GONADOTROPIN-RELEASING HORMONE AND KISSPEPTIN-10 REGULATE NUCLEAR RECEPTOR SUBFAMILY 5 GROUP A MEMBER 1/CATENIN BETA 1/ NUCLEAR RECEPTOR SUBFAMILY 0 GROUP B MEMBER 1 ACTIVITY IN FEMALE RAT ANTERIOR PITUITARY GLAND
INTRODUCTION
The neuroendocrine mechanism controlling reproductive functions in mammals is regulated by a complex network of hypothalamic neurons whose final output is the pulsatile release of gonadotropin-releasing hormone (Gnrh), a primary regulator of gonadotrope function (1). Gnrh-induced activation of several mitogen-activated protein kinase cascades regulates at least 75 genes (2), which are organized into a tiered hierarchy based on the kinetics of their response to Gnrh. The Gnrh transcriptional signal results in up-regulated expression of the following four tertiary gonadotrope signature genes: glycoprotein hormones, alpha polypeptide (Cga), luteinizing hormone beta polypeptide (Lhb), follicle stimulating hormone beta subunit (Fshb), and Gnrh receptor (Gnrhr). Although each gonadotrope signature gene contains its own specific set of transcription factors, response elements for the nuclear receptor subfamily 5 group A member 1, formerly known as steroidogenic transcription factor SF-1, are found in the promoter regions of all four genes (3). The gene encoding nuclear receptor subfamily 5, group A, member 1, Nr5a1, is highly conserved in vertebrates, in which it is critical for maintaining fertility. Nr5a1 transgenic mice with Nr5a1-deficient gonadotropes were hypogonadal, failed to express detectable levels of Cga, Lhb, Fshb, and Gnrhr, and were infertile (4). Nr5a1, which is classified as an orphan nuclear receptor, was first found to be refractory to regulation by Gnrh (5), according to studies on LβT2 gonadotrope cells (6, 7). However, evident Gnrh pulse-frequency dependent stimulation of Nr5a1 transcription in the pituitary gland was reported in female (8) and male (9) rats in vivo. Nr5a1 was not the primary determinant of hormone-induced transcriptional fluxes of gonadotrope signature genes. In fact, although it acts directly through its target DNA element, its activity is strongly influenced by interactions with other transcription factors and co-regulators. Its predominant interaction is with co-repressor nuclear receptor subfamily 0 group B member 1, known as the dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1 DAX1, encoded by the Nr0b1 gene (9). In vitro studies showed that Nr0b1 interacts directly with Nr5a1 via the carboxy-terminal regions of each protein, and co-expression of Nr0b1 and Nr5a1 inhibits Nr5a1- mediated transactivation of many target genes (10). Conversely, co-activation of Nr5a1 occurs through the binding of Ctnnb1 to amino acids 235 – 38, localized in the first helix of the putative ligand-binding domain of the Nr5a1 that includes the activation function domain (11). Ctnnb1 is a transcriptional co-activator typically associated with T cell factor/lymphoid enhancer factor-responsive genes that promote the transcription of Wnt target genes (12). It may serve as an essential co-activator for many Nr5a1-dependent genes (13).
Although Gnrh neurons are a critical component of the reproductive axis activity including ovulation and pregnancy (14), kisspeptin peptides (KiSS-1 metastasis-suppressor) have been recognized as vital upstream regulators that integrate central and peripheral signals with Gnrh release (15-18). In rodents, kisspeptin perikarya are localized in a few discrete hypothalamic regions: the arcuate nucleus, anteroventral periventricular nucleus, periventricular nucleus, and in the continuum of the third ventricle (19, 20). Kisspeptin signaling through its receptor is coupled to the Gq/11α G-protein subunit, activating phospholipase C and increasing inositol triphosphate and diacylglycerol levels in the cell (21); thus kisspeptin acts directly on Gnrh neurons to induce a sustained depolarization event and increase the rate of action potential firing (22). It also may exert an indirect effect on Gnrh neurons via synaptic input from other neurons in the hypothalamus that express kisspeptin receptor (17). Neurokinin B and dynorphin are co-transmitters of kisspeptin signaling. Since hypothalamic neurokinin B and dynorphin neurons are colocalized with kisspeptinergic neurons, and all such neurons project to Gnrh neurons, kisspeptin signaling may also play a role in modulating the pulsatile release of Gnrh (16). Furthermore, a galanin system as well as a humoral phototransduction for some of bright light's effects on Gnrh pulse generator activity were shown (23-25).
In this study, we tested the hypothesis that central Gnrh network stimulation affects anterior pituitary Nr5a1 gene and NR5A1 protein expression by altering Ctnnb1/CTNNB1 and Nr0b1/NR0B1 gene/protein regulation. To verify this, an in vivo model which preserves endogenous hypothalamo-pituitary connections and more closely resembles the physiological context of Gnrh function was employed (26). It has been shown that endogenously produced Gnrh, predominantly originated from the Gnrh axonal bed in the median eminence that lies in close proximity to the third cerebral ventricle, is present in mammalian cerebospinal fluid (27). Tanycytes are glial, ependymal cells lining the latero-ventral portion of the third ventricle (28). As a bipolar cells bridging the cerebrospinal fluid to the portal capillaries tanycytes can participate in the release of Gnrh to the portal blood by absorbing the molecules from the cerebospinal fluid and providing signals to Gnrh neurons (29). A presence of subpopulation of Gnrh neurons which express Gnrhr-1 and respond to Gnrh with altered firing suggested a physiological ultrashort loop feedback role for Gnrh (30). Indeed, Gnrh itself has been reported to activate M-type potassium currents hyperpolarizing membrane potential, what thus form an autoregulatory mechanism of negative feedback to Gnrh neurons activity (31, 32). Furthermore, studies on single Gnrh neurons obtained from slices derived from Gnrh-GFP transgenic mice revealed that Gnrh was also shown to exert a direct depolarizing actions upon its neurons, what indicates that positive ultrashort feedback loops exist among Gnrh neuronal population (33). Also trans-synaptic pathway for Gnrh to regulate the activity of Gnrh neurons via modifying GABA-ergic transmission to these cells has been reported (34).
