THE ROLE OF mTOR INHIBITORS AND HMG-CoA REDUCTASE INHIBITORS
ON YOUNG AND OLD ENDOTHELIAL CELL FUNCTIONS, CRITICAL
FOR
RE-ENDOTHELIALISATION AFTER PERCUTANEOUS CORONARY INTERVENTION:
AN IN VITRO STUDY
INTRODUCTION
In recent decades percutaneous coronary intervention (PCI) with stent implantation has been the predominant surgical procedure in patients with acute coronary syndromes (1, 2). Stent implantation has resulted in significant vessel remodelling at the site of intervention, leading to neointima formation (3). Inoue et al. reported that in vascular injury bone marrow-derived cells may differentiate into endothelial cells and also into smooth muscle cells, leading to both re-endothelialisation and neointimal thickening and restenosis (4). Even in successful PCI procedures, more than twenty percent of patients required additional invasive procedures over the following 3 years (2). To stop smooth muscle cell proliferation in intima, drug-eluting stents (DES) were used. The first generation of DES used sirolimus (rapamycin) and paclitaxel as antiproliferative agent. The second generation of DES, use sirolimus derivatives (everolimus, zotarolimus) (5). Despite stent improvement, in-stent re-endothelialisation is still insufficient. The mechanism of the inhibition of cell proliferation by rapamycin and its derivatives consists in the inhibition of mTOR kinase (mammalian target of rapamycin). mTOR kinase is responsible for cell proliferation, migration, regulation of translation and transcription and inhibition of the immune response. The effects of antimitotic drugs used in DES do not always seem to be beneficial to the vascular endothelium. They accelerate the aging process, potentiate stent thrombosis and suppress the proliferation of endothelial cells (6-8).
Statin therapy is also one of the pharmacological methods which prevent restenosis (9). In animal models stents coated with statins reduced the neointimal formation and improved endothelial function (10). The reduction of neointima is based on the inhibition of smooth muscle cell proliferation (11) but, depending on their concentration statins may also affect the endothelial cell proliferation (12, 13). Besides the prevention of restenosis, statins promote re-endothelialisation by increasing the number of circulating progenitor cells which colonize denuded parts of the vessel (11).
Aging is a relevant risk factor in any cardiovascular disease. Elderly people constitute a major group of patients treated with PCI and are much more prone to complications after angioplasty, owing to the senescence of the cardiovascular system (14-16). Cessation of replication is the hallmark of senescence (17). Although senescent cells stop proliferating, they remain metabolically active and synthesize high amounts of proinflammatory cytokines changing the local tissue homeostasis (18). Progressive accumulation of senescent endothelial cells, deregulation in cytokine production and the reduction in the number of circulating endothelial progenitor cells during the aging process all affect vascular wound healing (19). Senescent endothelial cells were found in arterial vessels in patients with atherosclerosis (20), coronary artery disease (21), and those exposed to various cardiovascular risk factors (22). Senescence of either vascular endothelial cells or endothelial progenitor cells is one of the most important reasons for restenosis in elderly patients who are particularly more prone to complications after the PCI procedure when compared with young patients (15).
Recently, we have demonstrated a model for the regeneration of young and old vascular endothelial cells after sustaining injuries mimicking vascular damage after PCI (23). Using permanent cell line HUVEC line EA.hy926 (24) we have also demonstrated that atorvastatin (AT) at clinically relevant doses does not affect vascular endothelial cell wound healing in vitro (12). Therefore, in the present paper we have focused on pharmacological methods preventing restenosis and their impact on young and old endothelial cell functions, critical for re-endothelialisation after percutaneous coronary intervention (PCI). Using young and old endothelial cells we have tested how acute exposure to atorvastatin, sirolimus and everolimus, at clinically relevant doses, affects wound healing in an in vitro model mimicking endothelial injuries occurring during angioplasty.
MATERIALS AND METHODS
Cell culture
in vitro studies were performed with pooled primary human umbilical endothelial cells purchased from Clonetics (Lonza, Switzerland). Cells were propagated in HEPES (25 mM) buffered M199 culture medium, supplemented with L-glutamine (2 mM), amphotericin (2.5 µg/ml), gentamycin (50 µg/ml), hydrocortisone (1 µg/ml ), heparin (10 U/ml), EGF (10 ng/ml), and 15% v/v foetal calf serum. All reagents were from Sigma (St. Louis, MO, USA) and cell culture plastics were from Costar (USA).
