IN VITRO EFFECTS OF LUTEINIZING HORMONE, PROGESTERONE
AND OESTRADIOL-17β ON LEPTIN GENE EXPRESSION AND LEPTIN
SECRETION BY PORCINE LUTEAL CELLS OBTAINED IN EARLY PREGNANCY
INTRODUCTION
Leptin (16 kDa protein) is an important factor linking metabolic status to reproduction (1). There is evidence that this hormone is implicated in the timing of the onset of puberty and in the maintenance of a normal reproductive cycle (1-5). It is also believed that leptin plays a role during pregnancy. Serum leptin concentration increases during pregnancy and may be due to the production of leptin by the placenta (6-8). It is also suggested that this peptide may participate in the implantation process and acts as a regulator for the apposition and adhesion phases of implantation (9-11). Leptin has an influence on reproductive processes through its own receptors. Leptin receptors (OB-R) belong to the interleukin-6 receptor family of class 1 cytokine receptors (12). One of the six alternatively spliced isoforms of the leptin receptor is the long form (OB-Rb). It is capable of full signal transduction, acting through two pathways: JAK-STAT and MAPK (13). Leptin receptors in the reproductive tract are localized in the ovary, oviduct, uterus and the placenta (3, 14-20). Ovarian leptin receptors have been found in theca and granulosa cells, and the corpus luteum (CL) and stroma (OS) in several species, including mice, rats, pigs, cattle, rabbits and humans (3, 14-17, 21). Our previous experiments showed different patterns of expression for leptin and OB-Rb mRNAs/proteins in the porcine ovarian tissue (CL and OS) during the oestrous cycle and early pregnancy, and that steroids are implicated in the regulation of leptin gene expression and secretion in porcine luteal cells during the mid-luteal phase of the cycle (15, 22, 23). The control of leptin gene expression and leptin secretion has not been examined in porcine luteal cells during early pregnancy. Therefore, the purpose of this study was to: 1) determine the level of leptin and OB-Rb transcript/protein in luteal cells on days 14–16 of gestation (the beginning of the implantation process) and 2) examine the effects of luteinizing hormone (LH), E2 and P4 on the level of leptin gene expression and leptin secretion in those cells during this period of pregnancy.
MATERIALS AND METHODS
Experimental animals
All experiments were approved by the Animal Ethics Committee at the University of Warmia and Mazury in Olsztyn, Poland. The experimental animals (n=6) were post-pubertal crossbred pigs (Polish Landrace x Pietrain), aged 9–10 months, with body weight of 110–130 kg and descended from private breeders. Females were naturally bred on the second day of oestrus naturally occurring. The pigs had been last fed in the afternoon before day of the slaughter. The animals were sacrificed on days 14–16 of the pregnancy. Pregnancy was detected by ultrasound scanning before killing of the gilts. Additionally, it was confirmed by the presence of embryos/foetuses after flushing of uterine horns with 20 mL of sterile saline. The choice of days 14–16 of pregnancy was connected with the beginning of the implantation process. The ovaries, the adipose tissue (positive control for leptin expression) and medial basal hypothalamus (MBH; positive control for OB-Rb expression) were collected within 10 minutes after slaughter. Adipose tissue and MBH were frozen in liquid nitrogen and stored at –80°C until real-time PCR analysis. The ovaries were transported to the laboratory with Eagle’s Medium (Biomed, Poland) with antibiotics (penicillin, streptomycin; Polfa, Poland) and nystatin (Sigma-Aldrich, USA).