Given Nr5a1 role in the activation of four gonadotrope genes essential for maintenance of reproduction, the present research was undertaken to determine whether and how endogenous Gnrh network activity could affect Nr5a /NR5A1 system in vivo. To this end, we performed pulsatile intracerebroventricular (i.c.v.) injections of kisspeptin and Gnrh, and analyzed mRNA as well protein expression of Nr5a1/NR5A1, Ctnnb1/CTNNB1 and Nr0b1/NR0B1 in the female rat anterior pituitary gland. Furthermore, to assess changes in the Ctnnb1 intracellular localization within gonadotrope cells, the immunofluorescence confocal microscopy was also performed.
METHODS
Peptides
Gnrh was obtained from Bachem AG (Bubendorf, Germany). Antide, a Gnrh antagonist, was obtained from Sigma-Aldrich (St. Louis, USA). Kisspeptin-10, a highly active kisspeptin agonist, and kisspeptin-234, a kisspeptin antagonist, were purchased from Tocris Cookson Ltd (Avonmouth, UK). All peptides were dissolved in 0.9% NaCl solution.
Animals and surgical procedures
All experimental procedures in this study were conducted in accordance with the Polish Guide for the Care and Use of Animals and were approved by the Local Ethics Committee of the Warsaw University of Life Sciences, Poland.
Fifty eight four-month-old female Wistar rats (Cmdb/WI), 250 to 280 g b.w., were obtained from the Center of Experimental Medicine (Bialystok, Poland). The animals were well adapted to the experimental conditions and kept under controlled light (14:10 h light/dark cycle, lights on at 06.00 a.m.) and temperature (22ºC) with free access to pelleted food and tap water.
All rats were bilaterally ovariectomized under general ketamine anesthesia (20 mg/100 g b.w.) and were allowed to recover for eight days before being used in one experiment. On day 9, using the same ketamine anesthesia, each rat was stereotactically implanted with a permanent stainless steel guide cannula (outer diameter, 0.8 mm) in the third ventricle of the brain. The coordinates (8.20 mm interaural, –0.80 mm to the bregma, gauge depth: 4.00 mm from the skull surface) were selected according to the rat brain atlas of Paxinos and Watson (35). The cannula was fixed in place with Duracryl dental cement (SpofaDental, Warsaw, Poland), and its location was confirmed by observing the flow of cerebrospinal fluid. The animals were allowed to recover for eight days prior to the start of the experiment.
Experimental design
After recovery, rats were randomly divided into five experimental groups (three groups n = 14 and two groups n = 8) and i.c.v. cannulae were connected to an automatic CMA/402 microinjection pump (CMA/Microdialysis AB, Stockholm, Sweden) by 45-cm long silicon tubing (internal diameter, 0.5 mm; outer diameter, 1 mm), allowing the rats to move freely. Rats received i.c.v. microinjections of 0.9% physiological saline (control with vehicle; n = 14), 2 nM Gnrh (n = 14), or 1 nM kisspeptin-10 (n = 14) alone; or a combined infusion of 2 nM Antide + 2 nM Gnrh (n = 8) or 2 nM kisspeptin-234 + 1 nM kisspeptin-10 (n = 8). All microinjections were given with a frequency of one pulse per 45 min over 6 hours and the flow rate was carefully balanced and set to deliver 10 µl/5 min (1 pulse). In the latter groups, the agonist/antagonist pulse was followed 30 min later by the Gnrh or kisspeptin-10 pulse. Moreover, additional Antide and kisspeptin-234 microinjections (10 µl/5 min) were given twice, at 16 and 12 h before starting the regular schedule of drug administration. Pulsatile microinjection patterns and doses of the agents were based of published data (9, 36). Thirty minutes after completing the last microinjection, animals were deeply anesthetized (ketamine 30 mg/100 g b.w.) and sacrificed by decapitation. Anterior pituitaries intended for RNA extraction (n = 8 from each group) were immediately excised, flash frozen in liquid nitrogen, and finally stored at –80ºC; other tissue samples (6 pituitaries from groups without antagonist infusions) were fixed in 4% paraformaldehyde for confocal microscopy. Trunk blood was collected, centrifuged in dry tubes, and the serum was stored at –20ºC for radioimmunoassay (RIA) use.
Relative gene expression assay
Anterior pituitaries were homogenized using a TissueLyzer LT bead mill (Qiagen, Valencia, CA, USA). Total RNA was extracted and purified using a NucleoSpin RNA/Protein kit (Macherey-Nagel GmbH & Co, Duren, Germany) with on-column DNase treatment, in accordance with the manufacturer’s recommendations. Total RNA concentration and purity were estimated using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and its integrity was validated electrophoretically by separation on an ethidium bromide-stained 1.5% agarose gel. Next, 3 µg of total RNA in a 20 µl final volume was reverse transcribed into first-strand cDNA using a Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol.
Pairs of primers specific for the reference genes: glyceraldehyde-3-phosphate dehydrogenase (Gapdh), succinate dehydrogenase complex flavoprotein subunit A (Sdha), and β-actin 2-microglobulin (Actb), as well as for target genes, Nr5a1, Ctnnb1, Nr0b1 and Lhb were designed to span over intron sequences using PRIMER3 open-source software (37) (IDT PrimerQuest; Integrated DNA Technologies Inc., Coralville, IA, USA). Their specificity was confirmed by a BLAST software-assisted search of a nonredundant nucleotide sequence database (National Library of Medicine, Bethesda, MD). HPLC-grade specific primers were synthesized by Genomed (Warsaw, Poland). All primer data are listed in Table 1.
Specific real-time polymerase chain reactions (qPCR) analysis were performed using Luminaris HiGreen qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) and high-performance liquid chromatography-purified oligonucleotide primers. Each qPCR reaction mixture contained 10 µl of PCR Master Mix, 10.8 µl of RNase-free water, 0.6 µl of each primer (0.3 µl final concentration) and 1 µl of five-fold diluted cDNA template (total reaction volume, 20 µl). The cycling conditions were as follows: 95ºC for 10 min to activate the Hot-Start DNA polymerase, followed by 35 cycles of denaturation at 95ºC for 15 s, annealing at 58ºC for 30 s and extension at 72ºC for 30 s. The reactions were run on a Rotor-Gene 6000 cycler software 1.7. (Qiagen, Valencia, CA, USA). After completion of the reaction, a final melting curve analysis with continuous fluorescence measurement was performed to confirm amplification specificity. Additionally, proper amplicon size and the absence of nonspecific products was confirmed by ethidium bromide-stained 2% agarose gel electrophoresis. All qPCR products were further confirmed by direct sequencing in both directions (Genomed, Warsaw, Poland). Relative Nr5a1, Ctnnb1, Nr0b1 and Lhb genes expression was calculated on the basis of the comparative quantitation option of the Rotor-Gene 6000 cycler software 1.7. (Qiagen, Valencia, CA, USA) with the use of Relative Expression Software Tool 2008 according to the literature (38). To compensate for variations in cDNA concentration and PCR efficiency between tubes, three endogenous control genes: Gapdh, Actb and Sdha were amplified for each sample. As the most stable among the three housekeeping genes tested, the Gapdh gene was chosen to be quantified in each sample and used for normalization. The average relative gene expression of the control group was set to 1.0 values represent means ± SEM for each group.
