MYOGENIC CELLS APPLICATIONS IN REGENERATION
OF POST-INFARCTION CARDIAC TISSUE
INTRODUCTION
Stem cells have been under investigations due to their remarkable potential of growth and differentiation into many cell types in the body, with the promise for treating variety of diseases and injuries. Stem cell technology could deliver tissue regeneration after injuries for which natural repair mechanisms are not able to restore functional recovery and for which current therapeutic strategies have minimal effectiveness. Stem cells hold great promise for regenerative medicine due to their potential to regenerate tissues without the production of scar tissue, which is commonly related to healing processes.
Myocardial infarction has become one of the leading causes of death among people in industrialized nations. The percentage of people suffering from heart infarctions is rising every year, which is related to the increasing age of population and with improper lifestyle (bad eating habits, stress etc.). Myocardial infarction leads to the loss of cardiomyocytes, which is caused by reduced blood flow triggering a cascade of events including inflammation, formation of non-contracting fibrous scars (leading to a decrease in systolic function and left ventricle remodeling), changes in the workload of the myocardium and, if the damaged area is large sufficiently, it may cause the congestive heart failure (1-3). In the place of cardiomyocytes which underwent apoptosis and necrosis a scar is formed, incapable of contraction. The scar tissue should be replaced by a new, functionally active cardiac tissue in order to restore the heart functions (4). Rescued cardiomyocytes may attempt to compensate the loss of function and repair the tissue after myocardial infarction. However, myocardial restoration is inadequate due to low regenerative capabilities of mature cardiac cells (they are postmitotic and incapable of replication) to replace dead cells after the process of ischemia (5).
Data mentioned above mobilized physicians to search for methods, which could reshape the dilated left ventricle (LV) through the use of drugs, heart transplantation and cardiac devices. Despite a significant improvement in patients’ survival, these therapies have been shown to be insufficient (3, 6, 7). Consequently, investigators are currently exploring the use of stem cells transplantation into myocardium to limit infarct size and reduce the subsequent cardiac failure incidence. At the moment, cell transplantation is a very promising and the most realistic method of treating heart disease (8). The main goal of this therapy is to repopulate post-infarction scar tissue with contractile cells, which could replace dead cardiac myocytes, restore systolic function and prevent the remodeling process (3, 9). Research has been conducted to find the optimal cell type which could be implanted in the post-infarct scar. Investigators have sought the mechanism by which the implanted cells improve cardiac function, and factors allowing their longer survival after transplantation (8, 10, 11).
So far the exact mechanism of stem cells activity in treating myocardial infarction remains unknown. Some reports indicate their differentiation potential into cardiomyocytes, which are capable of contraction. Others suggest the possible role of factors released from stem cells after transplantation, which act locally to stimulate angiogenesis and recruit other cells to the area of infarction scar, thus improving cardiac function by inhibiting apoptosis and fibrosis (12-14).
Many different cell types have been transplanted into myocardium in animal models and subsequently in humans (10, 14, 15). However, autologous skeletal myoblasts seem to be one of the best candidates for cardiac repair, due to their biological properties, structural and functional similarities between cardiomyocytes and myocytes, as well as lack of ethical and immunological problems (8, 16).
Therefore, in this paper we have tried to review the progress in skeletal myoblast-based treatment of myocardial infarction, summarize their current state of clinical application in the regeneration of cardiac tissue, as well as, discuss the complications resulting from skeletal myoblast transplantation into the infarcted area.
SKELETAL MYOBLASTS
Skeletal muscle stem cells (SMSC) are quiescent mononucletaled cells, which are normally located outside the sarcolemma within the basal lamina of the muscle fiber (8, 17). Their activation is a result of injury, which is manifested by mobilization, proliferation, differentiation and ultimately fusion into new muscle fibers (17-20) (Fig. 1). Jankowski et al. (21) described four different populations of SMSC: satellite cells (SC), muscle derived stem cells (MDSC), skeletal myoblasts (SMs) and their side population (SP) (8), however myoblasts are progeny of satellite cells obtained upon biopsy of skeletal muscle followed by in vitro culture (3).