Tissue isolation and culture of luteal cells
The healthy ovaries (both right and left of each sow) were selected for real-time polymerase chain reaction (RT-PCR) and radioimmunoassay (RIA). Luteal tissues from each animal were processed and cultured separately. Briefly, dissected corpora lutea (between 11 and 16 CLs/gilt) were cut into small fragments (1–2 mm) and digested with 0.15% trypsin (Biomed, Poland) in Ham’s F-12 medium (Sigma-Aldrich, USA) with gentamicin (50 µg/ml, KRKA, Slovenia) and nystatin (240 U/ml). Luteal tissue was dissociated in eight consecutive incubation periods (10 min each, 37°C). After digestion, cell suspensions were centrifuged (500 ×g, 10 min). The cells were rinsed three times with a fresh medium, pooled and filtered through a sterile nylon filter (60 µm mesh) to remove undigested tissue debris. The number and viability of luteal cells were determined using a haemocytometer and trypan blue (MP Biomedicals, LLC, Santa Ana, CA, USA) exclusion test, respectively. The viability of isolated cells exceeded ±95%. The luteal cells suspension (2 ml; 106 cells/ml) was placed in 6-well plates and cultured under a gas atmosphere of 95% air and 5% CO2 at 37°C for 48 hours. Subsequently, culture media were changed, plates were rinsed, and cells were cultured in Ham’s F-12 medium with gentamicin (50 µg/ml) and nystatin (240 U/ml) in the presence or absence (control) of LH (1; 10; 100 ng/ml, National Hormone and Pituitary Agency, University of Maryland, USA), E2 (0.02; 0.2; 2; 20 ng/ml, Sigma-Aldrich, USA) and P4 (20; 100; 200 ng/ml, Sigma-Aldrich, USA). The doses of LH and steroids were based on the results of pilot experiments (data unpublished) and they represent a physiological or higher blood levels. After 24 hours, media were collected, lyophilized and stored at –20°C until leptin concentration assay. The expression of leptin and OB-Rb mRNA as well as proteins in luteal cells was determined by quantitative real-time PCR and fluorescence immunocytochemistry (F-ICC), respectively.
Total RNA isolation, cDNA synthesis and quantitative real-time polymerase chain reaction analysis
Total RNA was extracted from the cells (n=6 cultures) and the tissues (adipose tissue or MBH) using PeqGOLD Trifast (Peg Lab, Erlangen, Germany) and fenozol (A&A Biotechnology, Polska), respectively, in accordance with the manufacturer’s instructions. RNA purity and yield were determined spectrophotometrically (Nanodrop ND-1000, NanoDrop Technologies Inc., DE, USA). Approximately 1 µg of total RNA was reverse-transcribed into cDNA with the high capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). A quantitative real-time PCR analysis was performed as described previously (23, 24). cDNA samples were amplified in a final reaction volume of 10 µL containing 1× SYBR Green PCR-Master Mix (Applied Biosystems, Foster City, CA, USA) and the respective primers (300 nmol/L; Table 1). PCR amplification was performed in duplicate in the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the following cycling program: 2 minutes at 50°C, 10 minutes at 95°C, followed by 15 second at 95°C and 1 minute at 59°C for a total of 46 cycles. Adipose tissue (leptin mRNA) and MBH (OB-Rb mRNA) were used as positive controls. In non-template control (NTC) samples, the template (cDNA) was replaced with nuclease free water. PCR products were analyzed by thermal dissociation to verify that a single specific PCR product had been amplified. Relative leptin gene expression level was calculated by the comparative cycle threshold (CT) method (25), normalized by GAPDH expression (26) and expressed as arbitrary units. To confirm homology to porcine leptin and OB-Rb sequences the obtained transcripts were sequenced (ABI Prism 3777 DNA sequencer) at the Institute of Biochemistry and Biophysics of the Polish Academy of Science.
Leptin radioimmunoassay assay
Leptin concentrations in the culture media (n=5) were determined by radioimmunology assay using the Millipore’s Multi-Species Leptin Radioimmunoassay Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. To increase the leptin detection limit, cell culture media were lyophilized in a vacuum concentrator (CHRIST, Germany) and dissolved in PBS (to obtain a 5-fold increase in concentration). All measurements were performed in duplicate. Assay sensitivity was 1 ng/ml; intra-assay coefficient of variation was 8.61% (in one series).
