INFLUENCE OF MELATONIN SUPPLEMENTATION ON SERUM ANTIOXIDATIVE PROPERTIES AND IMPACT OF THE QUALITY OF LIFE IN MULTIPLE SCLEROSIS PATIENTS
INTRODUCTION
Multiple sclerosis (MS) is the most prevalent autoimmune inflammatory demyelinating disease of the central nervous system (CNS) in young adults. Environmental factors play an important role in MS etiology. The relationship between the prevalence of MS, latitude gradient, and sunlight's ultraviolet radiation was proved (1). MS incidence is higher in geographic regions with sunlight exposure deficiency. Melatonin and vitamin D are directly connected with effects of sunlight in healthy individuals, and both are thought to play a role in MS pathophysiology (1, 2). It has been reported that vitamin D and melatonin may have related influences in patients with MS. Melatonin secretion is negatively correlated with vitamin D alterations in serum in MS patients treated with interferons (2).
To date, the exact mechanisms of MS pathophysiology remain to be fully explained, but a triad of neural tissue injury mechanisms: inflammation, demyelination, and axonal damage is still valid (3). Recent studies have suggested that oxidative stress can play a crucial role in the pathogenic traits of MS. The CNS is very susceptible to oxidative damage. Processes responsible for generation of reactive oxygen species (ROS) and lipid peroxidation, hallmarks of oxidative stress, have been studied in attempt to develop therapies that can diminish or stop CNS damage (4, 5). Pathogenesis of MS is associated with adaptive and innate immune cells. The adaptive immune CD4 T cells play an initial role in the autoimmune process. Under stimulation by CD4 T cells ingrained immune cells, such as macrophages, produce inflammatory cytokines, which results in causing damage to the myelin and neurons, and also leads to the generation of ROS (3, 6).
Melatonin (N-acetyl-5-methoxytryptamine) is a natural hormone mainly produced in the mammalian pineal gland during the dark phase. With its lipophilic and hydrophilic character, melatonin freely crosses the blood-brain barrier and enters all cells. Melatonin acts through G-protein coupled membrane receptors, MT1 and MT2 and through nuclear receptors RZR/ROR (7). Both receptors are widely present in the central and peripheral nervous system, and have been associated with cell differentiation and immune response regulation. Moreover, melatonin receptors are expressed on the membrane of CD4 T cells, CD8 T cells, and B cells (6). Melatonin and its metabolites have been demonstrated to possess multiple functions, including antioxidation, immunomodulatory and anti-inflammatory effects (3, 8, 9).
When the generation of ROS exceeds the ability of the endogenous antioxidant system to remove them, the oxidative stress in the brain occurs, subsequently leading to cellular damage. Melatonin is able to scavenge a variety of ROS and reactive nitrogen species (RNS), including hydroxyl radical (HO.), superoxide anion radical (O2.-), hydrogen peroxide (H2O2), and peroxynitrite anion (ONOO-), and thus terminate the initiation and propagation of lipid peroxidation (10, 11). Melatonin treatment also causes an important reduction in nitric oxide (NO) and malondialdehyde (MDA) levels, two compounds that are closely related to inflammation (10). Melatonin influences antioxidative defensive systems, up-regulates gene expression, and stimulates the activities of several antioxidant enzymes, including glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), as well as the levels of glutathione (GSH) (8-12).
Melatonin has been shown to have immunomodulatory properties involved in the regulation of both the cellular and humoral immunity. This indole stimulates the production of natural killer cells, monocytes and leukocytes, alters the balance of T helpers (Th)-1 and Th-2, and increases the production of relevant cytokines such as interleukin (IL)-2, IL-6, IL-10, IL-12, interferon-g, and modifies serum inflammatory parameters. The regulatory function of melatonin on immune mechanisms is seasonally dependent. As a consequence, melatonin improves the clinical course of illnesses with inflammatory etiology (3, 13). Moreover, it participates in neurogenesis, regulation of circadian rhythms, sleep, and also exerts an anti-tumor activity (3, 13, 14).
The important role of melatonin as a modulator of sleep is well documented. Changes in melatonin levels have been reported with regard to circadian rhythm sleep disorders (13). It is known that sleep disturbances are present more often in MS patients than in the general population and contribute to daytime fatigue (15, 16). There are only few original papers concerning sleep problems in MS patients. The prevalence of sleep problems was reported in 24–50% of MS patients (17, 18). The possible common pathomechanisms shared by MS and sleep disturbances may be connected with circadian rhythm disorders with compromised melatonin secretion reducing input to the suprachiasmatic nucleus due to impaired visual pathways, as well as increased levels of proinflammatory cytokines (19).