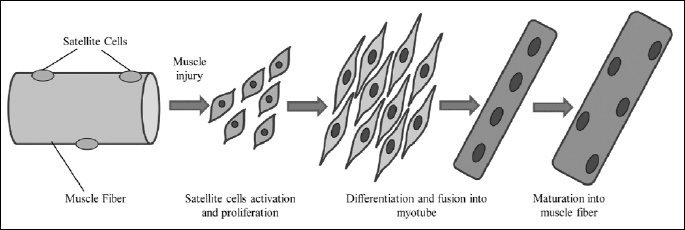
Due to the high regenerative potential of myoblasts, the ease of isolation, high in vitro proliferative capacity, their autologous origin (which solves the problem of rejection by organism) and unipotential characteristic (which prevents tumor formation), they are one of the most encouraging cell sources for clinical therapy including muscle regeneration (muscular dystrophies), heart disease and urinary incontinence (6, 8, 9, 22-24) (Fig. 2). Moreover, these cells are resistant to ischemia, which increases their chance of survival in the environment of infarction scar (10, 25).
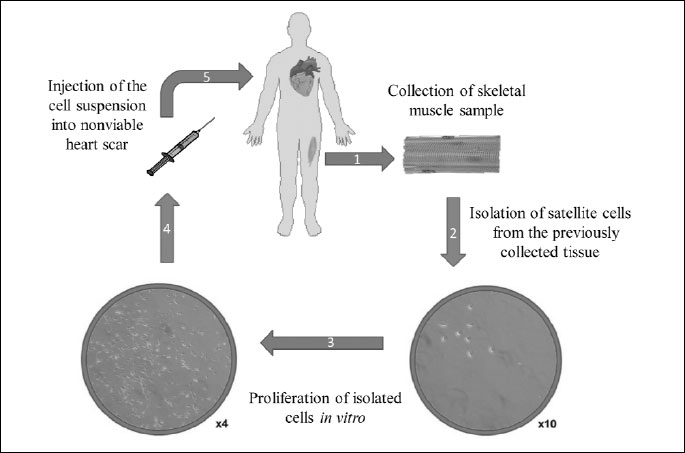
The transplantation of skeletal myoblasts has been used both experimentally and clinically in the attempts to restore cardiac function. Several clinical trials have been performed in order to evaluate the effectiveness of myoblasts in the treatment of heart failure. Research conducted by Menasche et al. (26) based on human autologous skeletal myoblasts transplantation into nonviable heart scar revealed after a 10-month follow-up study an increase in left ventricular ejection fraction (LVEF) and also improvement in systolic shortening in SMs-implanted scar. Moreover, their elastic properties provided the strengthening a scaffold of ventricular wall and reduction in size of post-infarction scar in examined patients. The only adverse event, probably related to the procedure, was the occurrence of sustained, monomorphic ventricular tachycardia (VT) in a short time after surgery in some patients but most were clinically well tolerated (26). Similarly, the trial conducted by Zhang et al. (27) also presented an increase in LVEF, improvement of ventricular wall thickness and perfusion in the area where the cells were injected after 4-month follow-up examination. Moreover, decreased left ventricular diastolic diameter was recognized. Positive outcomes were also noted by Dib et al. (28) during 24-months post-transplantation period. The group not only described improvement in LVEF and reduction in left ventricular systolic and diastolic volumes, but also observed new areas of viability within the infarction scar. Additionally, histological analysis revealed survival and engraftment of the implanted myoblast. However, in this trial authors also reported some adverse events in patients, such as ventricular fibrillation, atrial fibrillation, ventricular tachycardia, whereas the majority of those cases of arrhythmia was generally well tolerated clinically.
The results obtained in the aforementioned clinical trials have shown that autologous skeletal myoblast transplantation for treating heart failure is feasible and safe with some incidents of cardiac arrhythmia occurring in patients after cell transplantation. Furthermore, these results have demonstrated the survival and engraftment of transplanting skeletal myoblasts into the infarcted myocardium and also early benefit of cellular cardiomyoplasty, suggesting that this method offers a potential therapeutic use for heart failure treatment (26-28). Similar results were obtained by Siminiak et al. (29) in the first phase of clinical study with a 12 month follow-up. Researchers observed improvement in myocardial contractility and also an increase in the left ventricular ejection fraction. Hagege et al. (30) confirmed in the long-term follow-up study that autologous skeletal myoblast transplantation was safe (absence of tumor development), feasible and relatively straightforward procedure. Additionally, stable improvement of clinical status and ejection fraction was observed in examined patients and eventual arrhythmic risk could be controlled by medical therapy.