Fluorescence immunocytochemistry (F-ICC)
After culture, luteal cells were fixed in 4% paraformaldehyde. To decrease nonspecific binding, cells were blocked in 10% normal murine serum (Sigma-Aldrich, USA) diluted in 0.01 M PBS with 0.1% bovine serum albumin (BSA, Sigma-Aldrich, USA) and 1% triton X-100 (Sigma-Aldrich, USA) for 1 hour at room temperature (RT). Cell samples were left overnight at 4°C with primary antibodies: rabbit polyclonal anti-leptin PA1-052; (Affinity BioReagents, Golden, CO, USA) or goat polyclonal anti-OB-R (C-20 sc-1832) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), diluted 1:50. On the following day, the cells were kept in biotinylated goat anti-rabbit (for leptin) or rabbit anti-goat (for OB-Rb) IgG antibodies BA-1000 or BA-5000 (Vector Laboratories, USA) at 1:100 dilution for 1 hour at RT. The cells were incubated with the fluorescein streptavidin complex (Vector Laboratories, Burlingame, CA, USA) diluted 1:50 in 0.01 M PBS for 1 hour at RT. In negative control samples the primary antibody was replaced with 0.01 M PBS. Cell specimens were stained with propidium iodide to visualize cell nuclei. The cells were mounted in a fluorescent medium (Sigma-Aldrich, USA). The labeled cells were photographed with the DS-5Mc-U2 camera mounted on the Eclipse 80i fluorescence microscope with a dual filter cube for FITC and TRITC (Nikon Corporation, Japan).
Statistical analysis
The results were presented as the percentage (mean ±S.E.M.) of basic leptin mRNA expression and leptin secretion established in control cells (100%) from six and five independent experiments, respectively. Statistical analysis was performed using the Statistica program (Stat Soft Inc., USA) and significant differences were determined by one-way ANOVA followed by the least significant difference (LSD) post hoc test. A value of p<0.05 was considered to be statistically significant.
RESULTS
Localization of leptin and OB-Rb gene/protein expressions in porcine luteal cells on days 14–16 of pregnancy
Both leptin and OB-Rb transcripts were detected in luteal cells on days 14–16 of pregnancy. The obtained transcripts were sequenced at the Institute of Biochemistry and Biophysics of the Polish Academy of Science, and 100% and 95% homology to porcine leptin and OB-Rb sequences was confirmed, respectively. Leptin and OB-Rb proteins were found in luteal cells in the analyzed days of pregnancy and are shown in Fig. 1 and Fig. 2, respectively.
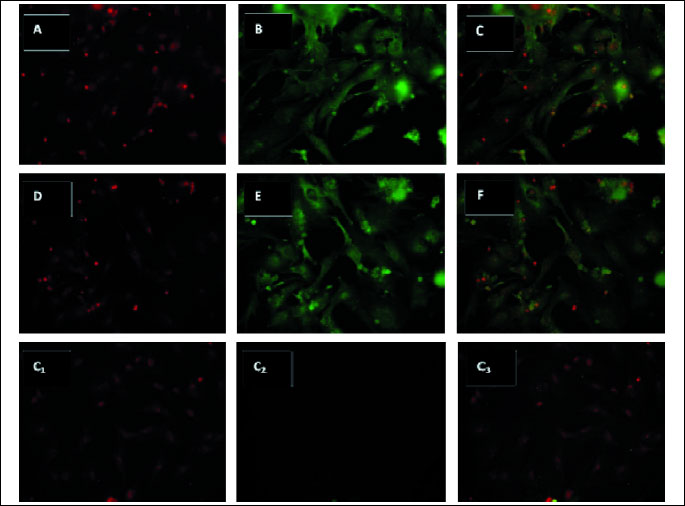
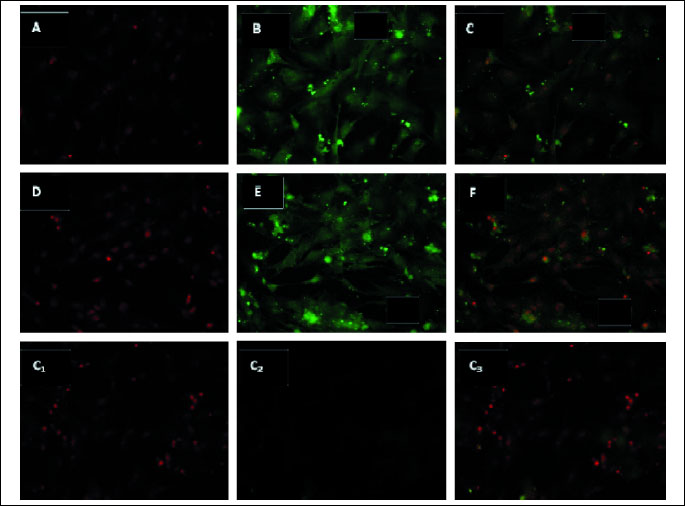
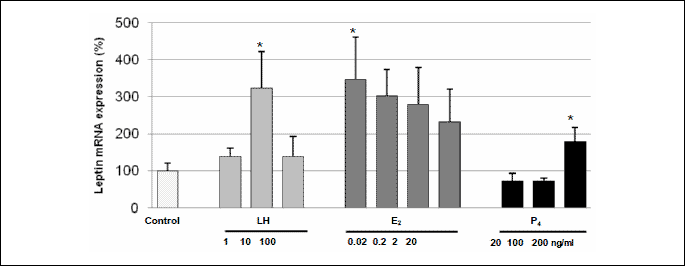
The effect of LH, E2 and P4 on the level of leptin gene expression in porcine luteal cells on days 14–16 of pregnancy
LH increased (p<0.05) leptin mRNA expression in luteal cells on days 14–16 of pregnancy only at the dose of 10 ng/ml (Fig. 3). The lowest dose of E2 (0.02 ng/ml) and the highest dose of P4 (200 ng/ml) stimulated (p<0.05) leptin gene expression in luteal cells in those days of gestation.