Western blot analysis
Total protein from anterior pituitary samples from the control, Gnrh, and kisspeptin-10 groups was extracted using a NucleoSpin® RNA/Protein kit (Macherey-Nagel GmbH & Co, Duren, Germany), in accordance with the recommendations of the manufacturer. Next, total protein concentrations for the samples were quantified using the Protein Quantification Assay Kit (Macherey-Nagel Gmbh & Co., Duren, Germany). Tissue protein extracts, with concentrations of 40 µg for NR5A1, 10 µg for Ctnnb1 and 40 µg for Nr0b1, were mixed with denaturing solution to a total volume of 20 µl (4 × Laemmli sample buffer with 2-mercaptoethanol) (Bio-Rad, CA, USA). Samples were electrophoresed in 10% polyacrylamide gels (Mini-Protean TGX Gels, Bio-Rad, CA, USA) in the presence of Precision Plus Protein Dual Color Standards molecular weight marker (Bio-Rad, CA, USA), and electrotransferred onto Hybond 0.2 µm PVDF membrane (Amersham, GE Healthcare Bio-Sciences, MA, USA). The following antibodies and predetermined dilutions were used: anti Nr0b1 primary mouse monoclonal antibody (Active Motif, CA, USA) 1:200, anti Nr5a1 primary mouse monoclonal antibody (Santa Cruz Biotechnology Inc., Dallas, USA) 1:100, anti Ctnnb1 primary mouse monoclonal antibody (Aviva Systems Biology, CA, USA) 1:1000, anti Actb primary mouse monoclonal antibody (Santa Cruz Biotechnology Inc., Dallas, USA) 1:1000; and anti-mouse secondary antibody conjugated with horseradish peroxidase (Abcam, Cambridge, UK) 1:5000. Visualization of membranes was performed using chromogenic detection by the Pierce Ultra 1-Step TMB-Blotting Substrate Solution (Thermo Fisher Scientific, Waltham, USA). Densitometric analysis of the scanned membrane was performed using ImageJ software (Research Services Branch, National Institute of Mental Health, Bethesda, USA).
Immunofluorescence assay
Paraformaldehyde-fixed tissue samples were dehydrated in alcohol, immersed in butanol, and embedded in paraffin; 4-µm-thick paraffin sections of the pituitary gland were cut on an HM 355S microtome (Carl Zeiss, Jena, Germany) and mounted on SuperFrost® Plus slides (Menzel-Glaser, Thermo Sc. Braunschweig, Germany). Sections were deparaffinized in xylene and rehydrated in alcohol, and antigens were unmasked with sodium citrate buffer (held just below boiling for 10 min). Next, sections were blocked in 0.1% Triton X-100 and 5% normal goat serum in PBS for 30 minutes in a humidity chamber at RT. Both primary and secondary antibodies were diluted in 1% BSA in PBS. Mouse monoclonal antibody to Ctnnb1 (Aviva Systems Biology, CA, USA), and rabbit polyclonal antibody to Gnrh-r (Aviva Systems Biology CA, USA) were applied at dilutions of 1:200 (for both the same), for overnight incubation at 4ºC in the humidity chamber. The next day, sections were incubated with goat anti-mouse AlexaFluor 488 (5 µg/ml) or goat anti-rabbit AlexaFluor 647 (5 µg/ml) for 2 hours at RT in the humidity chamber. Finally, nuclei were stained with DAPI (1 µg/ml) (Sigma Aldrich, St. Louis, USA) and sections were mounted under Fluoromount-G medium (Southern Biotech, Birmingham, USA). Immunohistochemically stained specimens were visualized using an Olympus FluoView FV1000 confocal microscope (Olympus Corporation Ltd, Tokyo, Japan). All images were taken using identical gain settings. The pictures were analyzed by the CellSens Dimension software (Olympus Corporation Ltd, Tokyo, Japan).
Plasma luteinizing hormone analysis
Analysis of luteinizing hormone concentration in rat serum was performed using a double-antibody radioimmunoassay (RIA), with anti-rat luteinizing hormone and anti-rabbit antisera and the rat luteinizing hormone standard generously supplied by Dr. A.F. Parlow and NIDDK (Baltimore, MD, USA). The assay sensitivity was 1.5 ng/ml, and intra- and inter-assay variations were 8.2% and 11.5%, respectively.
Statistical analysis
Statistical analysis was performed using the Statistica program (StatSoft, Inc., Tulsa, OK, USA); data are expressed as the mean ± SEM. Statistical evaluations involving comparisons of mRNA and protein expression among vehicle, Gnrh, Antide, kisspeptin-10 and kisspeptin-234 groups were performed using the non-parametric Mann-Whitney U-test. One-way ANOVA followed by Tukey’s post-hoc test was applied to evaluate differences in serum luteinizing hormone concentration. For all tests, statistical differences with P < 0.05 were considered significant.
RESULTS
Neurohormonal regulation of Nr5a1, Ctnnb1 and Nr0b1 genes transcriptional activity in the female rat anterior pituitary
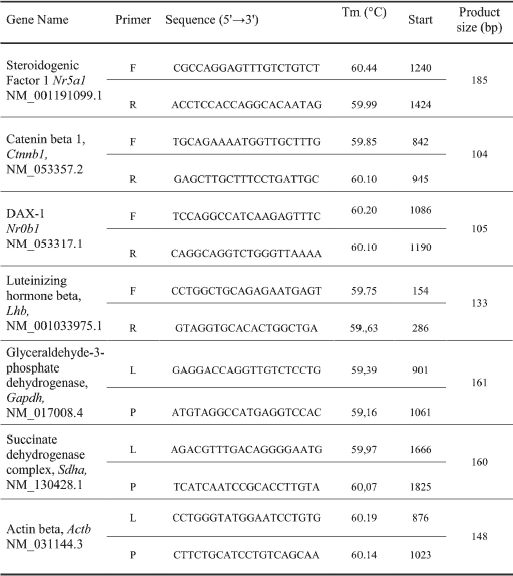
According to qPCR analysis, the pulsatile Gnrh i.c.v. microinjections resulted in a 39% increase in Nr5a1 mRNA expression (Fig. 1A), as well as in 48% elevation of Ctnnb1 mRNA level (Fig. 1B) as compared to the gene-respective vehicle-injection control group (P < 0.05). The effects attributed to Gnrh were clearly Gnrh-r-mediated, given the ability of Antide to significantly reduce the Gnrh-mediated stimulation of both Nr5a1 (43%) and Ctnnb1 (33%) transcription.