On the other hand large multicenter trial (MAGIC) did not confirm the optimistic results showing that myoblast transplantation can be a promising therapy for the treatment of heart failure. In this trial the myoblast-treated patients did not shown an incremental improvement in regional or global LV function. Moreover, in MAGIC trial a two times higher number of arrhythmias was noted in each of the myoblast-treated groups in comparison to the placebo group (31).
Obviously, apart from SMs transplantation, there were many studies conducted using other methods that help healing the infarcted area. Authors of these studies confirmed the improvement of cardiac function in patients with heart failure. Steendijk et al. (32) reported that implantation of cardiac resynchronization device, used in cardiac resynchronization therapy (CRT), improved LVEF, decreased end-diastolic pressure and reverse remodeling in patients with heart failure and intraventricular conduction delay. Moreover, Witkowski et al. (33) revealed that surgical ventricular restoration (SVR) in ischemic heart failure patients improves clinical symptoms and left ventricular systolic function as well as the quality of life. However, SMs transplantation therapy seems to be the most promising because it uses autologous SMs, which are most similar to cardiomyocytes, easy to collect from the patient’s muscle and show no immunological conflict. These characteristics of SMs transplantations give hope to complete or almost complete regression of post-infarction lesions and full heart recovery.
GENETIC MANIPULATIONS ON SKELETAL MYOBLASTS
Manipulations of adherence and gap junction proteins
Successful myoblast therapy depends on several factors, such as: delivery to the target tissue, long-term survival, effectiveness of engraftment, differentiation into cardiomyocytes and integration with the new microenvironment (3).
The mechanism by which implantation of skeletal myoblasts (SMs) may improve heart function is still unclear, in particular, because the transplanted cells are not functionally or electrically integrated with the host myocardium (10). Although, engrafted myoblasts improve post-infarct cardiac function, they do not transdifferentiate into cardiomyocytes and do not appear to express cardiac specific proteins (9, 26). The lack of cardiac-specific genes (N-cadherin and connexin43 (Cx43)), which are required for electro-mechanical coupling with cardiomyocytes, is the reason for inability of engrafted SMs to establish junction with host cardiac cells, which may indicate the post-implantation risk of ventricular arrhythmias (34-37). Studies performed by many investigators, have shown that the major adhesion and gap junction proteins are expressed in undifferentiated skeletal myoblasts, while after differentiation into myotubes the expression of these proteins decreases and is particularly absent in the skeletal muscle grafts in injured rat hearts (34, 35) (Fig. 3). Makkar et al. (38) also revealed that the expression of cardiac-specific proteins was decreased during differentiation of myoblasts to more contractile myotubes, which results in the potential for arrhythmia after transplantation (Fig. 3). This lack of cell-to-cell junctions like connexin43 on skeletal myotubes suggests that these cells do not beat in synchrony with the rest of the heart (23) (Fig. 3). The absence of electro-mechanical coupling contrasts with in vitro studies carried out by Reinecke et al. (39) and Formigli et al. (40). Authors demonstrated that myoblasts co-cultured with cardiomyocytes were coupled by functional gap junctions and also showed that cardiomyocytes enhanced gap junction communication and up-regulate expression of Cx43 in myoblasts. However after differentiation into myotubes the expression of N-cadherin and connexin43 was down-regulated (39) (Fig. 3). Many studies were performed in order to increase the expression of gap junction proteins in SMs, which could reduce the arrhythmogenic potential of transplanted cells. A significant enhancement of gap junction communication via connexin43, which expression is normally down-regulated during differentiation, was obtained in in vitro studies by gene transfer (41) and cyclic stretching (42). Roell et al. (43) using a transgenic mouse model have indicated that the transplantation of genetically modified skeletal myoblasts expressing Cx43 in myocardial infarction scar prevents post-infarction arrhythmia. Similar results were obtained by Fernandes et al. (44) via the cardiac engraftment of autologous connexin43-overexpressing myoblasts in infarcted rats. These results demonstrated that overexpression of Cx43 caused a significant improvement in cardiac function as well as reduction of the risk of post-transplantation arrhythmia. On the basis of the studies done on rat skeletal myoblasts by two research groups: Suzuki et al. (45) and Tolmahov et al. (46), it has been concluded that transgenic over-expression of Cx43 in skeletal myoblasts improves their electrical coupling with cardiac myocytes in vitro and prevents post-infarct arrhythmia. Moreover, these cells demonstrated more rapid differentiation which could also be advantageous in a graft for transplantation to the heart (45).
