Opioids are known to affect many physiological systems including neuroendocrine regulatory axes (1-3). The results of studies performed with various species indicate that EOPs and their receptors contribute to the functioning of the hypothalamo-pituitary-adrenal (HPA) axis and stress response (2, 3). Various effects (stimulation and/or inhibition) of opioids on HPA axis activity have been reported, depending on species and physiological status of experimental animals (2, 3). Previous studies carried out with pigs have shown that stress – besides the stimulation of the HPA major components (ACTH and cortisol) – may also enhance the pituitary release of ß-endorphin (4). In the cyclic gilts, systemic administration of naloxone (opioid receptor antagonist) was found to elevate plasma cortisol concentration, suggesting an inhibitory action of EOPs on the HPA-axis (5). The study of Esitenne et al. (6) implied that the naloxone effect on cortisol secretion is mainly evoked by its central action. Rushen et al. (7) and Janssens et al. (8) also demonstrated that endogenous opioids can inhibit the HPA axis response to stress in pigs.
The experiments performed with several mammalian species suggest that EOPs may also participate in the regulation of adrenocortical steroidogenesis at the local level. The presence of EOPs in the adrenal cortex has been reported; POMC and PDYN derivatives in rodents (9, 10) and PENK in sheep (11). Specific opioid binding sites have been found in the adrenal cortex of rats (12, 13) and cattle (14). The effects of opioids on the secretory function of this gland have been observed in various animals (15, 16). Nevertheless, available data concerning this problem are still fragmentary and far from satisfactory elucidation of the role of EOPs in the local regulation of adrenal cortex functions.
In our laboratory, the involvement of EOPs in the functioning of hypothalamo-pituitary-gonadal (HPG) axis – including expression of their genes in porcine pituitary and ovary as well as their effects on ovarian steroidogenesis – has been investigated (17-21). The present in vitro studies were undertaken to test whether porcine adrenocortical tissue may be a source of EOPs and which type of opioid receptors is potentially engaged in the regulation of adrenocortical steroidogenesis. In the first part of the study, the presence of mRNAs specific for EOP precursors (PENK, POMC and PDYN) and the influence of ACTH, angiotensin II (ANG II), CRH and epinephrine on the expression of opioid precursor genes in cells isolated from porcine adrenal cortex (experiment I) were determined. Moreover, in the second part of the study, the effects of selective opioid receptor agonists on the basal and ACTH- or ANG II-affected steroid secretion by porcine adrenocortical cells in vitro were evaluated (experiment II).
Animals
This study was carried out in accordance with the principles and procedures of the Animal Ethics Committee at the University of Warmia and Mazury in Olsztyn, Poland. The experiments were performed on dispersed adrenal cortex cells isolated from luteal-phase (days 7-10) cross-bred gilts. The stage of the estrous cycle was determined as described previously (22). Adrenals were recovered from gilts in a local slaughterhouse and transported to the laboratory on ice-cold Ham’s F-12 medium (with L-glutamine, HEPES, sodium bicarbonate; Sigma-Aldrich).
Isolation of adrenocortical cells
The cells were isolated and cultured individually for each experimental animal (N=2 experiments ×8 individuals). Cortical cells were isolated according to the method validated by Kaminska et al. (22, 23). The cortex was dissected from the medulla and minced into small pieces. Then, the tissue was rinsed several times with the medium and treated with 0.03% solution of collagenase type V (Sigma-Aldrich) in Ham’s F-12 medium (pH 7.4) with 5% bovine serum albumin (BSA; MP Biomedicals) at 37°C for 5 min. Cortical cells were collected after 5-6 consecutive digestions of adrenocortical tissue fragments (from both adrenals of individual gilt) and then centrifuged and rinsed three times with a fresh medium. The cells were passed through a nylon filter (60 µm mesh), then counted using a haemocytometer and their viability (>95%) was tested with trypan blue (Sigma-Aldrich). The resulting cells were used in the following two experiments.