Among three genes tested, exogenous kisspeptin-10 affected only Nr0b1 transcriptional activity. As shown on Fig. 1C, a significant 41% increase of Nr0b1 mRNA level was detected as compared to the level found in vehicle-treated control group (P < 0.05) An involvement of GPR54 neurotransmission was evident in that GPR54 antagonist (kisspeptin-234) reduced by 25% kisspetin-10 evoked effect (P < 0.05).
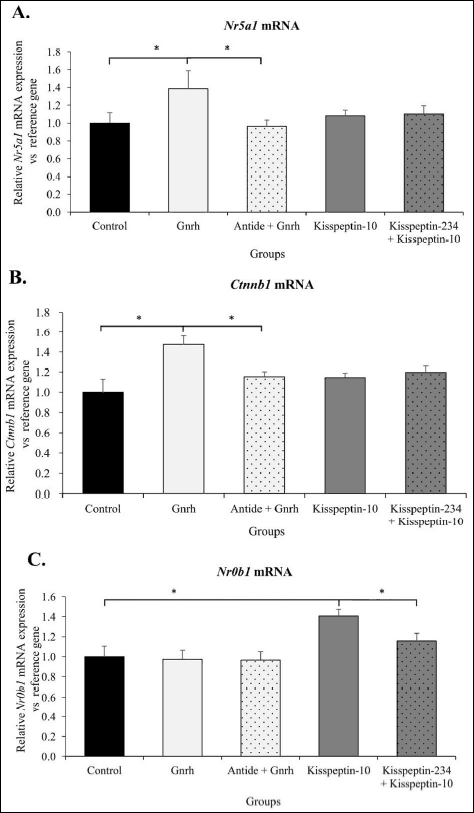 |
Fig. 1. Effects of neurohormonal stimulation on Nr5a1 (A), Ctnnb1 (B) and Nr0b1 (C) mRNA expression in the anterior pituitary gland of female rats in vivo. Ovariectomized rats were given pulsatile (1 pulse/45 min over 6 hours) i.c.v. microinjections of 0.9% NaCl (controls), 2 nM Gnrh, 1 nM kisspeptin-10, 2 nM Antide + 2 nM Gnrh or 2 nM kisspeptin-234 + 1 nM kisspeptin-10. Individual pituitaries were excised 30 minutes after the last micrioinjection. Nr5a1, Ctnnb1 and Nr0b1 mRNA levels were determined according to quantitative reverse transcription PCR method. The results represent a relative expression of the respective target gene versus reference gene. Values are expressed as the mean ± SEM (n = 8 per group). The significance of differences was assessed by Mann-Whitney U test. * P < 0.05. |
Intracellular expression and localization of specific proteins
Western blots of NR5A1, CTNNB1, and NR0B1 proteins were used to quantify translational activity (protein expression). Exogenous Gnrh decreased by 40% NR5A1 protein expression (P < 0.05; Fig. 2A), as well as enhanced by 35% Ctnnb1 protein expression (P < 0.05) (Fig. 2B) relative to the control group.
After kisspeptin-10 microinjections a 36% decrease in CTNNB1 protein expression (Fig. 2B), and a 46% increase in NR0B1 protein expression (Fig. 2C) were detected relative to the corresponding protein levels detected in controls (P < 0.05).
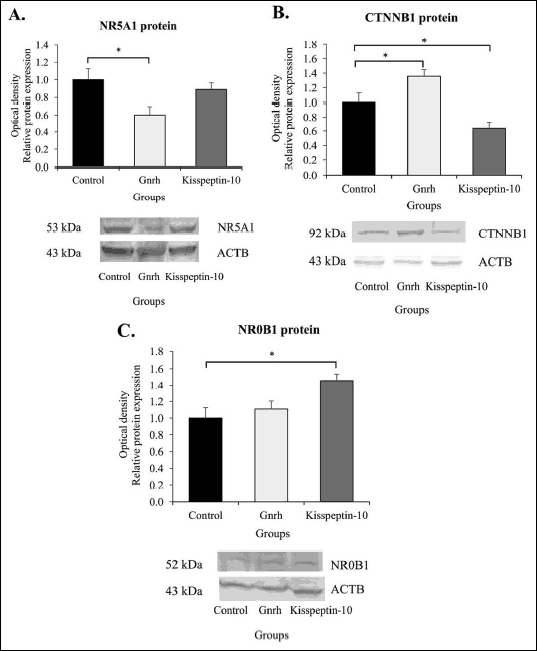 |
Fig. 2. Neurohormonal influence on NR5A1 (A), CTNNB1 (B), and NR0B1 (C) protein expression in female rat anterior pituitary gland in vivo. Ovariectomized rats received i.c.v. pulsatile (1 pulse/45 min over 6 hours) microinjections of 0.9% NaCl (control), 2 nM Gnrh, and 1 nM kisspeptin-10. For Western-blot analysis, individual pituitaries were collected 30 minutes after the last micrioinjection. NR5A1, CTNNB1 and NR0B1 expression levels were normalized by ACTB reference protein. Data are expressed as the mean ± SEM. * P < 0.05, ** P < 0.01 relative to the control group, as determined by the Mann-Whitney U test (n = 6). |
Furthermore, using the immunofluorescence confocal microscopy, an assessment of the CTNNB1 activation within gonadotrope cells was also performed. As shown in Fig. 3B, exogenous Gnrh evoked CTNNB1 relocation from cytoplasm to the nucleus. In contrast, exogenously given kisspeptin-10 resulted only in a weak labeling of CTNNB1 protein which has been detected mainly in the cytoplasm (Fig. 3C).