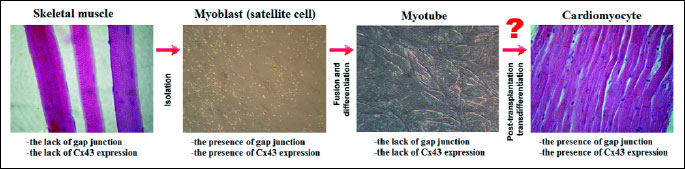
In order to achieve the best possible results of stem cell therapy, physicians have investigated the non-transgenic manipulation of connexin43 expression. Perumal Srinivascan et al. (10) performed an in vitro study, in which skeletal myoblasts were integrated into an engineered tissue construct, and demonstrated a long term survival, ordered alignment, as well as, a preserved ability to differentiate into contractile myotubes. Moreover, their studies showed that the passive longitudinal tensile stress caused maintained or elevated expression of gap junction and adherence proteins during differentiation, which could indicate that the mechanical loading of SMs may improve electromechanical integration with the myocardium. In vitro studies carried out by Zhang et al. (47) also demonstrated, that the mechanical forces promoted tissue morphogenesis, increased the rigidity of the extracellular matrix and formation of structurally organized cardiac muscle tissue.
Manipulations of growth factors expression
The major problem of the skeletal myoblasts therapy is the high degree of cell death induced by inflammatory process in response to transplanted cells, as well as, inadequate blood supply in the infarcted tissue. Currently, the genetic modifications of skeletal myoblasts seem to be particularly promising in increasing graft survival. The primary purpose of gene therapy is to increase blood circulation in the injured heart, resulting in a better delivery of oxygen and nutrients to the tissue, which could be beneficial in prevention of massive apoptosis of the transplanted cells (8, 9). In order to increase angiogenesis and vasculogenesis, researchers performed a number of studies on rabbit hind limb ischemia model, including the effect of pro-angiogenic factors, with particular reference to vascular endothelial growth factor (VEGF) and the fibroblast growth factor (FGF), due to their ability to stimulate angiogenesis, proliferation and migration of cells involved in organ repair and also their anti-apoptotic potential (48, 49). Recent studies on rat model of ischemic cardiomyopathy performed by Askari et al. (50), Yau et al. (51, 52), and Xia et al. (53) showed that SMs transfected with VEGF improved cardiac function, induced angiogenesis and also increased vascular density in infarcted area. Furthermore, it was demonstrated that cell-based delivery of VEGF suppressed the inflammatory response and limited apoptosis of transplanted cells and cardiomyocytes in the area of damaged tissue (Fig. 4). Similar observations on the same myocardial infarction animal model were made by Tambara et al. (54), who showed enhanced neovascularization, vessel density and increased survival of implanted cells, when myoblast expressing fibroblast growth factor were grafted into infarcted scar. In vitro research on murine myoblast, using simultaneous overexpression of two potent pro-angiogenic genes encoding the fibroblast growth factor 4 (FGF4) and vascular endothelial growth factor-A (VEGF-A), revealed the tendency to faster proliferation of murine C2C12 cells and increased capillary formation, which could improve the delivery of oxygen and nutrients to the infarcted zone, and probably prevent massive cell death after stem cells transplantation (8) (Fig. 4).