The Multiple Sclerosis Impact Scale (MSIS-29) was reported to be a valid and reliable method of assessing the quality of life in MS patients. It is a disease-specific instrument consisting of 29 questions, the first 20 of which address the physical impact scale (MSIS-29-PHYS) and the last 9 assess the psychological impact (MSIS-29-PSYCH) (20, 21).
The role of melatonin in MS has been suggested in several clinical studies. It has been reported to significantly decrease melatonin metabolite 6-sulphatoxy-melatonin (6-SMT) levels and disrupt circadian regulation of its secretion, which were increased with IFN-β treatment, caused by fatigue. In the MS group, sleep quality was significantly lower than in controls (22). It is known that shift work causes disrupted circadian rhythms and sleep restriction. It was found that shift work at a young age increases the risk of MS (23). Melatonin level in saliva was significantly low among patients with MS after controlling the effect of age. However, there were no correlations between the level of melatonin and duration of the disease, treatment, EDSS, sex or sleep quality (24). Being in clinical use for many years, melatonin is safe and well-tolerated even at high doses and long term treatment (9, 13), but appropriate melatonin doses in autoimmune diseases still need to be evaluated.
The aim of our present study was to evaluate the action of melatonin as a molecule with antioxidative properties on the serum SOD activity and MDA concentration as a marker of lipid peroxidation in patients with MS, a disease with well-documented oxidative stress disturbance pathology. Moreover, we also intended to ascertain the influence of melatonin on the physical and psychological impact on the quality of life of MS patients.
MATERIAL AND METHODS
Patients
A case-control prospective study was performed on 102 MS patients (69 women, 33 men) and 20 (13 women, 7 men) healthy subjects matched for age and sex, observed in 2013 in the Department of Neurology in Zabrze, Medical University of Silesia, Poland. The patients were divided into groups:
Group C (control group): 20 healthy controls observed in our Department due to undiagnosed headaches. Controls were matched for age and sex with the study group.
Group P (pre-treated MS group): 21 patients with de novo diagnosed, according to the McDonald criteria (25), relapsing-remitting form of MS (RRMS), without any immunomodifying MS treatment.
Group A (beta-1a interferons treated MS group): 25 patients with RRMS, diagnosed according to the McDonald criteria (2005). All of them received interferon beta-1a, applied once per week as an intramuscular injection.
Group B (beta-1b interferons treated MS group): 27 patients with RRMS, diagnosed according to the McDonald criteria (2005). All of them received interferon beta-1b, injected subcutaneously every other day.
Group G (glatiramer acetate treated MS group): 12 patients with RRMS, diagnosed according to the McDonald criteria (2005). All of them received daily subcutaneous glatiramer acetate injections.
Group MX (mitoxantrone treated MS group): 17 patients with secondarily progressive (SP) or progressive-relapsing (PR) form of MS, diagnosed according to the McDonald criteria (2005). All of them received 5 doses of mitoxantrone i.v. (12 mg/m2/dose) administered quarterly.
None of the patients received any antioxidative substances, vitamins, anti-inflammmatory, or hormonal treatment for at least 3 months prior to the study, and any sleeping pills two weeks prior to the study.
Study protocol
The study was approved by the local Ethics Committee of the Medical University of Silesia (KNW/0022/KB1/130/12).
After obtaining informed consent, demographic data, Kurtzke's Expanded Disability Status Scale (EDSS) (26), and MSIS-29 questionnaires were completed in all groups. MRI examinations were performed in all MS patients at the beginning of the study, in accordance with standard clinical protocols. The neurological examination was performed by a qualified neurologist using the EDSS before the therapy and after its completion. All MS patients were supplemented orally with melatonin, 5 mg per day, over a period of 90 days. The dose of melatonin and treatment duration was selected according the drug indication and our preliminary studies. We chose these doses because of its optimal and proved effects. Similar doses were also used by previous authors.
Before and after the melatonin supplementation, the MDA concentration as well as SOD activity in the serum were measured. Furthermore, validated Polish versions of the Multiple Sclerosis Impact Scale (MSIS-29), used as disease-specific instrument to assess the physical and psychological impact on the quality of life of MS patients were performed before and after the melatonin treatment.
