COMPARISON OF CEREBROCORTICAL MICROVASCULAR EFFECTS
OF DIFFERENT HYPOXIC-ISCHEMIC INSULTS IN PIGLETS:
A LASER-SPECKLE IMAGING STUDY
INTRODUCTION
The newborn piglet is an accepted large animal model to study the pathophysiology of hypoxic/ischemic (H/I) encephalopathy (HIE) of term infants, and to test putative neuroprotective strategies. HIE has multiple aetiology, and similar to this clinical heterogeneity, there have been numerous experimental methods in use to induce H/I stress in the newborn piglet. Although in the last three decades many experimental approaches have been tested ranging from acute total global cerebral ischemia induced by elevation of intracranial pressure above arterial pressure (1) to asphyxia induced by bilateral pneumothorax (2), however, more recently H/I stress is elicited by ventilating the piglets with hypoxic gas mixtures with (3-5) or without (6-8) bilateral occlusion of the common carotid arteries, suspending ventilation by occluding the endotracheal tube to elicit asphyxia (9), or sequential combination of the two methods (hypoxia/reoxygenation followed by asphyxia/reventilation) (10, 11). Understandably, all research groups adhere to their preferred H/I induction method in order to inflict consistent neurological damage and to be able to compare their results with the outcome of previous experiments. Therefore, direct comparisons between these H/I models are very scarce in the literature, and there is virtually no information to which extent these models actually elicit blood flow decreases in the brain, although the degree of ischemia is indeed a key determinant of neuronal damage.
In the present study, we set out to investigate the cerebrocortical microcirculatory effects of H/I insults that are often used in piglet models of HIE. More specifically, we were interested (I) how bilateral carotid artery occlusion (BCAO) affected the cortical perfusion in normoxic and in hypoxic conditions, and (II) how cerebrocortical perfusion altered during asphyxia/reventilation. We used the closed cranial window/laser-speckle imaging (LSI) technique that produces vivid two-dimensional perfusion maps, and these images were then evaluated with laser speckle contrast analysis (LASCA) allowing simultaneous assessment of parenchymal perfusion, pial arteriolar diameter and arteriolar flow velocity changes as shown previously (12).
MATERIALS AND METHODS
Animals
Newborn (1–2 days old, body weight: 1.5–2.5 kg) male Large-White piglets (n=7) were obtained on the day of experimentation from a local company (Pigmark Ltd. Co., Szeged, Hungary). All procedures were approved by the Animal Care and Use Committee of the University of Szeged.
Anesthesia was induced with intraperitoneal injection of sodium thiopental (45 mg/kg; Sandoz, Kundl, Austria) followed by intubation through tracheotomy and artificial ventilation with warmed and humidified medical air (21% O2, balance N2), using a pressure-controlled ventilator. The ventilation rate (25–35/min) and the peak inspiratory pressure (100–125 mmH2O) were set to keep blood gases and oxygen saturation in the physiological range. To elicit transient BCAO, remotely controlled vascular occlusion cuffs (OC 2A, in vivo Metric, Healdsburg, CA, USA) were secured around both exposed common carotid arteries. The right femoral artery and vein were catheterized to monitor arterial blood pressure, pH, pCO2, pO2, glucose, and to inject drugs and fluids, respectively. Anesthesia/analgesia was continued with intravenous bolus injection of morphine (100 mg/kg; Teva, Petah Tikva, Israel), and midazolam (250 mg/kg; Torrex Pharma, Vienna, Austria) then maintained with intravenous infusion of morphine (10 mg/kg/h), and midazolam (250 µg/kg/h) along with fluids (5% glucose, 0.45% NaCl, 2–5 ml/kg/h), with additional boluses if necessary. Body temperature was kept in the physiological range (38.5±0.5°C) using an electric heating pad.
The animals were then placed in a prone position with the head fixed in a stereotactic frame. Oxygen saturation, heart rate, arterial blood pressure, and body temperature were monitored with a Hewlett-Packard M1094 monitor (Palo Alto, California, USA); the data were on-line recorded using a PC (MecifView, Arlington, MA, USA). After retraction of the scalp, a ~20 mm diameter circular craniotomy was made in the left parietal bone, where a stainless steel closed cranial window with three needle ports was inserted after careful removal of the dura mater. The cranial window was sealed with bone wax, cemented in place with dental acrylic, and was filled with artificial cerebrospinal fluid (aCSF; containing KCl 220, MgCl2 132, CaCl2 221, NaCl 7710, urea 402, dextrose 665, and NaHCO3 2066 mg/l, warmed to 37°C and equilibrated with a gas mixture containing 6% O2, 6.5% CO2 and 87.5% N2). There was a 60 min stabilization period allowed after the implantation of the cranial window before commencing the experiments.