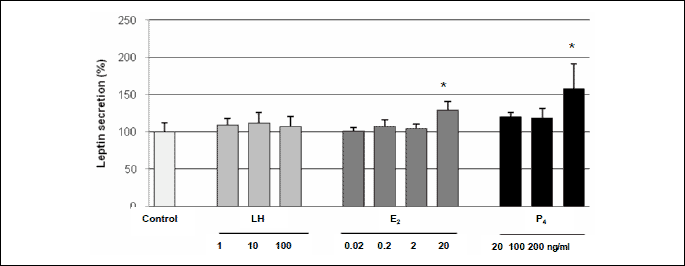
The effect of LH, E2 and P4 on the level of leptin secretion by porcine luteal cells on days 14–16 of pregnancy
Basal leptin level (mean ±S.E.M.) in control luteal cell media was 0.61 ±0.07 ng/mL. LH had no effect on leptin release by luteal cells on days 14–16 of pregnancy (Fig. 4). The highest doses of E2 (20 ng/ml) and P4 (200 ng/ml) stimulated (p<0.05) secretion of the analysed peptide by luteal cells on days 14–16 of gestation.
DISCUSSION
The present study, to the best of our knowledge, is the first ever experiment to demonstrate that on days 14–16 of the pregnancy: 1) leptin and OB-Rb genes/proteins are expressed in porcine luteal cells, and 2) LH and steroids (E2 and P4) participate in the regulation of leptin mRNA expression in porcine luteal cells, while steroids (E2 and P4) are engaged in the regulation of leptin secretion by porcine luteal cells. The present results are in agreement with our results from the previous study, which revealed the presence of leptin and OB-Rb transcripts/proteins in porcine luteal cells during the mid-luteal phase of the oestrous cycle (23). Moreover, the occurrence of leptin mRNA and protein in luteal cells confirms the results of our previous experiments in which we detected the leptin gene and protein expressions in the porcine corpus luteum (CL) during early pregnancy (days 14–16 and 30–32) as well as during the mid- and the late-luteal phase of the oestrous cycle (days 10–12 and 14–16) using semi-quantitative RT-PCR, in situ hybridization and Western blotting metods (22). Furthermore, we demonstrated fluctuations in the levels of the analyzed transcript and protein in the porcine CL during the examined periods. During gestation, leptin mRNA expression was higher on days 14–16 compared to days 30–32, although, conversely, the leptin protein level was higher on days 30–32. A comparison of leptin transcript and protein levels in CL between early pregnancy (days 14–16 or 30–32) and late-luteal phase of the oestrous cycle revealed higher levels of both expressions on days 14–16 of the cycle (22). Leptin mRNA and protein expressions were also detected in the bovine CL during pregnancy by Sarkar et al. (16). The researchers did not observe significant differences in the level of those expressions in CL during gestation (from 1 to 8 month), although they found differences between pregnancy and the oestrous cycle – a lower level of the expressions in cyclic early-luteal CL or corpus albicans (apart from mid- and late-luteal CL, in which expression levels were similar to those during pregnancy).