Experiment I: the in vitro expression of genes encoding EOP precursors
1. Cell incubation
Dispersed adrenocortical cells were resuspended to a concentration of 6×105 cells/0.9 ml in the incubation medium: DMEM/F-12 HAM’s medium, pH 7.4, with L-glutamine, HEPES, sodium bicarbonate (Sigma-Aldrich), gentamycin (0.05 U/ml; Krka) and 2% BSA. The cell suspension was seeded (2.7 ml) into each culture dish of a 6-well plate (Corning) and preincubated for 1 hour, followed by 20 h incubation with tested factors (diluted in 0.3 ml medium; final volume: 3 ml/well) in a humidified atmosphere of 95% air and 5% CO2 at 37°C (Heraeus incubator, type 6060). The incubations were performed without (control) or with the following hormones: ACTH1-24 (5 nM solution; Synacthen, Ciba), ANG II (100 nM; Sigma-Aldrich), CRH (10 nM; Sigma-Aldrich) or epinephrine (10 µM; Polfa). At the end of incubation, the media were recovered and centrifuged. Adrenocortical cells were harvested and subjected to RNA isolation. In addition, cortisol response to ACTH was tested by measuring (RIA) cortisol concentrations in the cultured media to confirm cell viability and reactivity.
2. RNA isolation and cDNA synthesis
Incubated cells were lysed in the RLT buffer (Qiagen) containing the RNase inhibitor, and they were homogenized by passing the lysate 10 times through a 20-gauge needle fitted to an RNase-free syringe. Total RNA isolation was carried out using an RNeasy Mini Kit in combination with an RNase-Free DNase Set (Qiagen). The quantity and purity of RNA were determined spectrophotometrically (Lambda Bio 10, Perkin Elmer). In addition, the integrity of randomly selected RNAs was examined by 1.5% agarose gel electrophoresis. Reverse transcription was performed using the Verte Kit (Novazym). A mixture containing M-MLV reverse transcriptase (100 U; Novazym), dNTP (0.24 mM each; Novazym), oligo(dT)15 (1 ng; Roche), RNase inhibitor (10 U, Eurx), 2 µg RNA template and Tris-HCl buffer pH 8.3 (Novazym), filled up with RNase-free water (Fermentas) to the final volume of 25 µl, was incubated for 1 hour at 37°C. Reverse transcription was terminated by heating the mixture to 70°C (10 min).
3. Polymerase chain reaction and relative quantification
The levels of PENK, POMC and PDYN cDNAs were monitored by real-time PCR (7500 Real-Time PCR System, Applied Biosystems) followed by the relative quantification protocol (7000 System SDS Software v. 1.2 with RQ Study Application, Applied Biosystem). The housekeeping gene, ß-actin, was used as a normalization control. Oligonucleotide primers specific for target genes were designed using Primer Express software (Applied Biosystems). The primers for porcine ß-actin were used according to Blitek et al. (25). In preliminary studies, the real-time PCR program, primers concentrations and the quantity of the cDNA template were optimized to produce the PCR efficiencies close to 100% for the amplification of both target and housekeeping genes.
Real-time PCRs were carried out in a 96-well plate (Micro Amp Optical Reaction Plate with Adhesive Films, Applied Biosystems) using a SYBR Green dsDNA binding dye-based detection (Power Sybr Green PCR 2×Master Mix, Applied Biosystems). A mixture containing the PCR master mix, uracil N-glycosylase (1 U per reaction; AmpErase, Applied Biosystems), a set of sense and antisense primers (details in Table 1) and 1.5 µl cDNA template, filled up with RNase-free water to the final volume 20 µl, was subjected to the following thermal program: activation of uracil N-glycosylase at 50°C (2 min), heat activation of polymerase at 95°C (10 min) followed by 38 cycles consisting denaturation at 95°C (15 s) and annealing/elongation at 61.5°C (60 s). The SYBR Green fluorescence, corresponding to the cDNA amplification level, was monitored during the second step of each cycle. After 38 cycles, a dissociation (melting) curve analysis was performed to confirm the specificity of PCR. Only a single specific product was obtained for each set of primers. Non-specific products were not present in the analyzed cDNA samples. Selected PCR products were additionally subjected to gel electrophoresis which confirmed their predicted size. Samples were amplified in duplicates for every: individual, gene and tested factor. Negative controls for RT and PCR were also included in the analysis as described elsewhere (26).