Luteinizing hormone secretion after Gnrh and kisspeptin agonist administration
We validated the experiments functionally by using pulsatile administration of Gnrh, kisspeptin-10 and their antagonists, and analyzing the resulting Lhb mRNA expression, and luteinizing hormone release. Gnrh appeared to significantly increase Lhb gene expression compared to control and Antide-treated animals (respectively 42% and 35%; P < 0.05). Kisspeptin-10 induced the positive significant effect on Lhb expression compared to control (58%; P < 0.05) (Fig. 4A).
As shown in Fig. 4B, significant increases in serum luteinizing hormone levels were evident in the response to Gnrh treatment (11.52 ± 1.61 ng/ml) compared to Antide-treated and control rats (4.43 ± 0.89 ng/ml and 4.98 ±1.03 ng/ml, respectively; P ≤ 0.05). Serial kisspeptin-10 infusions also resulted in a significant increase in mean plasma luteinizing hormone concentration (12.06 ± 2.48 ng/ml; P ≤ 0.01) in relation to control animals.
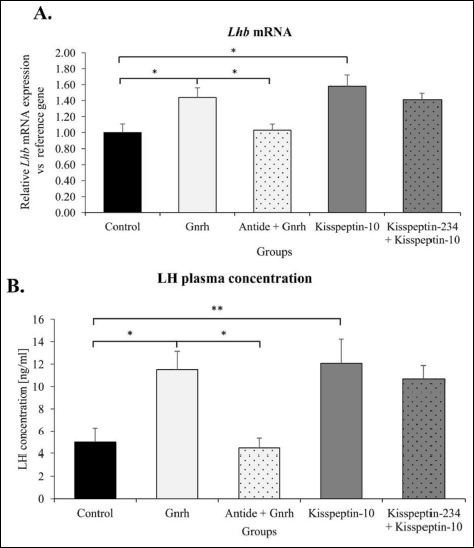 |
Fig. 4. Changes of anterior pituitary Lhb mRNA expression (A) and serum LH concentration (B) in female rats after i.c.v. Gnrh and kisspeptin-10 microinjections. Ovariectomized animals were treated according to legend given for Fig. 1. Relative Lhb mRNA expression was determined by qPCR analysis. Obtained data are expressed as the mean ± SEM. * P < 0.05 according to the Mann-Whitney test. LH serum level was evaluated by RIA method. Data represent the mean ± SEM *P < 0.05 and ** P < 0.01 as determined by one-way ANOVA followed by Tukey’s post-hoc test. |
DISCUSSION
To verify the hypothesis that central Gnrh network stimulation affects anterior pituitary Nr5a1/NR5A1 gene and protein expression by altering Ctnnb1/CTNNB1 and Nr0b1/NR0B1 gene/protein regulation, we employed an in vivo model which preserves endogenous hypothalamo-pituitary connections and more closely resembles the physiological context of Gnrh function. Using an i.c.v. pulsatile microinfusion strategy, a measurable Gnrh-induced and Gnrhr-dependent increase in Nr5a1 mRNA expression was found in the female rat anterior pituitary after pulsatile Gnrh treatment. The obtained result is in accordance with a previous report showing that both gonadectomy and intravenous Gnrh pulses administered 30 min apart increased pituitary Nr5a1 mRNA levels in male and female rats. This effect was reversed by suppression of endogenous Gnrh activity (8). More recently, Gnrh centrally applied to male rats at a frequency of 2 pulses every 30 min was shown to up-regulate and maintain within 24 h both Nr5a1 primary transcripts and its steady-state mRNA expression (9). Together with confirmation of the previously recognized Nr5a1 gene transcriptional dependency on Gnrh activity in vivo, the current study time provides novel data concerning the neurohormonal influence on intracellular mechanisms governing Nr5a1/NR5A1 activity in anterior pituitary female rats.
The mechanism(s) involved in Gnrh regulation of Nr5a1 gene transcription are not clear. However, deletional analysis of the rat Nr5a1 gene promoter revealed the presence of functional regulatory sequences between –92 bp and –60 bp, and a consensus E-box motif (CACGTG) at –82/–77 that was required for maximal expression of the Nr5a1 gene in steroidogenic cell types (39). Moreover, in αT3-1 and LβT2 gonadotropes, as well as in adrenocortical cell lines, the critical element for Nr5a1 expression is the E-box, which binds the helix loop helix (βHLH) protein’s upstream simulating factor 1 (USF1) and USF2 (40). The importance of the cis-acting USF binding site as well as the trans-acting USF protein for Gnrh sensitivity was reported for both Cga and Fshb promoters (41, 42). Nevertheless, whether and to what extent Gnrh operates to activate Nr5a1 gene mRNA expression via USF gene and/or protein synthesis requires further research.
Gnrh may also regulate Nr5a1 bioactivity via induction of Ctnnb1, an endogenous co-activator of Nr5a1. In gonadotrope cell lines, the up-regulatory effect of Gnrh on Ctnnb1 activity is expressed predominantly by an increase in CTNNB1 protein translocation from the cytoplasm into the nucleus (43-45). Recently, Jun N-terminal kinase pathway activation was shown to be involved in Gnrh-induced, sustained nuclear accumulation of Ctnnb1 in LβT2 cells (46). Our results showing a Gnrh-induced Ctnnb1 mRNA expression in the anterior pituitary in vivo indicate that regulatory input of this neurohormone is exerted also at the Ctnnb1 gene transcriptional level. Together with the parallel up-regulatory effects found at the CTNNB1 protein level (see further discussion), the presented data suggest that the co-activator’s response may represent an intracellular pathway involved in Gnrhr-dependent Nr5a1 activation in the anterior pituitary gland in vivo.
Furthermore, Gnrh-dependent regulation of Nr5a1 activity may also be exerted at the level of its co-repressor Nr0b1 (9). It has been reported that co-expression of Nr0b1 and Nr5a1 inhibits NR5A1-mediated transactivation of many target genes, including Lhb (47). In the LβT2 line, Gnrh administered at slower frequencies (1 pulse per hour) significantly increased Nr0b1 mRNA expression (48, 49) whereas in primary gonadotropes, Nr0b1 gene expression was activated after Gnrh pulses applied every 30 min (9). In our experimental model, however, exogenous Gnrh pulses given for 6 hours with a frequency of 1 pulse per 45 min were ineffective in stimulating Nr0b1 transcriptional activity. Since both Nr5a1 and Ctnnb1 mRNA were still up-regulated in our study, the Nr0b1-induced feedback mechanism that switches off Nr5a1 activity appears to be not operational yet. To determine whether anterior pituitary Nr5a1/Ctnnb1/Nr0b1 complex activity can be affected by neurohormonal stimulatory input exerted on the endogenous Gnrh neuron network, the influence of centrally applied kisspeptin-10 was the focus of interest in this study. The obtained results revealed kisspeptin’s efficiency in inducing responses for specific pituitary transcription factors, but unlike the results for Gnrh, neither Nr5a1 nor Ctnnb1 mRNA expression was affected.