The changes of leptin mRNA/protein levels in the CL during pregnancy (as in the oestrous cycle) suggest that leptin expression may be dependent on the concentrations of circulating hormones and factors produced locally in the corpus luteum. Numerous studies have revealed that the functions of the corpus luteum could be controlled by LH and steroids, such as E2 and P4. LH (a pulsatile released by anterior pituitary cells) is the principal luteotrophic signal in pigs, cows and sheep, and is necessary for normal CL development and maintenance of its functions (27). In pigs, oestrogen has also a luteotrophic activity and can increase P4 secretion (27-28). Oestradiol can prevent CL luteolysis by reducing PGF2α secretion from the uterus (29). Moreover, it has been proven that progesterone can stimulate its own secretion (30).
The present study indicates that LH is involved in the control of leptin gene expression in porcine luteal cells and stimulates the level of leptin mRNA in those cells on days 14–16 of pregnancy. However, in our previous experiment we did not observe the effect of LH on the leptin transcript level in porcine luteal cells on days 10–12 of the oestrous cycle (23). It is possible that the influence of this gonadotropin on leptin gene expression (and leptin secretion) in ovarian cells depends on the physiological status, the type of cells used, the species of the experimental animal and the experimental methods used. Karamouti et al. (31) using RT-PCR method did not detect ob mRNA transcript in human luteinized granulosa cells either hormonally stimulated (LH, FSH or A4) or without hormonal stimulation. Moreover, in contrast to our results, these researchers did not determine the leptin concentration in the media after cultures of those cells. The reason for the ability to detect leptin in the culture medium in our study was lyophilization of the culture samples and a corresponding increase in the measureable level of leptin. In turn, we did not find an influence of LH on leptin release by porcine luteal cells on days 14–16 of gestation, as in our previous studies relating to the mid-luteal phase of the oestrous cycle (23).
The results of the present experiment show that steroids (E2 and P4) increased both leptin mRNA expression and leptin secretion by porcine luteal cells on days 14–16 of pregnancy and are generally in accordance with the results of our previous study carried out on porcine dispersed luteal cells from the mid-luteal phase of the cycle (23). Hence, it can be assumed that both steroid hormones regulate the expression and secretion of leptin in porcine luteal cells during these periods of the cycle and pregnancy. It is interesting to note that 1) in vitro studies on adipocytes also revealed stimulating effect of E2 on leptin gene expression and leptin release in human and rat (32-33) and 2) the increased number of rat luteal cells containing leptin protein, the increased leptin expression in bovine CL and maximal serum leptin concentrations in women were all connected with high levels of P4 secretion (6, 16, 34).
Based on our results showing that luteal cells are leptin-immunoreactive and that leptin levels are defined in the culture media, we hypothesize that cells of CL are the site of leptin production. This hypothesis is supported by reports from experiments on mice (3), rats (14, 34), cows (16) and humans (35). Moreover, in addition to CL, uterus (endometrium, myometrium) and trophoblast may be also a source of leptin (36).
Our present and previous studies (23) have found that both the long, biologically active isoform (OB-Rb) gene and OB-Rb protein are expressed in porcine luteal cells during early pregnancy and the mid-luteal phase of the oestrous cycle. These findings confirm the results of our recent experiments concerning the detection of OB-Rb mRNA/protein expressions in the porcine CL during early pregnancy (days 14–16 and 30–32) as well as the mid- and the late-luteal phase of the oestrous cycle (days 10–12 and 14–16) (15). Leptin receptor mRNA and/or protein expressions were also found in the CL of immature gonadotropin-primed mice (3) and rats (14), pseudopregnant rabbits (17), pregnant and cyclic cows (16, 37), normal and polycystic women (35) and pregnant baboon (38).