Experimental design
Young and old endothelial cells were exposed to the cultured medium supplemented in mTOR inhibitors sirolimus (SR), everolimus (EV) and in HMG-CoA reductase inhibitor atorvastatin (AT) in the concentration obtained in serum after stent implantation (SR, EV) and (25, 26) typical medical treatment before PCI (AT) (27). We used the following concentration of the tested drugs: SR and EV 1 – 100 nmol/l and 0.01 – 0.1 µmol/l for AT.
Induction of senescence
Replicative senescence was induced by serial passages of HUVECs (23). Briefly, upon reaching confluency, cells from the culture were harvested using a tripsin-EDTA solution (0.05 – 0.02%) and seeded into a new culture flask at a density of 5000 cells/cm2. The next passages were performed at 5 – 9 day intervals with the same seeding density. The passages were carried out until cells (i) remained the same in their number for 4 weeks, (ii) exhibited a senescent morphology (irregular shape, hypertrophy, vacuolization), (iii) more than 70% of senescent endothelial cells stained positive for senescence-associated b-galactosidase (SA-β-Gal). Endothelial cells, after serial passages, reached final population doublings (PD) which resulted in cell senescence. The number of PDs was calculated using the equation PD = log2(Ct/C0), where C0 is the number of cells seeded, and Ct is the number of cells harvested after 5 – 9 days. Cells which were examined at their first passages and last passages were designated as 'young' and 'old' respectively.
Detection of senescence-associated β-galactosidase (SA-β-Gal)
The presence of senescence-associated β-galactosidase was visualized according to Dimri et al. (28), using Cell Staining Technology (USA). Cells were considered senescent when more than 70% of cells stained positive for SA-β-Gal.
Cell viability
Cell viability was measured using an MTT assay. Briefly, monolayers of 2 × 104 young and old endothelial cells were exposed to medium M199 with supplements ± SR, EV and AT for 24 hours. After the exposition, cells were incubated in a medium containing 1.25 mg/ml of the MTT salt (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) for 4 h at 37°C. The generated formazan product was dissolved with the addition of acidic solution of 20% w/v sodium dodecyl sulphate and 50% v/v N,N-dimethylformamide. The absorbance of the converted dye was recorded at 595 nm with a reference wavelength of 690 nm. The data were expressed as the percentage of control.
Proliferation assay
Young and old endothelial cells were seeded into a 48-well plate at a density of 2 × 104 cells, and allowed to attach for 4 hours. Then cells were exposed to a culture medium with supplements ± SR, EV and AT for 24 hours in the presence of [3H]-thymidine - 1µ Ci/ml (Institute of Radioisotopes, Prague, Czech Republic). After the incubation, cells were harvested and precipitated with 10% trichloroacetic acid (TCA) and dissolved in 0.1 mmol/l NaOH. The radioactivity was measured in a beta liquid scintillation counter (Wallac, Perkin Elmer). The data was expressed as CPM (Count Per Minute - value comes from the beta liquid scintillation counter).
Migration assay
QCMTM Chemotaxis 96-well cell migration assays (Chemicon, USA) with 8 µm pores were used to assess the migration of young and old HUVECs. Endothelial cells were grown to 80% confluency in a culture medium ± SR, EV and AT for 24 hours. Eighteen to twenty-four hours before the assay, cells were starved by incubating in the medium M199 with supplements and 2% v/v FCS and then washed twice with PBS, harvested using tripsin/EDTA solution, resuspended in a serum-free medium and placed in a migration chamber (5 × 104 cell/100 µl) covered with fibronectin (1 µg/ml overnight at 4°C). Cells were then stimulated for 24 hours with a standard 10% serum-containing medium with or without SR, EV and AT. Migrated cells were detached and treated for 15 minutes with the CyQuant GR dye in the lysis buffer, as per manufacturer's instructions. Fluorescence of cell lysates was measured with a fluorescence microplate reader (Victor 2, Perkin Elmer, USA) using 480 nm and 520 nm wavelengths for excitation and emission respectively.
Wound healing
Young and old endothelial cells were grown to confluence in 6-well dishes. Before the experiment, the cells were incubated in M199 with supplements ± SR, EV and AT for 24 hours. Under these conditions the cells remained viable in a nonproliferating state. The quiescent monolayer was injured by scraping with a disposable cell scraper (Nunc, Denmark). To compare the data from all experiments it was very important to make a comparable injury in all monolayers exposed to medications. The monolayer was washed twice with the culture medium and then incubated with M199 with supplements ± SR, EV and AT. The closure of the denuded area was monitored using a Zeiss Axio Observer D1 inverted microscope (Carl Zeiss, Germany) equipped with a CO2 module and an incubator. During the experiments, cells were maintained in a 5% CO2 humidified atmosphere at 37°C for the duration of the experiments. A video camera was attached to the microscope, and images of the wounded area were captured as a digitalized sequence every 30 minutes, using Axio-Vision Rel. 4.6.3. image analysis software. The results were calculated as a percentage of the healed area at six separate time intervals (2 h, 4 h, 6 h, 8 h, 10 h, 12 h) along the original margin of the wound. The assay is a well-established method to study wound healing in vitro and is thought to be particularly suited for measuring cell migration (29). In our study migration was additionally verified by using a Boyden chamber.