Enzymatic assays
10 mL samples of venous blood, before and after the 90 days of melatonin supplementation, were collected from the MS patients between 6.00 and 7.00 a.m., mixed for about 20–30 s and centrifuged at 1055 × g for 3–5 min. and frozen until laboratory measurements were performed. Control samples were collected at the beginning of the study.
The MDA concentration and SOD activity in the blood serum were assayed. MDA concentrations were determined according to the colorimetric methods by Ohkawa et al. using a reaction with thiobarbitiuric acid (27). Results were expressed as mol/L (27). The SOD (EC 1.15.1.1) activities were estimated according to Oyanagui and expressed in nitrite units per ml (NU/ml) (28). All laboratory analyses were performed at the Department of Biochemistry in Zabrze, Medical University of Silesia.
Questionnaires
The MSIS-29 questionnaire was used as a tool consisting of 29 questions to assessing the quality of life in MS patients. The responses are scored on a 1-5 scale and summed up to give a maximum of 100 points on the MSIS-29-PHYS scale and 45 points on the MSIS-29-PSYCH scale; a combined score can be generated, or both components can be reported separately (20). In our study, we used a validated Polish version of the questionnaire characterized by a high internal reliability with Cronbach's alpha values for each of the subscales of between 0.97 and 0.94 (21).
Magnetic resonance imaging examination
Head magnetic resonance imaging (MRI) was performed in all MS patients at the beginning of the study. The imaging was performed with the use of a General Electric HDx 1,5T scanner (USA). The patients were scanned with a standard head protocol (multiple planes, slice thickness 5 mm, contrast media: Gadovist [Gd]) and additional postcontrast 3DT1 sequences (1 mm slice thickness). An experienced radiologist reviewed the scans and assessed the approximate number of supratentorial and infratentorial plaques in T2 images. The number of enhancing T1 plaques was also reported.
Statistical analysis
All results expressed as means ± S.E.M. Normal data distribution was tested with the Kolmogorov-Smirnov's test. Comparisons between groups were performed using the Mann-Whitney U-test and Wilcoxon test. Differences between means were considered statistically significant at p<0.05. The Pearson correlation analysis was used to estimate the correlation. Results were statistically analyzed using STATISTICA v. 8.0 (StatSoft, Poland).
RESULTS
The demographic characteristics of the study groups are shown in Table 1.
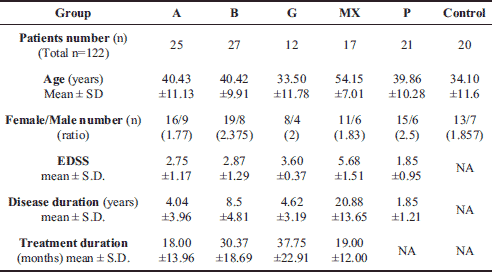
We observed significant increase in the MDA concentration in the serum (lipid peroxidation marker) in all MS patients groups - P, A, B, G, MX: 1.64±0.42, 2.06±0.51, 2.24±0.38, 2.06±0.55, 1.67±0.59 mol/L, respectively, as compared to healthy controls - group C: 0.97±0.2 µmol/L (p<0.05). No statistical differences in the MDA concentration were observed between the MS patient groups. After the 3 months of the melatonin treatment, the MDA concentration decreased significantly in groups A, B, and G accordingly: 1.05±0.29, 1.11±0.22, 0.91±0.06 mol/L; in MX group the MDA concentrations was similar (Fig. 1).
P (immunomodifying pre-treated RRMS group), C (control healthy group). NA - non applicable.
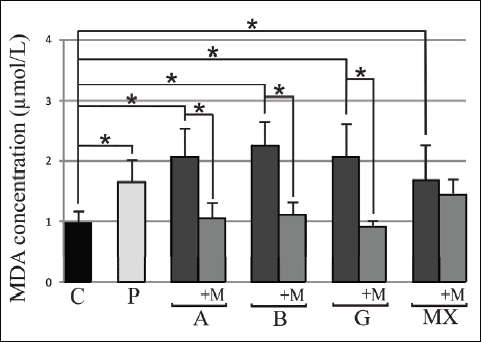 |
Fig. 1. Serum concentration of malondialdehyde (MDA) before and after 3 months 5 mg daily melatonin (M) supplementation in studied groups: A (beta-1a interferons treated RRMS group), B (beta-1b interferons treated RRMS group), G (glatiramer acetate treated RRMS group), MX (Mitoxantrone treated SP or PR MS group), P (immunomodifying pre-treated RRMS group), C (control healthy group). Data presented as mean ± S.D.; * p<0.05. |
The results from our present study marked an increase in SOD activity compared to control group only in G group 11.32±1.18 vs. 7.46±1.51. After 3 months melatonin supplementation the SOD activity increased compared to initial values in A group 9.67±2.6 vs. 7.98±1.82 and B group 10.22±1.31 vs. 8.12±1.38 (p<0.05). There were statistically significant differences between the studied groups only compared G with A and B groups (Fig. 2). The MDA and SOD results in the serum after the melatonin treatment for group P were inconsistent, and as such, difficult to interpret, requiring additional studies (data not presented).