As in the case of leptin, changes in leptin receptor expression level in the ovary during pregnancy and the oestrous cycle have been reported. In our previous study (15), we noticed a higher level of OB-Rb transcript in the porcine CL on days 30–32 of pregnancy compared to days 14–16 of pregnancy, whereas differences in OB-Rb protein expressions in the CL were not found. Moreover we observed an inverse relationship between OB-Rb gene expression and OB-Rb protein expression in the CL (the transcript level was higher, whereas the protein level was lower in early pregnancy than during the luteal phase of the cycle). Ruiz-Cortes et al. (39) showed that both OB-Rb mRNA and protein were low in the porcine postovulatory CL, maximal expressions were detected in the midcycle CL and lowest abundance was found in regressed CL. The authors indicated that those expressions correlate with the level of progesterone production (both maximal OB-R and P4 levels in the midcyle) and suggested a positive effect of leptin on luteal function. In studies on cow, fluctuations in OB-R mRNA expressions levels in CL were not found during pregnancy (from 1 to 8 month). Moreover OB-Rb expression in the CL during gestation was higher in comparison to days 1–2 or >18 of the oestrous cycle, while did not differ statistically in comparison to days 8–16 of the cycle (16).
Our results demonstrate the presence of leptin and its receptor in luteal cells and suggest that the leptin system is involved in the regulation of the CL cells function. Leptin affects the ovulation process (40), CL formation (including the process of apoptosis and angiogenesis) (5, 41) and regulates steroidogenesis in luteal cells during the oestrous cycle (5, 37). It is believed that leptin also may control CL steroidogenesis during pregnancy. in vitro studies carried out by Zerani et al. (17) showed leptin’s inhibitory influence on basal P4 secretion by rabbit luteal cells on day 9 of pseudopregnancy. From literature data, it emerges that leptin modulates P4 production by luteal cells in the presence of gonadotropins and growth factors (e.g. IGF-1) and its various effects on P4 secretion (5, 17, 37) may be associated with the using of different receptors (OB-Rb and/or OB-Rs) and intracellular signaling pathways (e.g. JAK/STAT and/or MAPK) (13, 17). P4 increases the uterine receptivity and induces conditions favorable for embryo implantation (42). It is likely that through the regulation of P4 secretion leptin may enhance the endometrial cell receptivity to the conceptus. It is possible that other ovarian steroid E2 may also be involved in the action of leptin on the implantation process. It is known that E2 decreases PGF2α release from uterus into the peripherial circulation and prevent CL luteolysis as well as increases P4 production; hence, the action of leptin may regulate pregnancy (27-29). Further study is needed to clarify this issue.
Leptin may be involved in the embryonic implantation by modulating of cytokine expression, such as interleukin-1 (IL-1), tumor necrosis factor (TNF), leukemia inhibitory factor (LIF) as well as by stimulating of VEGF secretion by endometrial cells (43). Exemplary, IL-1 increases prostaglandin E2 synthesis/release by the endometrium and the myometrium (44), which is also a hormone preventing luteolysis as well as this cytokine may initiate conceptus-uterine interaction during pregnancy (45). Leptin also upregulates the expression of relevant integrins for embryo adhesion in epithelial endometrial cells (43). Furthermore, it activates molecules involved in the tissue remodeling and also connected with the trophoblastic invasion matrix metalloproteinases (MMPs) (9, 41). Studies in mice ob/ob support that leptin is an essential factor in the process of implantation and pregnancy. It has been shown that exogenous leptin suplementation is required in leptin-null mice at least for 6.5 days post coitus to retain pregnancies and carry them to term (46).
The proposed physiological roles for leptin during pregnancy by Henson and Castracane (47, 48) and others researchers (8-11, 49), beyond its participation in the implantation, include: the regulation of preembryonic/embryonic/fetal growth and development, fetal/placental angiogenesis, embryonic hematopoiesis, and hormone biosynthesis within the maternal-fetoplacental unit. Leptin stimulates human chorionic gonadotropin (hCG) secretion by human placental explants (50). Moreover, leptin also affects the mother’s body by mobilizing its energy supplies (48).
In summary, the present study found that leptin and OB-Rb transcripts/proteins are expressed in porcine luteal cells on days 14–16 of pregnancy. These results indicate that there is participation of 1) LH, E2 and P4 in local modulation of leptin mRNA expression and 2) E2 and P4 in leptin secretion by porcine luteal cells obtained in early pregnancy.
Acknowledgements: This research was supported by the Ministry of Science and Higher Education (Project: N N311 098634). We are grateful to mgr E. Bohdan and mgr B. Sterbicka for their technical assistance in the experiment.
Author contributions: G. Siawrys has made substantial contribution to the laboratory work, the acquisition, analysis and interpretation of data, and to drafting the paper. N. Smolinska has made substantial contribution to the laboratory work.