Cytokine measurements
Young and old endothelial cells were cultured with M199 with supplements ± SR, EV and AT for 24 hours. Media were collected and analyzed for the constitutive concentrations of cytokines: IL-6, IL-8, MCP-1, sICAM-1, TGF-β, fibronectin, VEGF. Mediators were measured by using DuoSet Immunoassay Development Kits (R&D Systems) according to the manufacturer's instructions. The sensitivity of the assay was: 2.6 pg/ml for the IL-6, 4.4 pg/ml for the IL-8, 5.8 pg/ml for the MCP-1, 17.5 pg/ml for the sICAM, 17.6 pg/ml for the TGF-β, 0.96 ng/ml for the fibronectin and 12.2 pg/ml for the VEGF. The results were normalized per number of young and old cells in the culture wells.
Statistical analysis
The data were analyzed with repeated measures analysis of variance using GraphPad Prism 6.00 software (GraphPad Software Inc.). A post hoc test was used to make multiple comparisons. A P-value less than 0.05 was considered significant. Results are presented as means ± S.D.
RESULTS
Senescence of endothelial cells
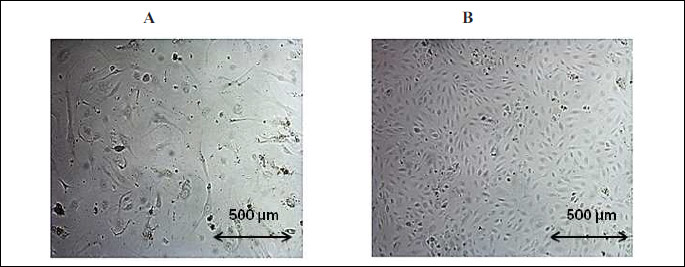
Endothelial cells, after approximately 28-31 passages, reached about 35 population doublings (PD), which resulted in cell senescence. Senescent cells increased in cell size (Fig. 1A) when compared with young cells (Fig. 1B). Young cells lost their proliferative capacity during serial passages (Fig. 2B). The senescence of endothelial cells was associated with the appearance of the senescence marker SA-β-Gal.
Effect of sirolimus, everolimus and atorvastatin on young and old endothelial cell viability and proliferation
Both young and old endothelial cells had the same viability entering the experimental protocol (OD at baseline: control young: 0.435 ± 0.016, control old: 0.444 ± 0.023) (Fig. 2A). Exposure of HUVECs to SR and EV resulted in a dose-dependent inhibition of young and old cells viability and proliferation. Figures show only results at the highest dose. The detrimental effect was more pronounced in young cells (Fig. 2A).
Using [3H] labelled thymidine we estimated young endothelial cell proliferation and DNA turnover in senescent cells. This assay confirmed that old cells lost their ability to proliferate and only a small amount of them was still in the cell-division cycle (control young: 5838 ± 310 CPM; control old: 1122 ± 146 CPM) (Fig. 2B). We observed a dose dependent decrease in DNA turnover in old cells exposed to the medium supplemented with SR and EV. The proliferation of young endothelial cells was also suppressed by the increasing concentration of mTOR inhibitors. In contrast, AT at clinically relevant doses did not impair young and old endothelial cell proliferation and viability (Fig. 2A and 2B).