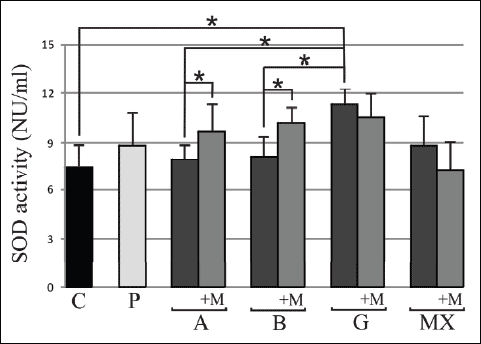 |
Fig. 2. Serum superoxide dismutase (SOD) activity before and after 3 months 5mg daily melatonin (M) supplementation in studied groups: A (beta-1a interferons treated RRMS group), B (beta-1b interferons treated RRMS group), G (glatiramer acetate treated RRMS group), MX (Mitoxantrone treated SP or PR MS group), P (immunomodifying pre-treated RRMS group), C (control healthy group). Data presented as mean ± S.D.; * p<0.05. |
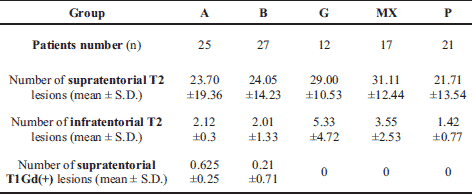
Numbers of MRI supratentorial and infratentorial T2 and T1 Gd(+) lesions for studied groups are shown in Table 2.
A positive relationship between supratentorial and infratentorial number of T2 lesions was reported in both the A (r=0.7, p<0.05) and B group (r=0.65, p<0.05). A significant negative relationship between the number of supratentorial plaques and mean MDA concentration (r= –0.53, p<0.05) in group B was observed. Furthermore, a positive correlation between the mean number of T2 supratentorial lesions and disease duration (r=0.76, p<0.05) was found in group B.
The annual relapse ratio (ARR) was significantly reduced in A, B, G groups: 0.65±1.06, 0.67±0.69, 0.75±1.91, respectively (p<0.05) compared to P group 1.34±2.77. No correlation between ARR and MDA or SOD results was observed.
MSIS-29 mean scores in the studied groups before and after the 3-month long melatonin treatment are presented as two impact subscales: physical (MSIS-29-PHYS) and psychological (MSIS-29-PSYCH) in Fig. 3. There was a significant increase in both MSIS-29-PHYS and MSIS-29-PSYCH items mean scores only in the MX group compared to other groups. There were no significant differences observed in terms of mean MSIS-29-PHYS results in the studied groups before and after the melatonin therapy. The statistical decrease in the mean MSIS-29-PSYCH scores after the melatonin treatment, compared to the initial values, was observed in two groups: group A - 14.71±5.25 vs. 21.71±8.12 and group B - 17.81±7.55 vs. 25.56±10.56. In group B, a positive correlation between mean MSIS-PHYS scores and the age of the patient was found: r=0.59, p<0.05, as well as between MSIS-PHYS and mean EDSS score: r=0.72, p<0.05.
