A1 promoter activity did not translate into decreased P4 levels (53). Here we also reported that steroidogenic genes expression was not correlated each other and to steroid hormones production as well. Such discrepancy may be a result of the absence of correlations between the protein concentration and gene transcript. This phenomenon could be attributed to transcriptional and post-transcriptional regulation (RNA processing and stability), differences in mRNA and protein stability and functioning feedbacks that suppress mRNA expression through high protein concentrations and attenuate post-transcriptional processes through high levels of gene expression. The absence of correlations between the protein concentration and gene expression of StAR was found by Blomberg and Zuelke (54) in developing porcine conceptuses. The authors suggested that StAR is regulated post-transcriptionally, either through mRNA stability, translation, or protein degradation. It cannot be ruled out that a similar phenomenon occurs in porcine uterus. Moreover, these inconsistencies may also derive from the activity of steroidogenic enzymes that can be modified post-translationally.
Specific binding sites for insulin have been identified in human endometrium (55). To our knowledge, there are very few studies investigating insulin’s effect on the uterus. It has been previously shown that insulin stimulates proliferation of mouse endometrial epithelial cell (56). In porcine endometrial epithelial cells, insulin has been demonstrated to stimulate transepithelial sodium transport by activation of a protein phosphatase that increases Na-K ATPase activity (57). Besides the fact that insulin modulated the effect of adiponectin on the expression of steroidogenic enzymes and secretion of hormones, our results revealed for the first time the influence of insulin itself on the steroidogenic activity of the uterus in mammals.
In summary, we have shown, for the first time to our knowledge, the expression of CYP11A1 gene in the porcine endometrium and myometrium during early pregnancy and the oestrous cycle. Our present observations are the first to disclose the role of adiponectin and insulin in the steroidogenic activity of the uterus. We found that adiponectin and insulin alone regulates the expression of StAR, CYP11A1 and HSD3B1 genes and secretion of P4 and A4 by the porcine endometrial and myometrial tissue explants during early pregnancy and the oestrous cycle. In this action adiponectin interacts with insulin. We documented that the influence of adiponectin on uterine steroidogenesis depends on the reproductive status, type of tissue and dose of adipokine. The present work provides new elements towards understanding the role of adiponectin within the porcine uterus. Most authors agree that 30 to 40% of porcine embryos are lost between days 12 to 18 of pregnancy. This critical period for embryos has not been fully understood. Periodic expression of genes for many factors controlling effective maternal recognition of pregnancy and implantation takes place during that time in various uterine structures. We proposed that adiponectin belongs to the group of factors that affect these processes by controlling the synthesis of steroid hormones.
Acknowledgements: This research was supported by National Science Centre (projects no: 2011/03/B/NZ9/04187).
Conflict of interests: None declared.
REFERENCES
- Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941-946.
- Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002; 8: 731-737.
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 1996; 120: 803-812.
- Hada Y, Yamauchi T, Waki H, et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun 2007; 356: 487-493.
- Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79-83.
- Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab 2004; 89: 1391-1396.
- Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 2001; 86: 3815-3819.
- Dranka-Bojarowska D, Lekstan A, Olakowski M, et al. The assessment of serum concentration of adiponectin, leptin and serum carbohydrate antigen-19.9 in patients with pancreatic cancer and chronic pancreatitis. J Physiol Pharmacol 2015; 66: 653-663.
- Kalisz M, Baranowska B, Wolinska-Witort E, et al. Total and high molecular weight adiponectin levels in the rat model of post-myocardial infarction heart failure. J Physiol Pharmacol 2015; 66: 673-680.
- Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423: 762-769.
- Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes 2010; 59: 1161-1170.
- Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction 2005; 130: 583-597.
- Campos DB, Palin MF, Bordignon V, Murphy BD. The ‘beneficial’ adipokines in reproduction and fertility. Int J Obes (Lond) 2008; 32: 223-231.
- Kos K, Harte AL, da Silva NF, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab 2007; 92: 1129-1136.
- Kaminski T, Smolinska N, Maleszka A, et al. Expression of adiponectin and its receptors in the porcine hypothalamus during the oestrous cycle. Reprod Domest Anim 2014; 49: 378-386.
- Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, et al. Regulation of pituitary cell function by adiponectin. Endocrinology 2007; 148: 401-410.