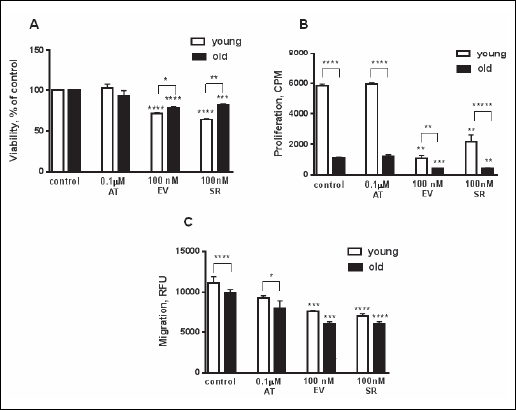 |
Fig. 2. The effect of the highest dose of atorvastatin (AT), everolimus (EV), and sirolimus (SR) on endothelial cell viability (A), proliferation (B) and migration (C). Young and old cells were treated with medications or vehicle control for 24 hours. After the exposure cell viability (MTT test), proliferation (3H-thymidine incorporation) and migration (Byden chambers) were tested. Results are expressed as mean ± S.D. The data were derived from 4 independent experiments. Asterisks represent a significant difference compared with representative control cells (*P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001). |
Effect of sirolimus, everolimus and atorvastain on young and old endothelial cell migration
mTOR inhibitors in a dose-dependent manner inhibited young and old endothelial cell migration (Fig. 2C, shows only the results at the highest dose). Young endothelial cells exhibited stronger response to the chemoattractant than old cells by approximately 30% (Fig. 2C). The response to the increasing concentration of SR and EV was similar in both groups (Fig. 2C). Migration of young and old endothelial cells exposed to AT did not differ significantly from the controls (Fig. 2C).
Effect of sirolimus, everolimus and atorvastatin on young and old endothelial cell wound closure
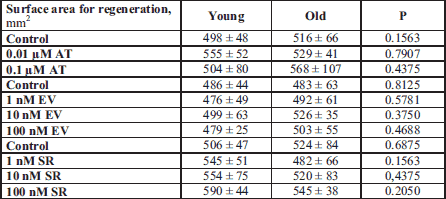
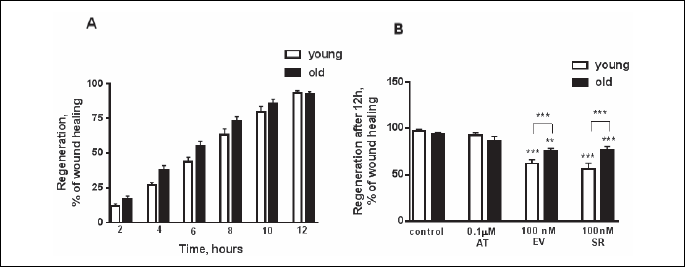
The closure of denuded area was monitored by the time lapse microscopy. Although control senescent HUVECs displayed markedly reduced potential to proliferate and migrate, they were still able to repopulate the denuded areas as fast as the young cells (Fig. 3A and 3B). The surface area of the wounds generated at the beginning of experiments did not differ between the groups and was confirmed by statistical analysis (Table 1). Images of the wounded area were taken at the beginning of the experiment (0 h), and after 2 h, 4 h, 6 h, 8 h, 10 h and 12 h of the progressive closure of the wounded area in both young and old endothelial cells. The results were calculated as a percentage of the healed area at six separate time intervals. The comparison of the kinetics of the regeneration processes in young and old cells revealed that both groups had similar patterns in the repopulation of wounded areas (Fig. 3A). They repopulated the denuded areas within comparable time of 12 hours (Fig. 3A). We observed that AT, at clinically relevant concentration did not disrupt young and old endothelial cell regeneration (Fig. 3B), while mTOR inhibitors attenuated wound healing in a dose dependent manner (data not shown). The percentage of healed wounds after the exposition to SR and EV was higher in old cells compared with young cells (Fig. 3B). This may be due to a better condition of old cells (viability) after exposition to mTOR inhibitors (Fig. 2A).
Effect of sirolimus, everolimus and atorvastatin on young and old endothelial cell cytokine production
Young and old endothelial cells produce various amounts of mediators. Senescent cells produce far more proinflammatory cytokines, such as IL-6, MCP-1, IL-8 and sICAM-1 when compared with young cells (Table 2). The production of angiogenic VEGF, and extracellular matrix proteins (fibronectin, TGF-β) is also more pronounced in senescent endothelial cells than in young cells (Table 2). The modification of cytokine production by the tested drugs was dose dependent. Fig. 4 presents the percentage change of cytokine production by the highest drug concentration. The modifying effect of rapalogs and AT on young and old endothelium was mostly comparable (Fig. 4A, 4B and 4C). Even after the exposition to the tested drugs, the detected concentrations of tested mediators was still significantly higher in the old endothelial cells in comparison with the young cells (Fig. 4A, 4B and 4C). AT, as an anti-inflammatory agent, reduces pro-inflammatory mediators proportionally to its concentration both in young and old cells. It also attenuates VEGF, fibronectin and TGF-β production (Fig. 4A).
VEGF, vascular endothelial growth factor; IL-8 (CXCL8), interleukin 8; IL-6, interleukin 6; MCP-1 (CCL2), monocyte chemoattractant protein; sICAM-1, the soluble form of intercellular adhesion molecule; TGF-β, transforming growth factor β.