| Table 1. Primers specific for genes encoding EOPs precursors (POMC, PENK, PDYN) and ß-actin used for real-time PCR. NCBI – mRNA sequence accession number, TA – the temperature of annealing, TM – melting point of PCR product. |
 |
Experiment II: the effect of opioid receptor agonists on corticosteroid secretion in vitro
1. Cell incubation
Dispersed adrenocortical cells were resuspended at a concentration of 3×105 cells/0.9 ml in the incubation medium: F-12 HAM’s medium, pH 7.4, with L-glutamine, HEPES, sodium bicarbonate (all: Sigma-Aldrich), 0.05 U/ml gentamycin (Krka) and 2% BSA. The cell suspension was seeded (1.8 ml) into each culture dish of 24-well plate (Corning) and preincubated for 0.5 h followed by 5 h incubation with tested factors (diluted in 0.2 ml medium; final volume: 2 ml/well) in a humidified atmosphere of 95% air and 5% CO2 at 37°C (Heraeus incubator, type 6060). The incubations were carried out without (controls) or with delta, mu or kappa opioid receptor agonists: FK 33-824, DPLPE and U50,488, respectively (all from Sigma-Aldrich). The agonists were used at different doses (0.1-100 nM), either alone (to establish their influence on basal steroidogenesis) or in combination with the respective secretagogues: 1 nM ACTH or 10 nM ANG II (to test their effect on stimulated steroidogenesis). At the end of the incubation period, the media were recovered and centrifuged. Medium supernatants were harvested and stored at -20°C until RIA analyses. All incubations were carried out in duplicates.
2. Radioimmunoassay of steroid hormones
The concentrations of cortisol, aldosterone and progesterone in the media were measured by RIA. The specificity of the antibodies against cortisol and progesterone has been described by Szafranska et al. (27) and Ciereszko et al. (28, 29), respectively. An aldosterone assay was performed using a commercially available kit (Active Aldosterone RIA, Diagnostic Systems Laboratories). The assay sensitivities were 15, 8 and 5 pg per tube for cortisol, aldosterone and progesterone, respectively. The intra- and inter-assay coefficients of variation were: 2.7% and 3.7% for cortisol, 4.8% and 9.8% for aldosterone, 3.2% and 4.0% for progesterone, respectively.
Data analysis
The mRNA contents of target genes was expressed as the mean ±S.E.M. (N=8). The values are relative to the housekeeping gene and they are shown in relation to the quantity obtained for non-treated cells (gene expression in control incubation =1). The mean values of eight replicates were compared using a paired two-sample t-test for the means (Statistica 6.0, StatSoft). The differences with P<0.05 were considered as statistically significant.
The results of corticosteroid concentrations were log transformed - due to the variability among their values for different cell cultures - and then subjected to one way analysis of variance for repeated measurements followed by LSD-test (Statistica 6.0, StatSoft). The final results are presented as the mean ±S.E.M. (N=8). The differences with P<0.05 were considered as statistically significant.
Experiment I: the in vitro expression of genes encoding EOP precursors
The results of real-time RT-PCR demonstrated that the genes encoding opioid precursors are expressed in cells isolated from the porcine adrenal cortex. The effects of ACTH and ANG II on the investigated process are presented in Fig. 1. The effects of CRH and epinephrine treatment are not shown.