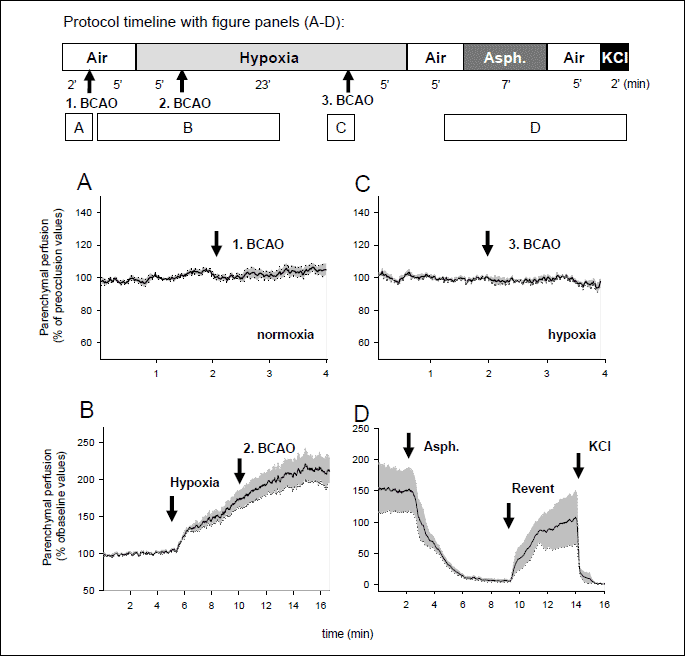
The experimental protocol was the following: after obtaining 2 min baseline, transient BCAO was elicited by inflating the occluders with air to 280 mmHg for 2 min. 5 min after the termination of the first BCAO, FiO2 was reduced to 0.1. Two more BCAOs (2–2 min) were elicited at the 5th and 30th minutes of hypoxic period. Five min after the last occlusion period, the animals were reoxygenated by ventilating them again with air for 5 min, followed by 7 min asphyxia induced by halting artificial ventilation and blocking the endotracheal tube. After asphyxia, the animals were reventilated with air for 5 min then the anesthetized animals were euthanized to obtain biological zero measurements. During these interventions LSI was performed, and the obtained images were analyzed by LASCA as described in the following section.
Laser-speckle imaging and contrast analysis
The cranial window was illuminated by the polarized light of a near infrared diode laser (l=808 nm, 200 mW; DL-8141-002 SANYO Electric Co., Japan) through an optical polarizing cube beamsplitter (850 nm, 35 mm; Edmund Optics Ltd, York, UK) approximately perpendicular to its surface (Fig. 1). The speckle images were recorded through the same polarizing cube that was attached through a custom-made bayonet adaptor to the objective of an operating microscope (Wild, Heerburg, Switzerland), coupled to a 1280×1024 pixel monochrome camera (PL-B741F; PixeLINK® Ottawa, Canada). Speckle images were recorded with 2 ms exposure time with 1 frame/s rate, and stored on a personal computer. Speckle contrast analysis was performed off line using custom-designed software written in LabVIEW (National Instruments Co., Austin, TX, USA). The local contrast maps were calculated from the recorded speckle images using rolling windows of 5×5 pixel areas. For each measurement in each animal, 4–4 regions of interests (ROIs) having an area of 5×5 pixels (~100 mm2) on the raw speckle images were identified over the cortical parenchyma and over pial arterioles. The t correlation times were calculated using eq.1, where K is the average speckle contrast of the corresponding ROI, T is the exposure time and b is the coherence factor:
The β coherence factor was previously determined from the speckle contrast of images recorded from a white Teflon® sheet as proposed in the literature (13). The 1/τ values were normalized to the respective baseline values as presented in the Results. 1/τ over parenchymal ROIs were determined in every image, however, in order to obtain better spatial resolution of arterioles, 1/τ values of ROIs over arterioles were determined at selected timepoints representing steady states of 30 s periods after averaging the contrast map of 31 consecutive images in order to decrease the native statistical noise of the images. On these images the internal diameter of the pial arterioles was also determined.
Statistical analysis
Parametric data are expressed as mean ±S.E.M. Data were analyzed with statistical software (SigmaPlot 11, Systat Software Inc., San Jose, CA, USA). Data were analyzed with one-way repeated measures ANOVA, followed by the Student-Newman-Keuls post hoc test where appropriate. P values <0.05 were considered statistically significant.