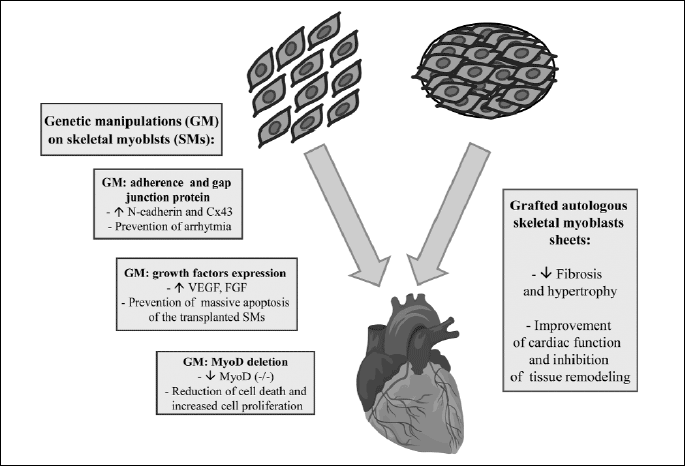
Additionally, studies by Niagara et al. (55) and Suzuki et al. (56) demonstrated that pharmacologic preconditioning of myoblasts, leading to increased viability of the cells under oxidative stress in vitro, enhanced cell survival after the injection into cardiac tissue (57).
MyoD deletion
Research performed by Nakamura et al. (14) on mouse model demonstrated that myoblast lacking the MyoD gene (MyoD–/–) exhibited high resistance to hypoxia, causing a superior efficacy of engraftment, as a large number of Myod–/– myoblast survived after transplantation. Moreover, the authors observed a reduction of cell death and increased cell proliferation, as well as improvement in systolic cardiac function (there were reductions of left ventricular end-diastolic and end-systolic dimensions, and increased ejection fraction) (Fig. 4). This study showed that transplantation of MyoD–/– myoblast into an infarcted mouse heart induced angiogenesis in the area of injury via the secretion of paracrine angiogenic factors including the stromal cell-derived factor-1 (SDF-1) and placental growth factor (PIGF) by MyoD–/– myoblast. The research performed earlier by Hirai et al. (58) on mice revealed that MyoD–/– myoblasts acquired a remarkable resistance to apoptosis by up-regulation of anti-apoptotic genes, including: Pax3, Bcl-2, Bcl-xL. The results presented above, suggest that myoblasts with suppressed MyoD function, might be useful in the stem cells-based treatment.
USE OF AUTOLOGOUS SKELETAL MYOBLAST SHEETS
To overcome problems related to the intramyocardial injection of cells, including cell loss and a limited graft area, a cell delivery system was described, which uses tissue-engineered myoblast grafts grown as sheets (59). Research conducted on canine (59), hamster (60) and rat heart failure model (61, 62) have shown, that transplantation of autologous skeletal myoblast sheets allowed better improvement of the global cardiac function compared with direct myocardial injection (Fig. 4). Skeletal myoblast sheet transplantation repaired the impaired myocardium, suppressed the ventricular fibrosis and increased capillary density, which may indicate that this method might become a novel therapeutic strategy for patients with severe heart failure (Fig. 4). It is considered, that the improved therapeutic effect of the myoblast sheet results from their production of paracrine factors, which locally stimulate the injured myocardium (61). Sekiya et al. (63) demonstrated, using rat model, that layered implementation of myoblast sheets attenuates adverse cardiac remodeling of infarcted heart. A substantial improvement of cardiac function was manifested by fewer fibrosis and less hypertrophy (Fig. 4). Moreover, after transplantation of layered myoblast sheets into the injured area the amount of elastic fibers significantly increased. This data suggests that the expression of elastin is one of the main factors inducing therapeutic effect of myoblast sheets engraftment and the overexpression of elastin could enhance these effects. Uchinaka et al. (11) has recently revealed that the elastin gene modification in the implanted myoblast sheets improves cardiac function and inhibits tissue remodeling and dilation of the left ventricular chamber. Researchers suppose that the improvement in the left ventricular performance may be due to the formation of elastin fibers in infarcted area. Moreover, elastin gene-transfected myoblast sheets improved the long term effects of cardiac function, caused by the secretion of paracrine growth factors in the early stage of treatment, and later by the formation of elastin fibers, which generate elasticity in the infarcted zone, as their lifespan is extremely high.