1. Proenkephalin
In comparison with control, none of the tested hormones had a significant influence on PENK gene transcription in studied cells (Fig. 1A). The content of PENK mRNA was significantly (P<0.05) higher in ANG II-treated cells in comparison with the cells incubated in the presence of ACTH.
 |
Fig. 1. Real-time RT-PCR analysis of in vitro proenkephalin (A), proopiomelanocortin (B) and prodynorphin (C) gene transcription in the porcine adrenocortical cells incubated for 20 h in control conditions and in the presence of ACTH (5 nM) or ANG II (100 nM). Proenkephalin, proopiomelanocortin and prodynorphin cDNA levels are shown in relation to the value obtained for respective control cell incubation (1.0). Asterisks indicate significant (*P<0.05) differences in comparison to control values (cells incubated without treatments). |
2. Proopiomelanocortin
POMC gene transcription was lowered (to 0.59±0.13 of control; P<0.05) in cells collected after incubation with ANG II, but it did not change significantly in response to ACTH (Fig. 1B), CRH and epinephrine.
3. Prodynorphin
Significant differences in PDYN mRNA levels were observed after ACTH and ANG II treatments, but these hormones exerted opposite effects (Fig. 1C). ACTH evidently reduced the yield of the PDYN transcript in the cells (to 0.56±0.10 of control; P<0.05). In turn, ANG II significantly stimulated (nearly two-fold; P<0.05) the transcription of PDYN gene in the cells. CRH and epinephrine did not significantly change PDYN mRNA cell content.
Experiment II: the effect of opioid receptor agonists on corticosteroid secretion in vitro
The influence of selective opioid agonists on basal and ACTH- or ANG II-affected steroidogenesis in isolated adrenocortical cells was studied. ACTH alone (1 nM) effectively elevated cortisol (P<0.01) and progesterone (P<0.01) concentrations in culture media. ANG II alone (10 nM) significantly increased aldosterone secretion (P<0.01). The effects of opioid receptor activation on basal and stimulated (with ACTH or ANG II) cortisol and aldosterone concentrations are presented in Fig. 2 and 3. The results of progesterone secretion are not shown.
1. Cortisol
DPLPE (delta agonist), at the concentration 10 nM, increased the basal secretion of cortisol from adrenocortical cells (P<0.05), but all tested doses of DPLPE failed to affect the ACTH-elevated release of the steroid (Fig. 2A). In turn, FK33-824 (mu agonist) significantly enhanced cortisol concentrations in the media, either alone (P<0.01) or in combination with ACTH (P<0.05; Fig. 2B). U50,488 (kappa agonist) increased the cortisol basal output (p<0.05), but it had no influence on cortisol secretion by ACTH-treated cells (Fig. 2C).
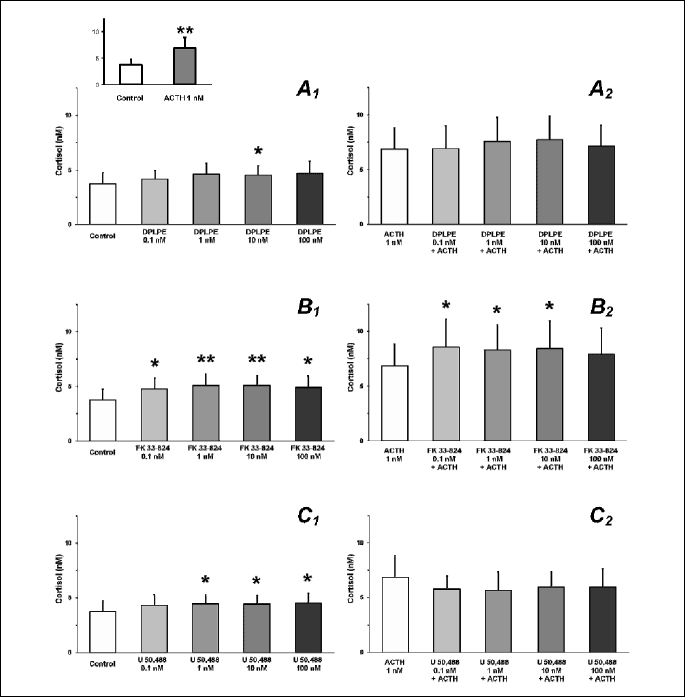 |
| Fig. 2. Effects of the opioid receptor agonists – DPLPE (A), FK33-824 (B) and U50,488 (C) – on the basal (A1, B1, C1) and ACTH-stimulated (A2, B2, C2) in vitro release of cortisol from the porcine adrenocortical cells. Asterisks indicate significant (* P<0.05; ** P<0.01) differences between treatments and respective control (cells incubated without treatment for basal secretion or ACTH-treated cells for stimulated secretion). Inset: positive control with ACTH. |
2. Aldosterone
The basal release of aldosterone from the cultured cells was not affected by any of the tested opioid agonists (Fig. 3A1-3C1). The treatment with 100 nM DPLPE decreased the output of ANG II-stimulated aldosterone (P<0.05; Fig. 3A2). In turn, U50,488 at concentrations 1, 10 and 100 nM attenuated aldosterone secretion by ANG II-treated cells (P<0.05; Fig. 3C2). FK33-824 did not affect aldosterone secretion in the presence of ANG-II (Fig. 3B).