Instead, an increase in Nr0b1 mRNA as well as its protein expression was found. To our knowledge this is the first report which indicates a relationship between central kissppetin-10 stimulation and Nr5a1/Ctnnb1/Nr0b1 complex transcriptional/translational response in anterior pituitary gland in vivo. The predominant pathway through which kisspeptin-10 could transmit its signal to pituitary target gene(s) is via stimulation of Gnrh neuron activity (19, 20, 50). To date, two major populations of kisspeptin neurons have been identified in the hypothalamus: one located in the arcuate nucleus and another in the preoptic area (51, 52). Some preoptic kisspeptin neurons are localized in the rostral periventricular area of the third ventricle, which in rodents represents the anteroventral periventricular nucleus as well as rostral and caudal portions of the periventricular preoptic nucleus (53). These neurons directly target the Gnrh neuron cell bodies/dendrites (54-56). Kisspeptin neurons projecting from arcuate nucleus are thought to regulate Gnrh neurons at nerve terminals in the median eminence, but were recently shown to also innervate Gnrh neuron cell bodies/dendrites (57). Furthermore, the regulatory effect of kisspeptin could also have been exerted at the level of the median eminence, where axo-axonal contacts between kisspeptin and Gnrh neurons in the internal zone of the median eminence implicate it in exerting stimulatory effects at Gnrh nerve terminals (58, 59). In our study, exogenous kisspeptin was applied directly into the third cerebral ventricle, and thus in the close proximity of both the rostral periventricular area of the third ventricle and the arcuate nucleus. The presented results, indicate, however, that kisspeptin effectively up-regulated both Nr0b1 mRNA and Nr0b1 protein expression. It indicates that mechanism responsible for consequent Nr5a1 down-regulation has been activated. The mechanism(s) that might be responsible for the disparate effects observed after Gnrh or kisspeptin-10 administration remain unknown at present. A kisspeptin-evoked Gnrh network activity desensitization, a phenomenon reported in in vivo studies performed on rats (60, 61) and monkey (62, 63) does not appear to be involved, since luteinizing hormone release increased after kisspeptin-10 administration. It cannot be ruled out, however, that induction of Nr0b1/NR0B1 mRNA/protein expression might result from an input of kisspeptin-induced regulatory signals adequate to affect Nr0b1 transcription and translation. Further in-depth research is required to highlight this problem.
Since the Lhb promoter is one of the gonadotrope target genes whose activity is regulated by the Nr5a1/Ctnnb1/Nr0b1 trans-acting transcription factor, the level of Lhb mRNA expression was also determined. Similarly to the observed stimulation of luteinizing hormone release, both Gnrh and kisspeptin-10 induced a statistically significant change in Lhb gene transcriptional activity, but differences detected in Nr5a1, Ctnnb1 and Nr0b1 expression profiles did not lead to differential Lhb mRNA expression. Although an orchestrated response of all transcription factors (Egr1, Nr5a1, Pitx1/Otx1, Sp1) is required for Gnrh-induced full Lhb promoter activation, an EGR1 protein is now well recognized as a crucial and indispensable mediator of Gnrh-stimulated Lhb transcription (44, 64). In our research an exogenous and i.c.v. given Gnrh and kisspeptin-10 pulses not only affected Nr5a1/Ctnnb1/Nr0b1 complex gene/protein expression, but paralelly up-regulated also Egr1 mRNA level (data not shown). In consequence, Lhb promoter was induced. In a such transcriptional context, an experimentally evoked changes of Nr5a1/Ctnnb1/Nr0b1 gene/protein activity were not adequate to be precisely reflected in a measurable changes of final Lhb mRNA expression in vivo. Furthermore, existing data indicate that in addition to transcriptional stimulation of specific target genes, neurohormones can also modify their mRNAs at a posttranscriptional level. In earlier studies, Gnrh, applied in a pulsatile manner to anterior pituitary cell culture (65) or in vivo directly into the third ventricle (66), was shown to increase polyadenylation and thus the size of α and Lhb subunit mRNAs. More recent data indicate a crucial role of microRNAs in affecting specific mRNAs translatability (67-69).
The second level of intracellular regulation we focused on concerned the actions of Gnrh and kisspeptin-10 on NR5A1, Ctnnb1, and Nr0b1 protein expression and subcellular localization of Ctnnb1. In our study Gnrh administration decreased NR5A1 protein expression as compared to control animals. Taking into account the multiple-level complexity of processes governing gene expression (70), the observed lack of correlation between NR5A1 protein concentration (down-regulation) and its gene transcription (up-regulation) found after i.c.v. Gnrh microinjections may result from the differences in mRNA and protein stability, transcriptional regulation, and attenuation of post-transcriptional processes through high levels of mRNA content (71, 72).
Furthermore, an intracellular ubiquitin-proteasome system linked to transcription via regulation of cyclic promoter associations by transcription factors (73-75) might be, at least in part, responsible for observed discrepancy between gene and protein expression level. Studies on gonadotrope cell line revealed that proteasome participates in the regulation of Gnrh-induced transcriptional-activity. Indeed, Egr1 as well as Nr5a1 transcription factors were shown to be ubiquinated after Gnrh stimulation and ubiquinated forms of these factors associated with Lhb promoter. In consequence, limiting of life-time of transcriptional activators appears to be vital to Gnrh-evoked transcriptional effectiveness (76).
A dichotomy between Nr0b1 mRNA and protein expression has been observed in LβT2 cells (48), as well as between adiponectin and Adipor1/r2 mRNA and protein expression in porcine anterior pituitary cells (77, 78). However, as shown in this study, exogenous Gnrh up-regulated both Ctnnb1, gene transcription and Ctnnb1 protein expression as well as effectively induced Ctnnb1 translocation from the cytoplasm to the nucleus. Our finding of in vivo translational Gnrh-induced Ctnnb1 activity is in full accordance with data obtained from studies on gonadotrope-derived cell lines (5). Moreover, in the case of Nr0b1, exogenous kisspeptin-10 stimulated gene and protein expression. Therefore, centrally applied neurohormones can utilize stimulation of both CTNNB1 and NR0B1 cellular bioavailability to alter NR5A1 transcription factor activity in the anterior pituitary gland in vivo.