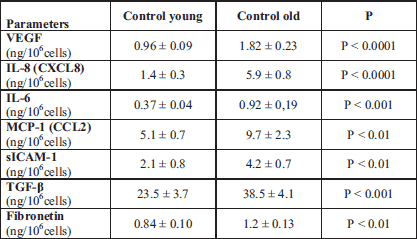
Anti-inflammatory effect of mTOR kinase inhibitors is more pronounced after EV exposition, especially in old HUVECs (Fig. 4B). The exposure to SR caused a decrease in IL-6, IL-8, MCP-1 and VEGF (Fig. 4C). The anti-inflammatory effect of mTOR kinase inhibitors was far less pronounced in young endothelial cells and concerned only statistically significant reduction in IL-6 and MCP-1, proportionally to the tested concentration (data not shown). mTOR kinase inhibitors exerted antiangiogenic effect by lowering VEGF production in both HUVECs groups and additionally had a weak modulatory effect on extracellular matrix proteins synthesis (Fig. 4B and 4C). Due to senescent cell hypertrophy, the effect of cytokine production was more evident when calculated per number of cells than per micrograms of cell protein (Table 2).
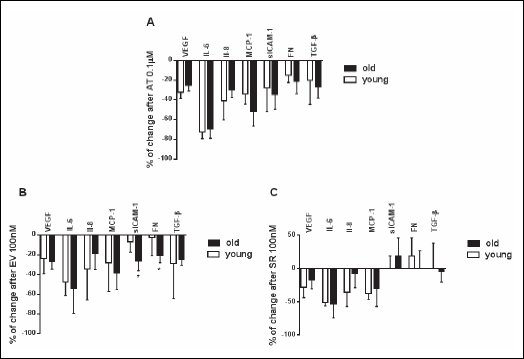 |
Fig. 4.The percentage change of mediators production by the highest drug concentration in cultured medium, obtained from young and old endothelial cells. Secreted mediators were measured in post-culture supernatants following a 24-hour exposure to AT, EV, SR. The data were derived from 8 independent experiments. (VEGF, vascular endothelial growth factor; Il-8 (CXCL8), interleukin 8; IL-6, interleukin 6; MCP-1 (CCL2), monocyte chemoattractant protein; sICAM-1, the soluble form of intercellular adhesion molecule; TGF-β, transforming growth factor β. *P < 0.05; young verus old. |
DISCUSSION
Endothelial denudation is considered to be a primary injury after stent implantation. Prompt regeneration of wounded endothelial cells is a precondition for protecting against vascular restenosis. Our previous study described a model for endothelial cell regeneration in tissue culture using a cell scraper. The repopulation of the wounded area was continuously monitored by time-lapse microscopy, with subsequent morphometric analysis (23). The healing of the endothelial cells after PCI is slow and takes time (30). After stent implantation, re-endothelialisation can be completed by the migration of vessel ECs, proliferation and from bone-marrow-derived progenitor endothelial cells (31, 32). The participation of progenitor endothelial cells in re-endothelialisation is still imperfectly understood (11, 33). However, we must take into consideration that this process could have been slowed down because of progenitor cell's senescence (34). Young cells can regenerate after injuries using both migration and proliferation, whereas in old cells migration seems to be the most plausible mechanism of wound healing since senescent cells have exhausted their replicative potential. In our paper we have proved that senescent endothelial cells produce more proinflammatory cytokines, angiogenic VEGF and extracellular matrix proteins. They also stop proliferating and have diminished migration. When compared to young endothelium, they have similar viability and can regenerate wounds in comparable time because they are hypertrophic. Their size is approximately five times larger than that of young cells, so in spite of a feeble migration and reduced cell proliferation they are still able to heal wounds (23). The tested drugs have anti-inflammatory properties and additionally alter extracellular matrix protein synthesis, but even after pretreatment old cells still produce significantly higher concentration of tested mediators in comparison with young cells. The mTOR inhibitors exhibit antiangiogenic properties by reducing the concentration of VEGF, proliferation and migration. For this reason they are used in tumour therapy (35, 36). Additionally, the modulating anti-inflammatory effect of the tested drugs seems to be more pronounced in senescent endothelium. Atorvastatin, at clinically relevant concentration, is safe for endothelium as we earlier proved using permanent cell line HUVECs EA.hy926 (12). The same effect was observed using primary young and old HUVECs. On the contrary, mTOR inhibitors used in concentration detected in the blood after stent implantation are harmful for both young and old endothelium. They slow down the cell cycle, restrain migration and regeneration, although old cells remain more viable after exposition to rapalogs, when compared to their young counterparts. Old cells are able to repopulate denuded areas faster than young cells after the exposition to SR and its derivative EV due to better viability and hypertrophy. It is worth emphasizing that the main mechanism by which wounds are regenerated is the migration process and only to a small degree it is caused by proliferation (23, 37).