Conflict of interests: None declared.
REFERENCES
- Schneider JE. Energy balance and reproduction. Physiol Behav 2004; 81: 289-317.
- Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab 2001; 12: 65-72.
- Ryan NK, Woodhouse CM, Van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod 2002; 66: 1548-1554.
- Duggal PS, Ryan NK, Van der Hoek KH, et al. Effects of leptin administration and feed restriction on thecal leucocytes in the preovulatory rat ovary and the effects of leptin on meiotic maturation, granulosa cell proliferation, steroid hormone and PGE2 release in cultured rat ovarian follicles. Reproduction 2002; 123: 891-898.
- Gregoraszczuk EL, Ptak A. in vitro effect of leptin on growth hormone (GH)- and insuline-like growth factor-I (IGF)-stimulated progesterone secretion and apoptosis in developing and mature corpora lutea of pig ovaries. J Reprod Dev 2005; 51: 727-733.
- Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol 1997; 47: 101-106.
- Linnemann K, Malek A, Sager R, Blum WF, Schneider H, Fusch C. Leptin production and release in the dually in vitro perfused human placenta. J Clin Endocrinol Metab 2000; 85: 4298-4301.
- Ashworth CJ, Hoggard N, Thomas L, Mercer JG, Wallace JM, Lea RG. Placental leptin. Rev Reprod 2000; 5: 18-24.
- Castellucci M, De Matteis R, Meisser A, et al. Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol Hum Reprod 2000; 6: 951-958.
- Craig JA, Zhu H, Dyce PW, Wen L, Li J. Leptin enhances porcine preimplantation embryo development in vitro. Mol Cell Endocrinol 2005; 229: 141-147.
- Yang YJ, Cao YJ, Bo SM, Peng S, Liu WM, Duan EK. Leptin-directed embryo implantation: leptin regulates adhesion and outgrowth of mouse blastocysts and receptivity of endometrial epithelial cells. Anim Reprod Sci 2006; 92: 155-167.
- Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995; 83: 1263-1271.
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J 2006; 393: 7-20.
- Ryan NK, Van der Hoek KH, Robertson SA, Norman RJ. Leptin and leptin receptor expression in the rat ovary. Endocrinology 2003; 144: 5006-5013.
- Smolinska N, Kaminski T, Siawrys G, Przala J. Long form of leptin receptor gene and protein expression in the porcine ovary during the estrous cycle and early pregnancy. Reprod Biol 2007; 7: 17-39.
- Sarkar M, Schilffarth S, Schams D, Meyer HH, Berisha B. The expression of leptin and its receptor during different physiological stages in the bovine ovary. Mol Reprod Dev 2010; 77: 174-181.
- Zerani M, Boiti C, Zampini D, et al. Ob receptor in rabbit ovary and leptin in vitro regulation of corpora lutea. J Endocrinol 2004; 183: 279-288.
- Zerani M, Boiti C, Dall’Aglio C, et al. Leptin receptor expression and in vitro leptin actions on prostaglandin release and nitric oxide synthase activity in the rabbit oviduct. J Endocrinol 2005; 185: 319-325.
- Smolinska N, Kaminski T, Siawrys G, Przala J. Long form of leptin receptor gene and protein expression in the porcine trophoblast and uterine tissues during early pregnancy and the oestrous cycle. Anim Reprod Sci 2009; 113: 125-136.
- Hoggard N, Hunter L, Lea RG, Trayhurn P, Mercer JG. Ontogeny of the expression of leptin and its receptor in the murine fetus and placenta. Br J Nutr 2000; 83: 317-326.
- Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod 1997; 3: 467-472.
- Smolinska N, Kaminski T, Siawrys G, Przala J. Leptin gene and protein expression in the ovary during the oestrous cycle and early pregnancy in pigs. Reprod Domest Anim 2010; 45: 74-83.
- Siawrys G, Smolinska N. Direct in vitro effect of LH and steroids on leptin gene expression and leptin secretion by porcine luteal cells during the mid-luteal phase of the estrous cycle. Reprod Biol 2012; 12: 317-323.