 |
| Fig. 3. Effects of the opioid receptor agonists – DPLPE (A), FK33-824 (B) and U50,488 (C) – on the basal (A1, B1, C1) and ANG II-stimulated (A2, B2, C2) in vitro release of aldosterone from the porcine adrenocortical cells. Asterisks indicate significant (* P<0.05; ** P<0.01) differences between treatments and respective control (cells incubated without treatment for basal or ANG II-treated cells for stimulated secretion). Inset: positive control with ANG II. |
3. Progesterone
None of the tested opioid agonists had a significant influence on basal or ACTH-affected progesterone secretion by isolated adrenocortical cells.
The results of this study indicate that the genes encoding the three major opioid precursors (PENK, POMC, PDYN) are transcribed in the porcine adrenal cortex and that adrenal steroidogenesis can be modulated by EOPs.
In isolated adrenocortical cells, mRNAs for PENK, POMC and PDYN has been found and their synthesis appeared to be affected by ACTH and/or AN II, but not by CRH or epinephrine. ANG II significantly attenuated synthesis of POMC mRNA, but stimulated that of PDYN, while ACTH only inhibited PDYN gene expression. There are very limited data regarding the presence of opioid peptides and/or opioid precursor mRNAs in adrenal cortex. Low, but detectable amounts of immunoreactive enkephalins were found in sheep adrenocortical tissue (11). POMC-derived peptides, ß-endorphin and ACTH, were immunohistochemically revealed in reticular zone of rat and mice adrenals (9). ACTH appeared to influence enkephalin release from calf adrenals (30). The studies of Day et al. (10), performed in vivo with hypophysectomized male rats, revealed the stimulatory effect of ACTH on PDYN gene transcription and contents of dynorphins (i.e. dynorphin A1-17 and dynorphin A1-8) in adrenal cortex. Our in vitro studies confirmed that ACTH is involved in the regulation of PDYN mRNA level, but this effect was opposite to that described by Day et al. (10). This discrepancy may result from distinct experimental protocols (in vivo vs. in vitro) and species or gender differences. It seems that in adrenal cortex ACTH and/or ANG II may directly affect the expression of genes encoding opioid precursors, because these hormones activate c-fos (31), which is known to participate in the regulation of PENK and PDYN gene expression (32, 33). On the other hand, ACTH and ANG II - major secretagogues of corticosteroid secretion - could also indirectly affect the transcription of genes coding for opioid precursors, since steroid hormones were reported to affect EOP synthesis in other tissues (34, 35). Collectively, the present and cited data suggest that the adrenal cortex is capable of synthesizing opioid peptides and thus their involvement in the local regulation of the adrenocortical functions might be suspected.