- Kiezun M, Maleszka A, Smolinska N, Nitkiewicz A, Kaminski T. Expression of adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) in the porcine pituitary during the oestrous cycle. Reprod Biol Endocrinol 2013; 11: 18. doi: 10.1186/1477-7827-11-18
- Lord E, Ledoux S, Murphy BD, Beaudry D, Palin MF. Expression of adiponectin and its receptors in swine. J Anim Sci 2005; 83: 565-578.
- Chabrolle C, Tosca L, Rame C, Lecomte P, Royere D, Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril 2009; 92: 1988-1996.
- Maleszka A, Smolinska N, Nitkiewicz A, et al. Expression of adiponectin receptors 1 and 2 in the ovary and concentration of plasma adiponectin during the oestrous cycle of the pig. Acta Vet Hung 2014; 22: 1-11.
- Maleszka A, Smolinska N, Nitkiewicz A, et al. Adiponectin expression in the porcine ovary during the oestrous cycle and its effect on ovarian steroidogenesis. Int J Endocrinol 2014; 2014: 957076. doi: 10.1155/2014/957076.
- Wen JP, Lv WS, Yang J, et al. Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun 2008; 371: 756-761.
- Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 2006; 147: 5178-5186.
- Takemura Y, Osuga Y, Yamauchi T, et al. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 2006; 147: 3203-3210.
- Kim ST, Marquard K, Stephens S, Louden E, Allsworth J, Moley KH. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum Reprod 2011; 26: 82-95.
- Smolinska N, Dobrzyn K, Maleszka A, Kiezun M, Szeszko K, Kaminski T. Expression of adiponectin and adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) in the porcine uterus during the oestrous cycle. Anim Reprod Sci 2014; 146: 42-54.
- Smolinska N, Maleszka A, Dobrzyn K, Kiezun M, Szeszko K, Kaminski T. Expression of adiponectin and adiponectin receptors 1 and 2 in the porcine uterus, conceptus, and trophoblast during early pregnancy. Theriogenology 2014; 82: 951-965.
- Knight JW, Jeantet MA. Effects of pregnenolone, cyclic adenosine monophosphate, and human chorionic gonadotropin on in vitro progesterone and estrone synthesis by the porcine placenta and endometrium. Domest Anim Endocrinol 1991; 8: 331-341.
- Franczak A, Kotwica G. Secretion of estradiol-17beta by porcine endometrium and myometrium during early pregnancy and luteolysis. Theriogenology 2008; 69: 283-289.
- Franczak A. Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs. Reprod Biol 2008; 8: 213-228.
- Wojciechowicz B, Kotwica G, Kolakowska J, Franczak A. The activity and localization of 3β-hydroxysteroid dehydrogenase/D(5)-D(4) isomerase and release of androstenedione and progesterone by uterine tissues during early pregnancy and the estrous cycle in pigs. J Reprod Dev 2013; 59: 49-58.
- Rhee HS, Oh SH, Ko BJ, et al. Expression of 3beta-hydroxysteroid dehydrogenase and P450 side chain cleavage enzyme in the human uterine endometrium. Exp Mol Med 2003; 35: 160-166.
- Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab 2009; 94: 623-631.
- Kiewisz J, Krawczynski K, Lisowski P, et al. Global gene expression profiling of porcine endometria on days 12 and 16 of the estrous cycle and pregnancy. Theriogenology 2014; 82: 897-909.
- Akins EL, Morrissette MC. Gross ovarian changes during estrouscycle of swine. Am J Vet Res 1968; 29: 1953-1957.
- Ciereszko RE, Petroff BK, Ottobre AC, Guan Z, Stokes BT, Ottobre JS. Assessment of the mechanism by which prolactin stimulates progesterone production by early corpora lutea of pigs. J Endocrinol 1998; 159: 201-209.
- Kurzynska A, Bogacki M, Chojnowska K, Bogacka I. Peroxisome proliferator activated receptor ligands affect progesterone and 17b-estradiol secretion by porcine corpus luteum during early pregnancy. J Physiol Pharmacol 2014; 65: 709-717.
- Dziadkowiec I, Danilczyk U, Rembiesa R. Biosynthesis of progesterone in the rat placenta. Endokrynologia Polska 1982; 33: 201-207.
- Szafranska B, Ziecik A, Okrasa S. Primary antisera against selected steroids or proteins and secondary antisera against g-globulins - as available tool for studies of reproductive processes. Reprod Biol 2002; 2: 187-204.