- Fluhr H, Krenzer S, Deperschmidt M, Zwirner M, Wallwiener D, Licht P. Human chorionic gonadotropin inhibits insulin-like growth factor-binding protein-1 and prolactin in decidualized human endometrial stromal cells. Fertil Steril 2006; 86: 236-238.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001; 25: 402-408.
- Bogacka I, Przala J, Siawrys G, Kaminski T, Smolinska N. The expression of short form of leptin receptor gene during early pregnancy in the pig examined by quantitative real time RT-PCR. J Physiol Pharmacol 2006; 57: 95-108.
- Stouffer RL. Structure, function, and regulation of the corpus luteum. In: Physiology of Reproduction, Knobil E, Neill JD. (eds). Elsevier Academic Press, 2006, pp. 475-527.
- Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPbeta in female reproduction. Genes Dev 1997; 11: 2153-2162.
- Ford SP, Magness RR, Farley DB, Van Orden DE. Local and systemic effects of intrauterine estradiol-17 beta on luteal function of nonpregnant sows. J Anim Sci 1982; 55: 657-664.
- Rothchild I. The corpus luteum revisited: are the paradoxical effects of RU486 a clue to how progesterone stimulates its own secretion? Biol Reprod 1996; 55: 1-4.
- Karamouti M, Kollia P, Karligiotou E, et al. Absence of leptin expression and secretion by human luteinized granulosa cells. J Mol Endocrinol 2003; 31: 233-239.
- Machinal-Quelin F, Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine 2002; 18: 179-184.
- Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. in vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology 1999; 140: 1567-1574.
- Archanco M, Muruzabal FJ, Llopiz D, et al. Leptin expression in the rat ovary depends on estrous cycle. J Histochem Cytochem 2003; 51: 1269-1277.
- Loffler S, Aust G, Kohler U, Spanel-Borowski K. Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod 2001; 7: 1143-1149.
- Smolinska N, Siawrys G, Kaminski T, Przala J. Leptin gene and protein expression in the trophoblast and uterine tissues during early pregnancy and the oestrous cycle of pigs. J Physiol Pharmacol 2007; 58: 563-581.
- Nicklin LT, Robinson RS, Masters P, Campbell BK, Mann GE, Hunter MG. Leptin in the bovine corpus luteum: receptor expression and effects on progesterone production. Mol Reprod Dev 2007; 74: 724-729.
- Green AE, O’Neil JS, Swan KF, Bohm RP, Ratterree MS, Henson MC. Leptin receptor transcripts are constitutively expressed in placenta and adipose tissue with advancing baboon pregnancy. Proc Soc Exp Biol Med 2000; 223: 362-366.
- Ruiz-Cortes ZT, Men T, Palin MF, Downey BR, Lacroix DA, Murphy BD. Porcine leptin receptor: molecular structure and expression in the ovary. Mol Reprod Dev 2000; 56: 465-474.
- Duggal PS, Van Der Hoek KH, Milner CR, et al. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology 2000; 141: 1971-1976.
- Park HY, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 2001; 33: 95-102.
- Bazer FW, Song G, Kim J, et al. Uterine biology in pigs and sheep. J Anim Sci Biotechnol 2012; 3: 23.
- van Mourik MS, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol 2009; 85: 4-19.
- Franczak A, Zmijewska A, Kurowicka B, Wojciechowicz B, Kotwica G. Interleukin 1β-induced synthesis and secretion of prostaglandin E2 in the porcine uterus during various periods of pregnancy and the estrous cycle. J Physiol Pharmacol 2010; 61: 733-742.
- Spencer TE, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol 2004; 2: 49.
- Malik NM, Carter ND, Murray JF, Scaramuzzi RJ, Wilson CA, Stock MJ. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology 2001; 142: 5198-5202.
- Henson MC, Castracane VD. Leptin in pregnancy. Biol Reprod 2000; 63: 1219-1228.
- Henson MC, Castracane VD. Leptin in pregnancy: an update. Biol Reprod 2006; 74: 218-229.
- Kawamura K, Sato N, Fukuda J, et al. The role of leptin during the development of mouse preimplantation embryos. Mol Cell Endocrinol 2003; 202: 185-189.
- Cameo P, Bischof P, Calvo JC. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol Reprod 2003; 68: 472-477.
A c c e p t e d : August 25, 2013