The mTOR kinase is serine/threonine protein kinase belonging to the kinase family that is highly concentrated in all eukaryotes. The dysregulation of mTOR signalling is often associated with cancer and several metabolic disorders (e.g. obesity, type 2 diabetes) (36). Experimental data show that statins and mTOR kinase inhibitors exhibit a biphasic effect depending on their concentration. The mechanism underlying the biphasic effect of statins is attributed to their impact on protein prenylation (38). In high concentrations (>1 µM) they inhibit endothelial cell proliferation, while in very low (nanomolar) concentrations they stimulate cell cycle (12, 13). While in high concentration (µM range) mTOR kinase inhibitors initiate cell death by the induction of autophagy-specific genes (36). The mTOR kinase is inhibited by rapalogs at nanomolar range, and Pallet et al. hypothesized that the majority of rapalogs-related adverse effects are not necessarily caused by mTOR inhibition, but by side effects that remain to be identified (39). Using atorvastatin, sirolimus and everolimus in concentration corresponding to those obtained during clinical therapy makes them close to the in vivo conditions (25-27).
A large body of evidence indicates that statins and mTOR inhibitors have a pleiotropic anti-atherosclerotic effect and can be used as an addition to therapy to prevent or delay the vascular disease (36, 40-42). By suppressing cell proliferation and promoting cell autophagy the mTOR inhibitors control plaque growth and destabilization but on the other hand they act adversely on endothelium (6-8). Rapamycin and everolimus accelerate the aging process in endothelial cells (8), but it has also been demonstrated that rapamycin extends life span in mice by delaying aging (43). Indeed, mTOR inhibition seems to be an innovative strategy for the treatment of age-associated diseases. The importance of mTOR inhibitors in progeria-related diseases is complex and consists mostly in: (i) activating autophagy, (ii) decreasing age-related inflammation and (iii) mimicking the effects of caloric restriction and its role in extending lifespan (44).
Contrary to rapalogs, statins exert a beneficial effect on endothelial cells improving their hemostatic, vasomotor, anti-inflammatory and angiogenic functions (13). The anti-atherosclerotic effect of rapalogs and statins is attributed to their anti-inflammatory effect. As we have demonstrated atorvastatin not only reduces the pro-inflammatory cytokine synthesis in endothelial cells but also reduces the production of extracellular matrix proteins and VEGF. Anti-inflammatory effect of rapalogs is more apparent in everolimus, especially in senescent endothelial cells. This anti-inflammatory effect of rapalogs has also been seen in macrophages (40). The observed effect is related to the inhibition of protein synthesis by cells exposed to mTOR kinase inhibitors (36). The inhibition of protein synthesis together with autophagy might lead to the gradual decrease of cell viability and cell death. Exposition to increasing concentration of mTOR kinase inhibitors resulted in progressive loss in cell viability, proliferation and migration in young and old endothelial cells. Anti-proliferative, anti-migratory, anti-inflammatory and immunomodulatory effects of rapalogs are well established (7, 8, 36). Hovever, they can also stimulate the production of pro-inflammatory cytokines in vascular smooth muscle cells (45), monocytes and neutrophils (46). This may also account for endothelial dysfunction (39, 45, 47). The adverse effects associated with mTOR inhibitors such as the deterioration of many essential functions of the endothelium and recently identified dyslipidemia can be improved by a combined use of statins (36). The improvement of cardiac contractility and protection against arrhythmia after ACS is one of the most important designations of the cardiac pharmacotherapy. Benova et al. using animal model of isolated rat hearts, demonstrated antiarrhythmic effect of atorvastatin. Isolated fibrillating hearts were protected from the lethal arrhythmia and the sinus rhythm was restored after atorvastatin treatment (48).