Another problem that needs to be approached in the field of autologous skeletal myoblasts transplantations is the number of implanted cells and the time of implantation after myocardial infarction. Simultaneous injection of large number of cells causes massive cell death and a substantial risk of tissue overgrowth (3). Research conducted on rat models (54, 64) have shown that repetitive injection of myoblast improved the function and contractility of left ventricular, and also increased the engraftment area, as compared to the single implantation. These results suggest that the repeated administration of myoblasts may reduce the risk of massive cell death more effectively than a single injection of their higher number and this method is also a safe therapeutic strategy for the infarcted myocardium treatment.
CONCLUSIONS
Skeletal myoblast-based therapy for heart failure is one of the most promising methods of treatment due to the fact that the implantation of SMs increases cardiac contractility, improves post-infarct cardiac function, limits infarct expansion and heart remodeling. Moreover, skeletal myoblasts seems to be one of the best candidates for cardiac repair because of their myogenic and contractile phenotype, high regenerative potential and proliferative capacity in vitro, ease of isolation, availability for autologous transplantation as well as resistance to tissue ischemia.
Although skeletal myoblasts are significantly tolerant to poor graft environment, a large number of cells transplanted into myocardium do not survive due to inadequate blood supply in infarcted tissue. Nowadays, the genetic modifications of SMs seem to be a very effective method of increasing their survival, thereby resulting in a better delivery of oxygen and nutrients in the damaged tissue, which could be beneficial in prevention of massive apoptosis of engrafted cells (Fig. 4).
Despite encouraging data related to clinical applications of SMs into post-infarction scar tissue, the mechanism by which these cells improve cardiac function is still unknown. Studies have shown that SMs do not form electromechanical connections with host cariomyocytes, which results in ventricular arrhythmias. In order to reduce the arrhythmogenic potential of transplanted cells the transgenic and non-transgenic manipulations were conducted, leading to significant improvement of heart function and reduction of post-transplantation arrhythmia (Fig. 4).
It is undeniable that stem cells therapy brings hope to many patients with myocardial infarction who, otherwise, may suffer from the continuing progress of heart disease, finally leading to death.
Acknowledgements: This study was supported by National Science Centre Grants No. N N311 123538 and UMO-2011/03/B/NZ9/03987.
The authors thank Dr. Malgorzata Gajewska for help with manuscript preparation.
Conflict of interests: None declared.
REFERENCES
- Czarnowska E, Gajerska-Dzieciatkowska M, Kusmierski K, et al. Expression of SDF-1-CXCR4 axis and an anti-remodelling effectiveness of foetal-liver stem cell transplantation in the infarcted rat heart. J Physiol Pharmacol 2007; 58: 729-744.
- Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 2003; 92: 139-150.
- Seidel M, Borczynska A, Rozwadowska N, Kurpisz M. Cell-based therapy for heart failure: skeletal myoblasts. Cell Transplant 2009; 18: 695-707.
- Nilsson JC, Groenning BA, Nielsen G, et al. Left ventricular remodeling in the first year after acute myocardial infarction and the predictive value of N-terminal pro brain natriuretic peptide. Am Heart J 2002; 143: 696-702.
- Taylor DA. Cell-based myocardial repair: how should we proceed? Int J Cardiol 2004; 95(Suppl. 1): S8-S12.
- Menasche P. Skeletal muscle satellite cell transplantation. Cardiovasc Res 2003; 58: 351-357.
- Zhang F, Pasumarthi KB. Embryonic stem cell transplantation: promise and progress in the treatment of heart disease. BioDrugs 2008; 22: 361-374.
- Bialas M, Krupka M, Janeczek A, et al. Transient and stable transfections of mouse myoblasts with genes coding for pro-angiogenic factors. J Physiol Pharmacol 2011; 62: 219-228.
- Burdzinska A, Berwid S, Orzechowski A. Muscle cell transplantations: the ups and downs. Post Hig Med Dosw 2005; 59: 299-308.
- Perumal Srinivasan S, Neef K, Treskes P, et al. Enhanced gap junction expression in myoblast-containing engineered tissue. Biochem Biophys Res Commun 2012; 422: 462-468.
- Uchinaka A, Kawaguchi N, Hamada Y, et al. Transplantation of elastin-secreting myoblast sheets improves cardiac function in infarcted rat heart. Mol Cell Biochem 2012; 368: 203-214.