In the second part of the study mu receptor agonist was shown to markedly increase both, basal and ACTH-stimulated cortisol outputs, while activation of kappa receptor modestly stimulated basal release of this glucocorticoid. ANG-II elevated secretion of aldosterone was effectively reduced by kappa receptor agonist only. Avaiable data, concerning the role of EOPs in the regulation of adrenal steroidogenesis, are fragmentary and sometimes contradictory (15, 16). Kapas et al. (36) reported that agonists of opioid mu and kappa receptors (DAMGO and U50,488, respectively) increased basal corticosterone secretion by isolated adrenocortical cells of rats, whereas delta receptor agonist (DPDPE) was ineffective. In contrast, other in vitro studies demonstrated the inhibitory effect of EOPs on glucocorticoid release from adrenocortical cells of humans (37), guinea-pigs (38) and rats (39). Selective mu opioid receptor agonists also increased basal aldosterone output from cultured rat (36) and bovine (40) adrenocortical cells. On the other hand, Neri et al. (39) reported an inhibitory effect of dynorphins on both, basal and ACTH-elevated aldosterone secretion by adrenocortical cells isolated from rats. These authors have suggested that dynorphins influence post-progesterone adrenocortical steroidogenesis. Consistently, in our experiment, progesterone secretion was affected by none of the opioid agonists tested. Overall, our results confirm the modulatory action of opioids on adrenocortical steroid secretion in the pig, indicating predominant involvement of mu and kappa opioid receptors in this process.
The relationship between the influence of ANG II on PDYN gene expression and the modulatory effect of kappa opioid receptor activation on aldosterone secretion, stated in our study, seems to have the most important physiological impact. ANG II is generally known to stimulate aldosterone secretion. We demonstrated that ANG II also increased PDYN gene expression, suggesting a possibility of elevated production of dynorphins - opioid peptides acting mainly through kappa receptors. On the other hand, the selective stimulation of kappa receptors decreased the aldosterone response to ANG II. Our findings suggest that ANG II exhibits parallel direct (stimulatory) and indirect (inhibitory, mediated by PDYN-derived peptides) effects on adrenocortical aldosterone secretion in pigs. Considering possible modulation of glucocorticoid secretion by opioids it seems that activation of mu receptors may play a primary role in this process since it increased both basal and ACTH-stimulated cortisol output. Therefore, it can be concluded that ß-endorphin, a major endogenous mu receptor ligand, may act synergically with ACTH.
In summary, our results suggest that endogenous opioids can be produced in the porcine adrenal cortex, and that this process might be affected by ACTH (PDYN) and ANG II (PDYN, POMC). The study also proved a possibility of opioid action on adrenocortical steroidogenesis in pigs. It should be emphasized that cortisol secretion appeared to be stimulated mainly by the mu receptor agonist, but aldosterone secretion inhibited by the kappa agonist. It can be generally concluded that endogenous opioid systems may participate in the local, paracrine and/or autocrine, modulation of adrenocortical steroidogenesis in pigs. The studies, employing an imaging techniques, are required to precisely delineate the localization of opioid precursor mRNAs and/or opioid peptides within the porcine adrenal cortex.
Acknowledgments: This study was supported by research grants No. 0206-0208 and 0206-0207 from the University of Warmia and Mazury in Olsztyn. This work was also supported by European Social Fund (ESF).
Conflict of interests: None declared.
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci 1984; 7: 223-255.
- Pechnick RN. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu Rev Pharmacol Toxicol 1993; 33: 353-382.
- Jessop DS. Central non-glucocorticoid inhibitors of the hypothalamo-pituitary-adrenal axis. J Endocrinol 1999; 160: 169-80.
- Roozen AW, Tsuma VT, Magnusson U. Effects of short-term restraint stress on plasma concentrations of catecholamines, b-endorphin, and cortisol in gilts. Am J Vet Res 1995; 56: 1225-7122.
- Barb CR, Kraeling RR, Rampacek GB, Whisnant CS. Influence of stage of the estrous cycle on endogenous opioid modulation of luteinizing hormone, prolactin, and cortisol secretion in the gilt. Biol Reprod 1986; 35: 1162-1167.