- Ben-Zimra M, Koler M, Melamed-Book N, Arensburg J, Payne AH, Orly J. Uterine and placental expression of steroidogenic genes during rodent pregnancy. Mol Cell Endocrinol 2002; 187: 223-231.
- Schmidt T, Fischer S, Tsikolia N, et al. Expression of adipokines in preimplantation rabbit and mice embryos. Histochem Cell Biol 2008; 129: 817-825.
- Gamundi-Segura S, Serna J, Oehninger S, Horcajadas JA, Arbones-Mainar JM. Effects of adipocyte-secreted factors on decidualized endometrial cells: modulation of endometrial receptivity in vitro. J Physiol Biochem 2015; 71: 537-546.
- Dos Santos E, Serazin V, Morvan C, et al. Adiponectin and leptin systems in human endometrium during window of implantation. Fertil Steril 2012; 97: 771-778.e1.
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction 2009; 138: 195-209.
- Tillson SA, Erb RE. Progesterone concentration in peripheral blood plasma of the domestic sow prior to and during early pregnancy. J Anim Sci 1967; 26: 1366-1368.
- Robertson HA, King GJ. Plasma concentrations of progesterone, oestrone, oestradiol-17beta and of oestrone sulphate in the pig at implantation, during pregnancy and at parturition. J Reprod Fertil 1974; 40: 133-141.
- Stefanczyk-Krzymowska S, Grzegorzewski W, Wasowska B, Skipor J, Krzymowski T. Local increase of ovarian steroid hormone concentration in blood supplying the oviduct and uterus during early pregnancy of sows. Theriogenology 1998; 50: 1071-1080.
- Stone BA, Seamark RF. Steroid hormones in uterine washings and in plasma of gilts between days 9 and 15 after oestrus and between days 9 and 15 after coitus. J Reprod Fertil 1985; 75: 209-221.
- Simpson ER, Clyne C, Speed C, Rubin G, Bulun S. Tissue-specific estrogen biosynthesis and metabolism. Ann NY Acad Sci 2001; 949: 58-67.
- Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod 1982; 27: 925-939.
- Geisert RD, Zavy MT, Moffatt RJ, Blair RM, Yellin T. Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil Suppl 1990; 40: 293-305.
- Ramanjaneya M, Conner AC, Brown JE, et al. Adiponectin (15-36) stimulates steroidogenic acute regulatory (StAR) protein expression and cortisol production in human adrenocortical cells: role of AMPK and MAPK kinase pathways. Biochim Biophys Acta 2011; 1813: 802-809.
- Landry D, Pare A, Jean S, Martin LJ. Adiponectin influences progesterone production from MA-10 Leydig cells in a dose-dependent manner. Endocrine 2015; 48: 957-967.
- Blomberg LA, Zuelke KA. Expression analysis of the steroidogenic acute regulatory protein (STAR) gene in developing porcine conceptuses. Mol Reprod Dev 2005; 72: 419-429.
- Nagamani M, Stuart CA, Dunhardt PA, Doherty MG. Specific binding sites for insulin and insulin-like growth factor I in human endometrial cancer. Am J Obstet Gynecol 1991; 165: 1865-1871.
- Shiraga M, Takahashi S, Miyake T, Takeuchi S, Fukamachi H. Insulin-like growth factor-I stimulates proliferation of mouse uterine epithelial cells in primary culture. Proc Soc Exp Biol Med 1997; 215: 412-417.
- Deachapunya C, Palmer-Densmore M, O’Grady SM. Insulin stimulates transepithelial sodium transport by activation of a protein phosphatase that increases Na-K ATPase activity in endometrial epithelial cells. J Gen Physiol 1999; 114: 561-574.
- Rusovici R, Hui YY, Lavoie HA. Epidermal growth factor-mediated inhibition of follicle-stimulating hormone-stimulated StAR gene expression in porcine granulosa cells is associated with reduced histone H3 acetylation. Biol Reprod 2005; 72: 862-871.
- Spagnuolo-Weaver M, Fuerst R, Campbell ST, et al. A fluorimeter-based RT-PCR method for the detection and quantitation of porcine cytokines. J Immunol Methods 1999; 230: 19-27.
A c c e p t e d : April 29, 2016