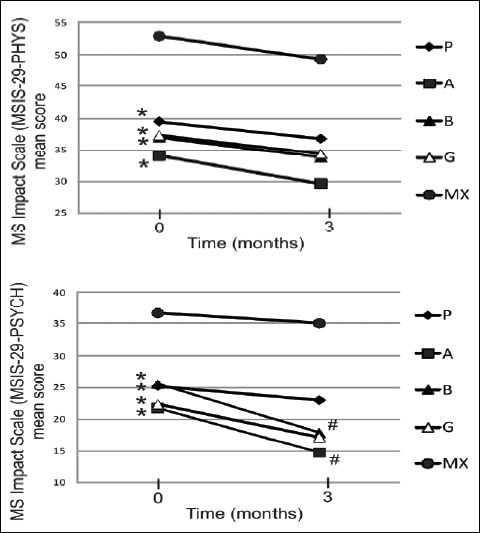 |
Fig. 3. The Multiple Sclerosis Impact Scale (MSIS-29) mean score presented as two subscales: physical impact scale (MSIS-29-PHYS) and psychological impact scale (MSIS-29-PSYCH). Datapoints show mean scores before and after 3 months 5 mg daily melatonin (M) supplementation in studied groups: A (beta-1a interferons treated RRMS group), B (beta-1b interferons treated RRMS group), G (glatiramer acetate treated RRMS group), MX (Mitoxantrone treated SP or PR MS group), P (immunomodifying pre-treated RRMS group). Data presented as mean ± S.D.; # p<0.05 after melatonin (M) supplemented group vs. before melatonin supplemented group; * p<0.05 MX MSIS-29-PHYS and MSIS-29-PSYCH mean score vs. A, B, G, P MX MSIS-29-PHYS and MSIS-29-PSYCH mean score. |
DISCUSSION
MS incidence is higher in geographic regions with sunlight deficiency. Both melatonin and vitamin D are important factors in the effect of sunlight in healthy individuals, and recent studies proved that they could play a key role in the pathophysiology of MS (1, 2). There exist only a few papers concerning the role of melatonin in MS pathophysiology, all of them published in recent years (2, 3, 22-24, 29-33).
Results from our study confirm a marked increase in serum lipid peroxidation marker - MDA concentration, similarly in all MS patient groups compared to controls. Data from our previous work also show that MDA concentration in serum and the cerebrospinal fluid are significantly higher in MS patients, in both de novo diagnosed RRMS and in the worsening MS form than in the controls (10). Several studies have demonstrated a significant increase in lipid peroxidation products in the brain, plasma, and cerebrospinal fluid in MS patients (34, 35). It is known that oxidative stress characterized by excessive production of ROS and reduction of antioxidant defense mechanisms has been implicated in the pathogenesis of MS (4, 5). The impairment of antioxidant systems, or an increase in the production of ROS could contribute to lipoprotein peroxidation in MS. Lipoprotein lipid peroxidation products are neurotoxic, have proinflammatory properties, and could be involved in demyelination and axonal injury in MS (35). In our previous research, we found protein oxidative and glycoxidative damage in MS patients, and an indication that markers of these kinds of damage, especially the advanced protein oxidation products level, may be useful in monitoring oxidative stress in the course of MS therapy (29).
In our present study we found, after the 3 months of the melatonin treatment, an MDA concentration decrease in interferons- and glatiramer acetate-treated groups, but not in the MX-treated and MS-immunomodulatory untreated patient groups. Similarly, a recent study suggests that 1 month of 10 mg melatonin supplementation caused a decrease in MDA concentration in the red blood cells in SPMS patients (31). It is known that melatonin and its metabolites scavenge ROS, and thus terminate the initiation and propagation of lipid peroxidation (10, 11).
Significant negative correlations between the number of supratentorial plaques and mean MDA concentration in group B were found. Furthermore, positive correlation between the mean number of T2 supratentorial lesions and disease duration was found in group B.
In our study the disease activity was evaluate by annual relapse ratio and by MRI changes estimation. In RRMS patients treated with interferons and glatiramer acetate the ARR was significantly reduced compared to pretreated patients, but the number of supratentorial MRI changes are similar. These results may explain that the changes in MDA and SOD levels in our report might be influenced also by change in clinical disease activity related to IFN-treatments. Recent reports suggest a negative linear correlation between bilirubin and uric acid levels and MRI in clinically isolated syndrome. There was also a significant correlation between bilirubin level, MRI findings, uric acid levels, and EDSS in RRMS patients (36).
The results from our present study marked an increase in SOD activity compared to control group only in Glatiramer acetate treated MS group. In previous reports blood antioxidant enzymes: SOD, CAT, and glutathione reductase (GR) activity was found to be elevated in RRMS against SPMS and controls and negative correlation established between SOD and CAT and EDSS in RRMS. Multiple logistic regressions toward the brain MRI injury volume proved the significance of C reactive protein, γ-interferon, and CAT. These results suggest that the state of the endogenous protection system and blood content of antioxidant enzymes (CAT, SOD) in MS patients could play a significant role in early progression of RRMS in SPMS (34). Similarly, in the Tasset et al. study values for total glutathione, SOD, GR were significantly higher in blood samples in RRMS patients compared to healthy controls (37).