- Estienne MJ, Kesner JS, Barb CR, Kraeling RR, Rampacek GB. On the site of action of naloxone-stimulated cortisol secretion in gilts. Life Sci 1988; 43: 161-166.
- Rushen J, Schwarze N, Ladewig J, Foxcroft G. Opioid modulation of the effects of repeated stress on ACTH, cortisol, prolactin, and growth hormone in pigs. Physiol Behav 1993; 53: 923-928.
- Janssens CJ, Helmond FA, Loyens LW, Schouten WG, Wiegant VM. Chronic stress increases the opioid-mediated inhibition of the pituitary-adrenocortical response to acute stress in pigs. Endocrinology 1995; 136: 1468-1473.
- Arefolov VA, Dmitriev AD, Tennov AV, Val’dman AV. Detection of the pro-opiomelanocortin peptide fragments -b-endorphin and ACTH - in the adrenals of rats and mice by immunohistochemistry. Biull Eksp Biol Med 1986; 101: 445-447.
- Day R, Schafer MK, Collard MW, Watson SJ, Akil H. Atypical prodynorphin gene expression in corticosteroid-producing cells of the rat adrenal gland. Proc Natl Acad Sci USA 1991; 88: 1320-1324.
- Dunlap CE, Sundberg DK, Rose JC. Characterization of opioid peptides from maternal and fetal sheep adrenal glands. Peptides 1985; 6: 483-489.
- Quirion R, Finkel MS, Mendelsohn FA, Zamir N. Localization of opiate binding sites in kidney and adrenal gland of the rat. Life Sci 1983; 33: 299-302.
- Dave JR, Rubinstein N, Eskay RL. Evidence that b-endorphin binds to specific receptors in rat peripheral tissues and stimulates the adenylate cyclase-adenosine 3’,5’-monophosphate system. Endocrinology 1985; 117: 1389-1396.
- Gelfand RA, Bobrow A, Pham L, Young C, Parker L. b-endorphin binding in cultured adrenal cortical cells. Endocrine 1995; 3: 201-207.
- Nussdorfer GG. Paracrine control of adrenal cortical function by medullary chromaffin cells. Pharmacol Rev 1996; 48: 495-530.
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 1998; 19: 101-143. Erratum in: Endocr Rev 1998; 19: 301.
- Kaminski T, Gawronska B, Derecka K, Okrasa S, Przala J. Gene expression and peptide localization for LH/hCG receptor in porcine small and large luteal cells: possible regulation by opioid peptides. J Physiol Pharmacol 2000; 51: 359-368.
- Szafranska B, Tilton JE. Free intracellular calcium ([CA2+]i) in opioid sensitive cells of the porcine anterior pituitary. J Physiol Pharmacol 2000; 51: 541-554.
- Staszkiewicz J, Skowronski MT, Kaminski T, et al. Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine theca and granulosa cells. Anim Reprod Sci 2007; 101: 97-112.
- Staszkiewicz J, Skowronski MT, Siawrys G, et al. Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine luteal cells. Acta Vet Hung 2007; 55: 435-449.
- Wylot B, Staszkiewicz J, Okrasa S. The expression of genes coding for opioid precursors, opioid receptors, beta-LH subunit and GnRH receptor in the anterior pituitary of cyclic gilts. J Physiol Pharmacol 2008; 59: 745-758.
- Wylot B, Staszkiewicz J, Okrasa S. The expression of genes coding for opioid precursors, opioid receptors, beta-LH subunit and GnRH receptors in the anterior pituitary of cyclic gilts. J Physiol Pharmacol 2008; 59: 745-758
- Kaminska B, Opalka M, Ciereszko RE, Dusza L. The involvement of prolactin in the regulation of adrenal cortex function in pigs. Domest Anim Endocrinol 2000; 19: 147-157.