Vascular aging is associated with EC dysfunction which predisposes to increased risk of cardiovascular events in the elderly (16). A significant number of elderly patients require PCI and suffer from the complications of the procedure. We have previously examined how senescence of ECs in vitro affects the regeneration after injuries mimicking those occurring during angioplasty. Our experiments were performed using HUVECs which revealed a great convergence with endothelial cells from coronary arteries (49). It was important to assess the reaction of young and old endothelial cells after exposition to atorvastatin and rapalogs. Because human coronary artery endothelial cells are usually derived from adult donors we used very young endothelial cells derived from umbilical veins. Following serial passages in culture, ECs entered the state of senescence. We found it surprising that senescent cells repopulated denuded areas as fast as young cells in spite of growth inhibition and reduced migratory potential. To reconcile the results we performed a morphometric analysis, which reveals that old cells effectively covered the wound by virtue of their increased size. An additional explanation of this phenomena could be an increased expression of eNOS by senescent HUVECs (23). eNOS-mediated NO production enhanced endothelial cell migration (50) and stimulated wound healing in elderly patients (51). Zemakova et al. documented that atorvastatin prevented inflammatory-mediated downregulation of eNOS protein level. This effect was regulated via endoglin, a transmembrane glycoprotein, that is part of the TGF-beta receptor complex (52). This endoglin supporting effect on eNOS synthesis could be particularly important for senescent endothelium, since TGF-beta is known to be involved in the aging process and tumorigenesis (53, 54).
Taken together, our results indicate that atorvastatin at clinically relevant concentrations and rapalogs in the concentration obtained in the serum after stent implantation may weaken the inflammatory response associated with angioplasty. Rapalogs delay young and old cell regeneration after injuries mimicking those occurring after PCI, while atorvastatin does not delay endothelial recovery from injuries.
Conflict of interests: None declared.
REFERENCES
- Kiemeneij F, Serruys PW, Macaya C, et al. Continued benefit of coronary stenting versus balloon angioplasty: five-year clinical follow-up of Benestent-I trial. J Am Coll Cardiol 2001; 37: 1598-1603.
- Laarman G, Muthusamy TS, Swart H, et al. Direct coronary stent implantation: safety, feasibility, and predictors of success of the strategy of direct coronary stent implantation. Catheter Cardiovasc Interv 2001; 52: 443-448.
- Topol EJ, Serruys PW. Frontiers in interventional cardiology. Circulation 1998; 98: 1802-1820.
- Inoue T, Sata M, Hikichi Y, et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation 2007; 115: 553-561.
- Pilgrim T, Windecker S. Drug-eluting stent technology: progress beyond the polymer. Eur Heart J 2014; 35: 1991-1995.
- Camici GG, Steffel J, Amanovic I, et al. Rapamycin promotes arterial thrombosis in vivo: implications for everolimus and zotarolimus eluting stents. Eur Heart J 2010; 31: 236-242.
- Finn AV, Nakazawa G, Joner M, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol 2007; 27: 1500-1510.
- Ota H, Eto M, Ako J, et al. Sirolimus and everolimus induce endothelial cellular senescence via sirtuin 1 down-regulation: therapeutic implication of cilostazol after drug-eluting stent implantation. J Am Coll Cardiol 2009; 53: 2298-2305.
- Kamishirado H, Inoue T, Sakuma M, et al. Effects of statins on restenosis after coronary stent implantation. Angiology 2007; 58: 55-60.
- Miyauchi K, Kasai T, Yokayama T, et al. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J 2008; 72: 832-838.
- Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 2002; 22: 1567-1572.
- Korybalska K, Kawka E, Breborowicz A, Witowski J. Atorvastatin does not impair endothelial cell wound healing in an in vitro model of vascular injury. J Physiol Pharmacol 2012; 63: 389-395.
- Medina RJ, O'Neill CL, Devine AB, Gardiner TA, Stitt AW. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS One 2008; 3: e2584. doi: 10.1371/journal.pone.0002584
- Gennaro G, Menard C, Michaud SE, Rivard A. Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation 2003; 107: 230-233.
- Torella D, Leosco D, Indolfi C, et al. Aging exacerbates negative remodeling and impairs endothelial regeneration after balloon injury. Am J Physiol Heart Circ Physiol 2004; 287: H2850-H2860.
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol 2009; 94: 317-321.
- Hayflick L. The lymited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37: 614-636.
- Campisi J. The biology of replicative senescence. Eur J Cancer 1997; 33: 703-709.
- Minamino T, Komuro I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat Clin Pract Cardiovasc Med 2008; 5: 637-648.
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002; 105: 1541-1544.
- Ogami M, Ikura Y, Ohsawa M, et al. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 2004; 24: 546-550.
- Voghel G, Thorin-Trescases N, Farhat N, et al. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev 2007; 128: 662-671.
- Korybalska K, Kawka E, Kusch A, et al. Recovery of senescent endothelial cells from injury. J Gerontol A Biol Sci Med Sci 2013; 250: 250-257.
- Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 1983; 80: 3734-3737.
- Thakkar AS, Abhyankar AD, Dani SI, et al. Systemic exposure of sirolimus after coronary stent implantation in patients with de novo coronary lesions: supralimus-Core(R) pharmacokinetic study. Indian Heart J 2012; 64: 273-279.
- Wiemer M, Seth A, Chandra P, et al. Systemic exposure of everolimus after stent implantation: a pharmacokinetic study. Am Heart J 2008; 156: 751-757.
- Bahrami G, Mohammadi B, Mirzaeei S, Kiani A. Determination of atorvastatin in human serum by reversed-phase high-performance liquid chromatography with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 826: 41-45.
- Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 1995; 92: 9363-9367.
- Liang CC, Park AY, Guan JL. in vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007; 2: 329-333.
- Grewe PH, Deneke T, Holt SK, Machraoui A, Barmeyer J, Muller KM. Scanning electron microscopic analysis of vessel wall reactions after coronary stenting [in German]. Z Kardiol 2000; 89: 21-27.
- Albuquerque ML, Waters CM, Savla U, Schnaper HW, Flozak AS. Shear stress enhances human endothelial cell wound closure in vitro. Am J Physiol Heart Circ Physiol 2000; 279: H293-H302.
- Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 2003; 93: e17-e24.
- Purhonen S, Palm J, Rossi D, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 2008; 105: 6620-6625.
- Matsuo Y, Imanishi T, Hayashi Y, et al. The effect of senescence of endothelial progenitor cells on in-stent restenosis in patients undergoing coronary stenting. Intern Med 2006; 45: 581-587.
- Gangloff YG, Mueller M, Dann SG, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 2004; 24: 9508-9516.
- Martinet W, De LH, De Meyer GR. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 2014; 233: 601-607.
- Yung S, Davies M. Response of the human peritoneal mesothelial cell to injury: an in vitro model of peritoneal wound healing. Kidney Int 1998; 54: 2160-2169.
- Urbich C, Dernbach E, Zeiher AM, Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res 2002; 90: 737-744.
- Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf 2013; 12: 177-186.
- Castro C, Campistol JM, Sancho D, Sanchez-Madrid F, Casals E, Andres V. Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27 Kip1-independent pathway. Atherosclerosis 2004; 172: 31-38.
- Hughes AD. The role of isoprenoids in vascular smooth muscle: potential benefits of statins unrelated to cholesterol lowering. J Hum Hypertens 1996; 10: 387-390.
- Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res 2000; 47: 648-657.
- Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460: 392-395.
- Evangelisti C, Cenni V, Lattanzi G. Potential therapeutic effects of the MTOR inhibitors for preventing ageing and progeria-related disorders. Br J Clin Pharmacol 2016; 82: 1229-1244.
- Chibana H, Kajimoto H, Ueno T, et al. Interleukin-1beta is associated with coronary endothelial dysfunction in patients with mTOR-inhibitor-eluting stent implantation. Heart Vessels 2017; Jan 23: doi: 10.1007/s00380-017-0947-x. [epub ahead of print]
- Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008; 29: 565-577.
- Chen TG, Chen JZ, Wang XX. Effects of rapamycin on number activity and eNOS of endothelial progenitor cells from peripheral blood. Cell Prolif 2006; 39: 117-125.
- Benova T, Knezl V, Viczenczova C, Bacova BS, Radosinska J, Tribulova N. Acute anti-fibrillating and defibrillating potential of atorvastatin, melatonin, eicosapentaenoic acid and docosahexaenoic acid demonstrated in isolated heart model. J Physiol Pharmacol 2015; 66: 83-89.
- Chi JT, Chang HY, Haraldsen G, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 2003; 100: 10623-10628.
- Murohara T, Witzenbichler B, Spyridopoulos I, et al. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol 1999; 19: 1156-1161.
- Kirk SJ, Hurson M, Regan MC, Holt DR, Wasserkrug HL, Barbul A. Arginine stimulates wound healing and immune function in elderly human beings. Surgery 1993; 114: 155-159.
- Zemankova L, Varejckova M, Dolezalova E, et al. Atorvastatin-induced endothelial nitric oxide synthase expression in endothelial cells is mediated by endoglin. J Physiol Pharmacol 2015; 66: 403-413.
- Ksiazek K, Korybalska K, Jorres A, Witowski J. Accelerated senescence of human peritoneal mesothelial cells exposed to high glucose: the role of TGF-beta1. Lab Invest 2007; 87: 345-356.
- Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev 2002; 12: 22-29.
A c c e p t e d : May 31, 2017