After 3 months melatonin supplementation the SOD activity increased compared to initial values in interferon beta-1a and interferon beta-1b groups. Antioxidative properties of melatonin are well-documented. This indolamine could act indirectly by stimulating antioxidant enzymes, enhancing the activities of other antioxidants and directly inactivating reactive oxidant species (3, 11, 12). Melatonin upregulates antioxidative defensive systems, including the activities of SOD and GSH-Px, as well as the levels of GSH and these actions further reduce the oxidation state of cells. It has been reported that the endogenous antioxidant, bilirubin and uric acid, levels were lower in clinically isolated syndrome and RRMS patients than in the control group. The obtained values of bilirubin and uric acid were higher in both study groups in patients with lower EDSS, lower MRI lesion number and shorter disease duration (36). Furthermore, 1 month of 10mg melatonin supplementation caused a statistically significant increase in SOD and GSH-Px activities in erythrocytes in SPMS patients (31). Interestingly, Bahamonde C et al. reported that treatment RRMS patients with natalizumab caused a significant increases in serum melatonin concentrations and simultaneous rise in increase of antioxidants and a reduction in oxidative stress biomarkers. These results may confirm role of antioxidative processes in beneficial effects of natalizumab in MS therapy (38).
Many abovementioned biochemical parameters in interferons- and glatiramer acetate-treated MS patients were different than in the P and MX patient groups. These observations are very difficult to interpret, because even many years of therapy with these drugs in MS brought no response about detailed mechanisms of their therapeutic actions. It has been demonstrated that this therapy decreases antigen presentation, has potential modulatory effects on costimulatory molecules present on dendritic and other cells, suppresses proliferation of the Th1 cells, increases expression of IL-10 (a major anti-inflammatory cytokine), and shifts the inflammatory environment from proinflammatory to anti-inflammatory (39).
It is known that sleep disturbances are more often present in MS patients than in the general population (15, 16). Still, there are relatively few original papers concerning sleep problems in MS patients. The prevalence of sleep problems was reported in 24-50% of MS patients (17, 18). The possible common pathomechanisms shared by MS and sleep disturbances may be connected with circadian rhythm disorders with compromised melatonin secretion reducing input to the suprachiasmatic nucleus due to impaired visual pathways, as well as increased levels of proinflammatory cytokines (22). Najafi et al. reported that sleep phase syndrome and irregular sleep wake patterns in MS patients with mild and severe fatigue were compared with healthy subjects. Circadian rhythm sleep disorders were significantly higher in MS patients in relation to healthy subjects (40). In the study of Braley et al., decreased sleep efficiency in MS patients was observed. Furthermore, it is correlated with increased symptoms of tiredness, fatigue, and lack of energy in patients with MS (41). Melatonin is one of the major regulators of the sleep-wake cycle. There was an association observed between shift work at a young age and the occurrence of MS. Consequences of shift work, such as circadian disruption and sleep restriction, were associated with disturbed melatonin secretion and enhanced proinflammatory responses (23). Melamud et al. reported significantly lower sleep efficiency in the MS patients connected with dysregulation of melatonin secretion in MS patients, which may be influenced by treatment with interferons beta (22). Exogenous melatonin has somniferous properties in normal subjects and can improve sleep quality in several clinical conditions. Recent studies have shown that melatonin may play a role in improving sleep in patients (13). Our work provides information about significant increase in both MSIS-29-PHYS and MSIS-29-PSYCH items mean scores only in the MX group as compared to other groups. There were no significant differences in mean MSIS-29-PHYS studied groups observed before and after melatonin therapy. Melatonin supplementation caused a decrease in mean MSIS-29-PSYCH scores compared to initial values in interferons beta-treated groups. Moreover, as expected, a positive correlation between mean scores of MSIS-PHYS and age, and between MSIS-PHYS and mean EDSS score was found, but only in the interferon beta-1b group. Several studies in Poland and other countries have reported that the quality of life is worse in patients with MS than in healthy controls, with a higher prevalence of depression and fatigue (42-43). Our data in both MSIS-29-PHYS and MSIS-29-PSYCH items scores were lower than those obtained during the Polish MSIS-29 validation study (21). On the other hand, our results in the MSIS-29-PSYCH item were similar to those published in 2013 obtained via the web portal of the UK MS Register by Jones et al. concerning MSIS-29 mean scores among 4558 MS patients. The mean MSIS-29-PSYCH score in this study was established at 24.8±8.9 and MSIS-29-PHYS at 60.5±21.6 (44). The MSIS-29-PSYCH scores in our A group was 21.71±8.12 and in B group 25.56±10.56. Our mean MSIS-29-PHYS item scores were lower than those mentioned in the UK data. It was previously suggested that melatonin deficiency can play an important role in fatigue and mood disorders, but not directly affecting the impact on the quality of life (22, 45, 46). Our present work strongly suggests that exogenous melatonin supplementation can adjusts psychological aspects of quality of life in MS patients, some of which are probably connected with better sleep.