- Pelacho B, Nakamura Y, Zhang J, et al. Multipotent adult progenitor cell transplantation increases vascularity and improves left ventricular function after myocardial infarction. J Tissue Eng Regen Med 2007; 1: 51-59.
- Shintani Y, Fukushima S, Varela-Carver A, et al. Donor cell-type specific paracrine effects of cell transplantation for post-infarction heart failure. J Mol Cell Cardiol 2009; 47: 288-295.
- Nakamura Y, Asakura Y, Piras BA, et al. Increased angiogenesis and improved left ventricular function after transplantation of myoblasts lacking the MyoD gene into infarcted myocardium. PLoS One 2012; 7: e41736.
- Laflamme MA, Murry CE. Heart regeneration. Nature 2011; 473: 326-335.
- Siminiak T, Kalmucki P, Kurpisz M. Autologous skeletal myoblasts for myocardial regeneration. J Interv Cardiol 2004; 17: 357-365.
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 2001; 91: 534-551.
- Grounds MD, White JD, Rosenthal N, Bogoyevitch, MA. The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem 2002; 50: 589-610.
- Burdzinska A, Gala K, Paczek L. Myogenic stem cells. Folia Histochem Cytobiol 2008; 46: 401-412.
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 2006; 54: 1177-1191.
- Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther 2002; 9: 642-647.
- Burdzinska A, Bartoszuk-Bruzzone U, Godlewski MM, Orzechowski A. Sodium ascorbate and basic fibroblast growth factor protect muscle-derived cells from H2O2-induced oxidative stress. Comp Med 2006; 56: 493-501.
- Siminiak T, Meliga E, Jerzykowska O, Serruys PW. Percutaneous transplantation of skeletal myoblast in the treatment of post-infarction injury. Eur Heart J 2006; 8(Suppl.): H57-H64.
- Bartoszuk-Bruzzone U, Burdzinska A, Orzechowski A, Klos Z. Protective effect of sodium ascorbate on efficacy of intramuscular transplantation of autologous muscle-derived cells. Muscle Nerve 2012; 45: 32-38.
- Menasche P. Skeletal myoblasts and cardiac repair. J Mol Cell Cardiol 2008; 45: 545-553.
- Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 2003; 41: 1078-1083.
- Zhang F, Yang Z, Chen Y, et al. Clinical cellular cardiomyoplasty: technical considerations. J Card Surg 2003; 18: 268-273.
- Dib N, McCarthy P, Campbell A, et al. Feasibility and safety of autologous myoblast transplantation in patients with ischemic cardiomyopathy. Cell Transplant 2005; 14: 11-19.
- Siminiak T, Kalawski R, Fiszer D, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J 2004; 148: 531-537.
- Hagege AA, Marolleau JP, Vilquin JT, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation 2006; 114(Suppl. 1): I108-I113.
- Menasche P, Alfieri O, Janssens S, et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial. First randomized placebo-controlled study of myoblast transplantation. Ciculation 2008; 117: 1189-1200.
- Steendijk P, Tulner SA, Bax JJ, et al. Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure-volume loops. Circulation 2006; 113: 1295-1304.
- Witkowski TG, ten Brinke EA, Delgado V, et al. Surgical ventricular restoration for patients with ischemic heart failure: determinants of two-year survival. Ann Thorac Surg 2011; 91: 491-498.
- Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol 2002; 34: 241-249.
- Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci USA 2003; 100: 7808-7811.
- Fernandes S, Amirault JC, Lande G, et al. Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res 2006; 69: 348-358.
- Gandolfi F, Vanelli A, Pennarossa G, Rahaman M, Acocella F, Brevini TA. Large animal models for cardiac stem cell therapies. Theriogenology 2011; 75: 1416-1425.
- Makkar RR, Lill M, Chen PS. Stem cell therapy for myocardial repair: is it arrhythmogenic? J Am Coll Cardiol 2003; 42: 2070-2072.
- Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol 2000; 149: 731-740.
- Formigli L, Francini F, Tani A, et al. Morphofunctional integration between skeletal myoblasts and adult cardiomyocytes in coculture is favored by direct cell-cell contacts and relaxin treatment. Am J Physiol Cell Physiol 2005; 288: C795-C804.
- Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res 2005; 97: 159-167.
- Iijima Y, Nagai T, Mizukami M, et al. Beating is necessary for transdifferentiation of skeletal muscle-derived cells into cardiomyocytes. FASEB J 2003; 17: 1361-1363.
- Roell W, Lewalter T, Sasse P, et al. Engraftment of connexin43-expressing cells prevents post-infarct arrhythmia. Nature 2007; 450: 819-824.
- Fernandes S, van Rijen HV, Forest V, et al. Cardiac cell therapy: overexpression of connexin43 in skeletal myoblasts and prevention of ventricular arrhythmias. J Cell Mol Med 2009; 13: 3703-3712.
- Suzuki K, Brand NJ, Allen S, et al. Overexpression of connexin 43 in skeletal myoblasts: relevance to cell transplantation to the heart. J Thorac Cardiovasc Surg 2001; 122: 759-766.
- Tolmachov O, Ma YL, Themis M, et al. Overexpression of connexin 43 using a retroviral vector improves electrical coupling of skeletal myoblasts with cardiac myocytes in vitro. BMC Cardiovasc Disord 2006; 6: 25.
- Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M, Labouesse M. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 2011; 471: 99-103.
- Rissanen TT, Markkanen JE, Arve K, et al. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J 2003; 17: 100-102.
- Rissanen TT, Korpisalo P, Markkanen JE, et al. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation 2005; 122: 3937-3946.
- Askari A, Unzek S, Goldman CK, et al. Cellular, but not direct, adenoviral delivery of vascular endothelial growth factor results in improved left ventricular function and neovascularization in dilated ischemic cardiomyopathy. J Am Coll Cardiol 2004; 43: 1908-1914.
- Yau TM, Fung K, Weisel RD, Fujii T, Mickle DA, Li RK. Enhanced myocardial angiogenesis by gene transfer with transplanted cells. Circulation 2001; 104(Suppl. 1): I218-I222.
- Yau TM, Li G, Weisel R, et al. Vascular endothelial growth factor transgene expression in cell-transplanted hearts. J Thorac Cardiovasc Surg 2004; 127: 1180-1187.
- Xia JH, Xie AN, Zhang KL, Xu L, Zheng XY. The vascular endothelial growth factor expression and vascular regeneration in infarcted myocardium by skeletal muscle satellite cells. Chin Med J 2006; 119: 117-121.
- Tambara K, Tabata Y, Komeda M. Factors related to the efficacy of skeletal muscle cell transplantation and future approaches with control-released cell growth factors and minimally invasive surgery. Int J Cardiol 2004; 95(Suppl. 1): S13-S15.
- Niagara MI, Haider HKh, Jiang S, et al. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res 2007; 100: 545-555.
- Suzuki K, Murtuza B, Beauchamp JR, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J 2004; 18: 1153-1155.
- Burdzinska A, Bartoszuk U, Orzechowski A. Preincubation with bFGF but not sodium ascorbate improves efficiency of autologous transplantation of muscle-derived cells into urethral wall. Urology 2009; 73: 736-742.
- Hirai H, Verma M, Watanabe S, Tastad C, Asakura Y, Asakura A. MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J Cell Biol 2010; 191: 347-365.
- Hata H, Matsumiya G, Miyagawa S, et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J Thorac Cardiovasc Surg 2006; 132: 918-924.
- Kondoh H, Sawa Y, Miyagawa S, et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res 2006; 69: 466-475.
- Memon IA, Sawa Y, Fukushima N, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg 2005; 130: 1333-1341.
- Hoashi T, Matsumiya G, Miyagawa S, et al. Skeletal myoblast sheet transplantation improves the diastolic function of a pressure-overloaded right heart. J Thorac Cardiovasc Surg 2009; 138: 460-467.
- Sekiya N, Matsumiya G, Miyagawa S, et al. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J Thorac Cardiovasc Surg 2009; 138: 985-993.
- Premaratne GU, Tambara K, Fujita M, et al. Repeated implantation is a more effective cell delivery method in skeletal myoblast transplantation for rat myocardial infarction. Circ J 2006; 70: 1184-1189.
A c c e p t e d : July 19, 2013