- Kotwica G, Kaminska B, Franczak A, et al. The effect of oxytocin on cortisol and corticosterone secretion in cyclic gilts - in vivo and in vitro studies. Reprod Biol 2004; 4: 35-50.
- Blitek A, Waclawik A, Kaczmarek MM, Stadejek T, Pejsak Z, Ziecik AJ. Expression of cyclooxygenase-1 and -2 in the porcine endometrium during the oestrous cycle and early pregnancy. Reprod Domest Anim 2006; 41: 251-257.
- Kaminski T, Smolinska N, Nitkiewicz A, Przala J. Expression of orexin receptors 1 (OX2R) and 2 (OX2R) in the porcine hypothalamus during the oestrous cycle. J Physiol Pharmacol 2010; 61: 363-371
- Szafranska B, Ziecik A, Okrasa S. Primary antisera against selected stoids or proteins and secondary antisera against gamma-globulins - as avaiable tool for studies of reproductive processes. Reprod Biol 2002; 2: 187-203.
- Ciereszko R, Opalka M, Kaminska B, Wojtczak M, Okrasa S, Dusza L. Luteotrophic action of prolactin during the early luteal phase in pigs: the involvement of protein kinases and phosphatases. Reprod Biol 2001; 1: 63-83.
- Nynca A, Jablonska O, Slomczynska M, Petroff BK, Ciereszko RE. Effects of phytoestrogen daidzein and estradiol on steroidogenesis and expression of estrogen receptors in porcine luteinized granulosa cells from large follicles. J Physiol Pharmacol 2009; 60: 95-105.
- Jones CT, Edwards AV, Tindell D. Inhibitory effects of proopiomelanocortin on cortical and medullary activity in the calf adrenal. J Dev Physiol 1992; 17: 69-73.
- Penhoat A, Ouali R, viard I, Langlois D, Saez JM. Regulation of primary response and specific genes in adrenal cells by peptide hormones and growth factors. Steroids 1996; 61: 176-183.
- Kitahara T, Kaneko T, Horii A, et al. Fos-enkephalin signaling in the rat medial vestibular nucleus facilitates vestibular compensation. J Neurosci Res 2006; 83: 1573-1583.
- Campillo A, Gonzalez-Cuello A, Cabanero D, et al. Increased spinal dynorphin levels and phospho-extracellular signal-regulated kinases 1 and 2 and c-Fos immunoreactivity after surgery under remifentanil anesthesia in mice. Mol Pharmacol 2010; 77: 185-194.
- Civelli O, Birnberg N, Comb M, et al. Regulation of opioid gene expression. Peptides 1983; 4: 651-656.
- Pierzchala-Koziec K, Zubel J, Rzasa J. Effect of prolonged progesterone treatment on the proenkephalin mRNA gene expression and enkephalins concentration in the sheep brain. Reprod Biol 2006; 6: 37-46.
- Kapas S, Purbrick A, Hinson JP. Action of opioid peptides on the rat adrenal cortex: stimulation of steroid secretion through a specific mu opioid receptor. J Endocrinol 1995; 144: 503-510.
- Clarke D, Fearon U, Cunningham SK, McKenna TJ. The steroidogenic effects of b-endorphin and joining peptide: a potential role in the modulation of adrenal androgen production. J Endocrinol 1996; 151: 301-307.
- O’Connell Y, McKenna TJ, Cunningham SK. Effects of pro-opiomelanocortin-derived peptides on adrenal steroidogenesis in guinea-pig adrenal cells in vitro. J Steroid Biochem Mol Biol 1993; 44: 77-83.
- Neri G, Andreis PG, Malendowicz LK, Nussdorfer GG. Inhibitory effect of dynorphins on the secretory activity of isolated rat adrenocortical cells. Biomed Res 1990; 11: 277-281.
- Bevilacqua M, Vago T, Raggi U, et al. in vitro steroidogenic properties of FK 33 824, a stable analog of methionine-enkephalin. Opiate-dopamine interaction in the control of aldosterone production. J Endocrinol Invest 1982; 5: 277-280.