Findings from our study provided clinical evidence to support experimental data suggesting that melatonin can act as antioxidant and improves the reduced quality of life in MS patients. The results obtained from our research point to the importance of endogenous antioxidants, such melatonin in the outbreak and course of MS. On the one hand, melatonin acts as an antioxidant in a disease with documented oxidative stress pathophysiology. On the other hand, however, its ability to regulate the circadian rhythm in a disease associated with sleep disturbances, such as MS, could be beneficial. What is important, melatonin exhibits a high level of safety, is well tolerated, and generates no side effects even in high doses. Therefore, more investigation is needed to ascertain the exact role of melatonin in the treatment and pathophysiology of MS.
Conflict of interest: None declared.
REFERENCES
- Mehta BK. New hypotheses on sunlight and the geographic variability of multiple sclerosis prevalence. J Neurol Sci 2010; 292: 5-10.
- Golan D, Staun-Ram E, Glass-Marmor L, et al. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun 2013; 32: 180-185.
- Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol 2010; 8: 228-242.
- Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 2004; 251: 261-268.
- Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain 2011; 134: 1914-1924.
- Tully M, Shi R. New insights in the pathogenesis of multiple sclerosis - role of acrolein in neuronal and myelin damage. Int J Mol Sci 2013; 14: 20037-20047.
- Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor independent actions. Adv Med Sci 2007; 52: 11-28.
- Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med 2009; 15: 43-50.
- Gonciarz M, Gonciarz Z, Bielanski W, et al. The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. J Physiol Pharmacol 2012; 63: 35-40.
- Adamczyk-Sowa M, Sowa P, Pierzchala K, Polaniak R, Labuz-Roszak B. Antioxidative enzymes activity and malondialdehyde concentration during mitoxantrone therapy in multiple sclerosis patients. J Physiol Pharmacol 2012; 63: 683-690.
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 2007; 42: 28-42.
- Tomas-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res 2005; 39: 99-104.
- Kostoglou-Athanassiou I. Therapeutic applications of melatonin. Ther Adv Endocrinol Metab 2013; 4: 13-24.
- Srinivasan V, Spence DW, Trakh I, Pandi-Perumal SR, Cardinali DP, Maestroni GJ. Immunomodulation by melatonin: its significance for seasonally occurring diseases. Neuroimmunomodulation 2008; 15: 93-101.
- Boe Lunde HM, Aae TF, Indrevag W, et al. Poor sleep in patients with multiple sclerosis. PLoS One 2012; 7: e49996. doi: 10.1371/journal.pone.0049996.
- Cote I, Trojan DA, Kaminska M, et al. Impact of sleep disorder treatment on fatigue in multiple sclerosis. Mult Scler 2013; 19: 480-489.
- Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler 2008; 14: 1127-1130.
- Caminero A, Bartolome M. Sleep disturbances in multiple sclerosis. J Neurol Sci 2011; 309: 86-91.
- Hagan RM, Oakley NR. Melatonin comes of age? Trends Pharmacol Sci 1995; 16: 81-83.
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29). Brain 2001; 124: 962-973.
- Jamroz-Wisniewska A, Papuc E, Bartosik-Psujek H, Belniak E, Mitosek-Szewczyk K, Stelmasiak Z. Validation of selected aspects of psychometry of the Polish version of the Multiple Sclerosis Impact Scale 29 (MSIS-29). Neurol Neurochir Pol 2007; 41: 215-222.
- Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci 2012; 314: 37-40.