In conclusion, the presented data revealed that the pituitary Nr5a1/Ctnnb1/Nr0b1 system is controlled by endogenous Gnrh network activity, and hypothalamic-derived regulatory inputs can affect the processes responsible for gene transcription, translation, and protein intracellular translocation. Taken together, an orchestrated activity of Nr5a1 and its co-activator (Ctnnb1) and co-repressor (Nr0b1) genes, while receiving specific central regulatory input, appears to be involved in gonadotrope-signature transcription factor Nr5a1 induction within this Gnrh-dependent gonadotropic gene network.
Abbreviations: ACTB, β-actin; Cga, glycoprotein hormones, alpha polypeptide; Ctnnb1, catenin beta 1; Fshb, follicle stimulating hormone beta subunit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Gnrh, gonadotropin-releasing hormone; Gnrhr, Gnrh receptor; i.c.v., intracerebroventricular; Lhb, luteinizing hormone beta polypeptide; Nr0b1, nuclear receptor subfamily 0, group B member 1; Nr5a1, nuclear receptor subfamily 5 group A member 1; SDHA, succinate dehydrogenase complex flavoprotein subunit A.
Acknowledgements: This research was founded by the National Science Centre of Poland, decision number DEC-2012/05/D/NZ9/02292.
Conflict of interests: None declared.
REFERENCES
- Ojeda SR, Skiner MK. Puberty in the rat. In: Knobil and Neill’s Physiology of Reproduction. Elsevier, 2006.
- Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHβ-subunit promoter. Endocrinology 2002; 143: 1018-1025.
- Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ. Transcriptional control during mammalian anterior pituitary development. Gene 2003; 319: 1-19.
- Zhao L, Bakke M, Krimkevich Y, et al. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 2001; 128: 147-154.
- Salisbury TB, Binder AK, Grammer JC, Nilson JH. Maximal activity of the luteinizing hormone β-subunit gene requires β-catenin. Mol Endocrinol 2007; 21: 963-971.
- Kaiser UB, Halvorson LM, Chen MT. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (Egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-β gene promoter: an integral role for SF-1. Mol Endocrinol 2000; 14: 1235-1245.
- Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol Cell Biol 1999; 19: 2567-2576.
- Haisenleder DJ, Yasin M, Dalkin AC, Gilrain J, Marshall JC. GnRH regulates steroidogenic factor-1 (SF-1) gene expression in the rat pituitary. Endocrinology 1996; 137: 5719-5722.
- Burger LL, Haisenleder DJ, Marshall JC. GnRH pulse frequency differentially regulates steroidogenic factor 1 (SF1), dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1 (DAX1), and serum response factor (SRF): potential mechanism for GnRH pulse frequency regulation of LH. Endocrine 2011; 39: 212-219.
- Iyer AK, McCabe ERB. Molecular mechanisms of DAX1 action. Mol Genet Metab 2004; 83: 60-73.
- Desclozeaux M, Krylova IN, Horn F, Fletterick RJ, Ingraham HA. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol Cell Biol 2002; 22: 7193-7203.
- Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol 2018; 69: 185-196.
- Parakh TN, Hernandez JA, Grammer JC, et al. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc Natl Acad Sci 2006; 103: 12435-12440.
- Jakimiuk AJ, Nowicka MA, Zagozda M, Koziol K, Lewandowski P, Issat T. High levels of soluble vascular endothelial growth factor receptor 1/sflt1 and low levels of vascular endothelial growth factor in follicular fluid on the day of oocyte retrieval correlate with ovarian hyperstimulation syndrome regardless of the stimulation protocol. J Physiol Pharmacol 2017; 68: 477-484.
- Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update 2014; 20: 485-500.
- d’Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology (Bethesda) 2010; 25: 207-217.
- Herbison AE, d’Anglemont de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010; 151: 312-321.
- Li XF, Kinsey-Jones JS, Cheng Y, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One 2009; 4: e8334. doi: 10.1371/journal.pone.0008334
- Clarkson J, d’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 2009; 21: 673-682.
- Iijima N, Takumi K, Sawai N, Ozawa H. An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti-kisspeptin antibody. J Mol Neurosci 2011; 43: 146-154.
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 2009; 30: 713-743.
- Quaynor S, Hu L, Leung PK, et al. Expression of a functional G protein-coupled receptor 54-kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol 2007; 21: 3062-3070.
- Gajewska A, Zwierzchowski L, Kochman K. Stimulation of luteinizing hormone subunit gene expression by pulsatile intracerebroventricular microinjection of galanin in female rats. J Neuroendocrinol 2004; 16: 558-565.
- Gajewska A, Zielinska-Gorska M, Wasilewska-Dziubinska E, Baran M, Kotarba G, Gorski K. Pituitary galaninergic system activity in female rats: the regulatory role of gonadal steroids. J Physiol Pharmacol 2016; 67: 423-429.
- Romerowicz-Misielak M, Tabecka-Lonczynska A, Koziol K, et al. Changes in gonadotropin-releasing hormone and gonadotropinreleasing hormone receptor gene expression after an increase in carbon monoxide concentration in the cavernous sinus of male wild boar and pig crossbreed. J Physiol Pharmacol 2016; 67: 431-442.
- Gajewska A, Herman AP, Wolinska-Witort E, Kochman K, Zwierzchowski L. in vivo oestrogenic modulation of Egr1 and Pitx1 gene expression in female rat pituitary gland. J Mol Endocrinol 2014; 53: 355-366.
- Caraty A, Skinner DC. Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid: endogenous distribution and exogenous uptake. Endocrinology 2008; 149: 5227-5234.
- Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol 2008; 20: 732-742.
- Rodriguez EM, Blazquez JL, Pastor FE, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 2005; 247: 89-164.
- Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology 2004; 145: 728-735.
- Xu C, Roepke TA, Zhang C, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone (GnRH) activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology 2008; 149: 2459-2466.
- Norberg R, Campbell R, Suter KJ. Ion channels and information processing in GnRH neuron dendrites. Channels 2013; 7: 135-145.
- Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 2005; 132: 703-712.
- Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration- dependent manner engaging multiple signaling pathways. J Neurosci 2009; 29: 9809-9818.
- Paxinos G, Watson C. Atlas of Anatomy of Rat Brain. The Rat Brain in Stereotaxic Coordinates. San Diego, Academic Press Inc., 1986.
- Plant T, Witchel S. Puberty in Nonhuman Primates and Humans. In: Knobil and Neill’s Physiology of Reproduction. Elsevier, 2006.
- Untergasser A, Cutcutache I, Koressaar T, et al. Primer3-new capabilities and interfaces. Nucleic Acids Res 2012; 40: e115. doi: 10.1093/nar/gks596
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002; 30: e36.
- Nomura M, Bartsch S, Nawata H, Omura T, Morohashi KI. An E box element is required for the expression of the ad4bp gene, a mammalian homologue of ftz-f1 gene, which is essential for adrenal and gonadal development. J Biol Chem 1995; 270: 7453-7461.
- Harris AN, Mellon PL. The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol 1998; 12: 714-726.
- Jackson SM, Gutierrez-Hartmann A, Hoeffler JP. Upstream stimulatory factor, a basic-helix-loop-helix-zipper protein, regulates the activity of the alpha-glycoprotein hormone subunit gene in pituitary cells. Mol Endocrinol 1995; 9: 278-291.
- Ciccone NA, Lacza CT, Hou MY, et al. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone β gene. Mol Endocrinol 2008; 22: 1908-1923.
- Gardner S, Maudsley S, Millar RP, Pawson AJ. Nuclear stabilization of beta-catenin and inactivation of glycogen synthase kinase-3beta by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol 2007; 21: 3028-3038.
- Salisbury TB, Binder AK, Nilson JH. Welcoming β-catenin to the gonadotropin-releasing hormone transcriptional network in gonadotropes. Mol Endocrinol 2008; 22: 1295-1303.
- Salisbury TB, Binder AK, Grammer JC, Nilson JH. GnRH-regulated expression of Jun and JUN target genes in gonadotropes requires a functional interaction between TCF/LEF family members and β-catenin. Mol Endocrinol 2009; 23: 402-411.
- Wang Q, Chikina M, Zaslavsky E, Pincas H, Sealfon SC. β-catenin regulates GnRH-induced FSH β gene expression. Mol Endocrinol 2013; 27: 224-237.
- Suzuki T, Kasahara M, Yoshioka H, Morohashi KI, Umesono K. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol 2003; 23: 238-249.
- Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol 2007; 21: 1175-1191.
- Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J 2009; 56: 729-737.
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 2008; 149: 1979-1986.
- Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073-4077.
- Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol 2013; 784: 27-62.
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 2008; 57: 277-287.
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817-5825.
- Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol 2011; 23: 293-301.
- Liu X, Porteous R, d’Anglemont de Tassigny X, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci 2011; 31: 2421-2430.
- Yeo SH. Neuronal circuits in the hypothalamus controlling gonadotrophin-releasing hormone release: the neuroanatomical projections of kisspeptin neurons. Exp Physiol 2013; 98: 1544-1549.
- Uenoyama Y, Inoue N, Pheng V, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 2011; 23: 863-870.
- Kallo I, Vida B, Deli L, et al. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol 2012; 24: 464-476.
- Thompson EL, Murphy KG, Patterson M, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats Am J Physiol Endocrinol Metab 2006; 291: E1074-E1082.
- Roa J, Vigo E, Garcia-Galiano D, et al. Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab 2008; 294: E1088-E1096.
- Seminara SB, DiPietro MJ, Ramaswamy S, Crowley WF, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 2006; 147: 2122-2126.
- Ramaswamy S, Seminara SB, Pohl CR, Dipietro MJ, Crowley WF, Plant TM. Effect of continuous intravenous administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 2007; 148: 3364-3370.
- Jorgensen JS, Quirk CC, Nilson JH. Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev 2004; 25: 521-542.
- Weiss J, Crowley WF, Jameson JL. Pulsatile gonadotropin-releasing hormone modifies polyadenylation of gonadotropin subunit messenger ribonucleic acids. Endocrinology 1992; 130: 415-420.
- Gajewska A, Kochman K, Lerrant Y, Kochman H, Counis R. Modulation of luteinizing hormone subunit gene expression by intracerebroventricular microinjection of gonadotropin-releasing hormone or β-endorphin in female rats. Biochim Biophys Acta 2000; 1523: 217-224.
- Ye RS, Xi QY, Qi Q, et al. Differentially expressed miRNAs after GnRH treatment and their potential roles in FSH regulation in porcine anterior pituitary cell. PLoS One 2013; 8: e57156. doi: 10.1371/journal.pone.0057156
- Ahmed K, LaPierre MP, Gasser E, et al. Loss of microRNA-7a2 induces hypogonadotropic hypogonadism and infertility. J Clin Invest 2017; 127: 1061-1074.
- Cao C, Ding Y, Kong X, et al. Reproductive role of miRNA in the hypothalamic-pituitary axis. Mol Cell Neurosci 2018; 88: 130-137.
- Schwanhausser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature 2011; 473: 337-342.
- Gry M, Rimini R, Stromberg S, et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 2009; 10: 365.
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst 2009; 5: 1512-1526.
- Kang Z, Pirskanen A, Janne OA, Palvimo JJ. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 2002; 277: 48366-48371.
- Dace A, Zhao L, Park KS, et al. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci USA 2000; 97: 8985-8990.
- van der Horst A, de Vries-Smits AM, Brenkman AB, et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 2006; 8: 1064-1073.
- Walsh HE, Shupnik MA. Proteasome regulation of dynamic transcription factor occupancy on the GnRH-stimulated luteinizing hormone β-subunit promoter. Mol Endocrinol 2009; 23: 237-250.
- Smolinska N, Dobrzyn K, Maleszka A, Kiezun M, Szeszko K, Kaminski T. Expression of adiponectin and adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) in the porcine uterus during the oestrous cycle. Anim Reprod Sci 2014; 146: 42-54.
- Kiezun M, Smolinska N, Maleszka A, Dobrzyn K, Szeszko K, Kaminski T. Adiponectin expression in the porcine pituitary during the estrous cycle and its effect on LH and FSH secretion. AJP Endocrinol Metab 2014; 307: E1038-E1046.
A c c e p t e d : June 30 2018