- Hedstrom AK, Akerstedt T, Hillert J, Olsson T, Alfredsson L. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol 2011; 70: 733-741.
- Ghorbani A, Salari M, Shaygannejad V, Norouzi R. The role of melatonin in the pathogenesis of multiple sclerosis: a case-control study. Int J Prev Med 2013; 4 (Suppl. 2): S180-S184.
- Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 2005; 58: 840-846.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444-1452.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 1984; 142: 290-296.
- Sadowska-Bartosz I, Adamczyk-Sowa M, Galiniak S, Mucha S, Pierzchala K, Bartosz G. Oxidative modification of serum proteins in multiple sclerosis. Neurochem Int 2013; 63: 507-516.
- Lin GJ, Huang SH, Chen SJ, Wang CH, Chang DM, Sytwu HK. Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int J Mol Sci 2013; 14: 11742-11766.
- Miller E, Walczak A, Majsterek I, Kedziora J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol 2013; 257: 97-101.
- Natarajan R, Einarsdottir E, Riutta A, et al. Melatonin pathway genes are associated with progressive subtypes and disability status in multiple sclerosis among Finnish patients. J Neuroimmunol 2012; 250: 106-110.
- Miller E, Mrowicka M, Malinowska K, Kedziora J, Majsterek I. The effects of whole-body cryotherapy and melatonin supplementation on total antioxidative status and some antioxidative enzymes in multiple sclerosis patients. Pol Merkur Lekarski 2011; 31: 150-153.
- Davitashvili D, Beridze M, Shakarishvili R, Kiziria M, Sanikidze T. The role of endogenous antiradical protective system in multiple sclerosis. Georgian Med News 2012; April: 11-19.
- Ferretti G, Bacchetti T. Peroxidation of lipoproteins in multiple sclerosis. J Neurol Sci 2011; 311: 92-97.
- Ljubisavljevic S, Stojanovic I, Vojinovic S, et al. Association of serum bilirubin and uric acid levels changes during neuroinflammation in patients with initial and relapsed demyelination attacks. Metab Brain Dis 2013; 28: 629-638.
- Tasset I, Aguera E, Sanchez-Lopez F, et al. Peripheral oxidative stress in relapsing-remitting multiple sclerosis. Clin Biochem 2012; 45: 440-444.
- Bahamonde C, Conde C, Aguera E, et al. Elevated melatonin levels in natalizumab-treated female patients with relapsing-remitting multiple sclerosis: relationship to oxidative stress. Eur J Pharmacol 2014; 730: 26-30.
- Minagar A. Current and future therapies for multiple sclerosis. Scientifica (Cairo) 2013; 2013: 249101. doi: 10.1155/2013/249101.
- Najafi MR, Toghianifar N, Etemadifar M, Haghighi S, Maghzi AH, Akbari M. Circadian rhythm sleep disorders in patients with multiple sclerosis and its association with fatigue: a case-control study. J Res Med Sci 2013; 18 (Suppl. 1): S71-S73.
- Braley TJ, Chervin RD, Segal BM. Fatigue, tiredness, lack of energy, and sleepiness in multiple sclerosis patients referred for clinical polysomnography. Mult Scler Int 2012; 2012: 673936. doi: 10.1155/2012/673936.
- Nagaraj K, Taly AB, Gupta A, Prasad C, Christopher R. Prevalence of fatigue in patients with multiple sclerosis and its effect on the quality of life. J Neurosci Rural Pract 2013; 4: 278-282.
- Papuc E, Stelmasiak Z. Factors predicting quality of life in a group of Polish subjects with multiple sclerosis: accounting for functional state, socio-demographic and clinical factors. Clin Neurol Neurosurg 2012; 114: 341-346.
- Jones KH, Ford DV, Jones PA, et al. The physical and psychological impact of multiple sclerosis using the MSIS-29 via the web portal of the UK MS Register. PLoS One 2013; 8: e55422. doi: 10.1371/journal.pone.0055422.
- Akpinar Z, Tokgoz S, Gokbel H, Okudan N, Uguz F, Yilmaz G. The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res 2008; 161: 253-257.
- Chojnacki C, Walecka-Kapica E, Mokwinska M, et al. Influence of tianeptine on melatonin homeostasis and psychosomatic symptoms in patients with irritable bowel syndrome. J Physiol Pharmacol 2013; 64: 177-183.
A c c e p t e d : May 11, 2014