RESULTS
The assessed physiological parameters were in the normal range in all animals before the onset of hypoxia, the values were: MABP: 79±3 mmHg; heart rate 163±11 1/s; core body temperature 38.5±0.1°C; pH: 7.42±0.03; pCO2: 38±3 mmHg, pO2: 79±8 mmHg, oxygen saturation: 94±1%; glucose: 5.4±0.6 mmol/L. Hypoxia decreased oxygen saturation to 37±4%, and after 30 min blood gas values were: pH: 7.29±0.04; pCO2: 37±2 mmHg, pO2: 22±2 mmHg. The systemic cardiovascular response to hypoxia was quite variable: MABP was maintained or even increased in 5 piglets (from 78±12 to 85±19 mmHg), however, in two animals, MABP was severely depressed: from 84 to 51 and from 71 to 39 mmHg, respectively. Asphyxia resulted in severe acidosis, hypercapnia, hypoxia: the values were: pH: 6.73±0.02*; pCO2: 87±6* mmHg, pO2: 14±3* mmHg, respectively (* significantly different from baseline values).
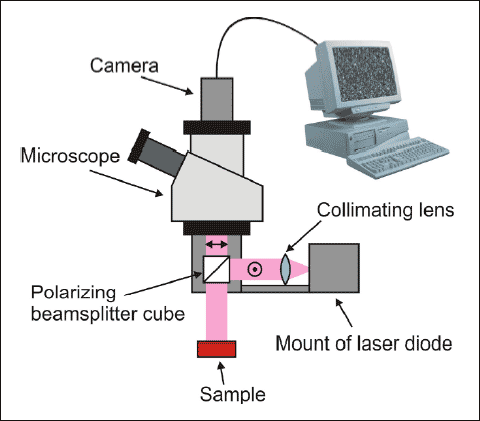 |
Fig. 1. The technical setup of the LSI device. The S-polarized light (direction of polarization is indicated by ť) of the laser diode was collimated, and then reflected towards the sample by the polarizing beamsplitter cube. The polarizing beamsplitter was designed as to reflect one polarization direction (S) while to transmit the other one (P-polarized light indicated by |
BCAO did not affect cortical parenchymal perfusion in either normoxic conditions (Figs. 2A, 3A, 3B), or the early/late phase of hypoxic ventilation (Fig. 2B, 2C). Hypoxia elicited in marked increases in cortical perfusion in the animals that maintained or increased their MABP after the initiation of hypoxia (Figs. 2B, 3C, 3D). In these animals, the hyperaemic response to hypoxia reached a steady state approximately after ~10 min and the dynamics of this blood flow increase appeared unaffected by the onset of the 2nd BCAO period elicited 5 min after the induction of hypoxia (Fig. 2B). In the two animals responding with hypotension, at the 10th minute of hypoxia cortical perfusion was reduced to 63% and 62% of baseline values, respectively. After the restoration of normoxia, elevated cortical perfusion corresponding with reactive hyperemia was observed, however, asphyxia quickly resulted in severe, but upon reventilation reversible cortical ischemia (Figs. 2D, 3E).
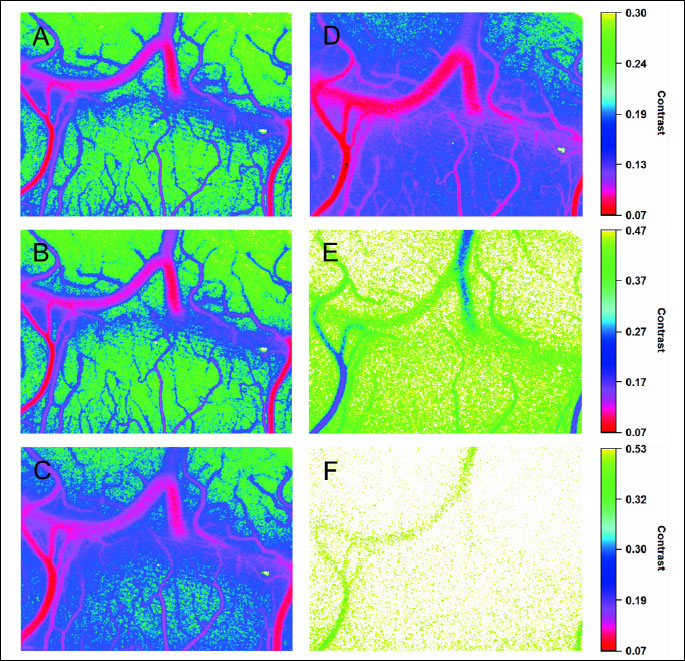
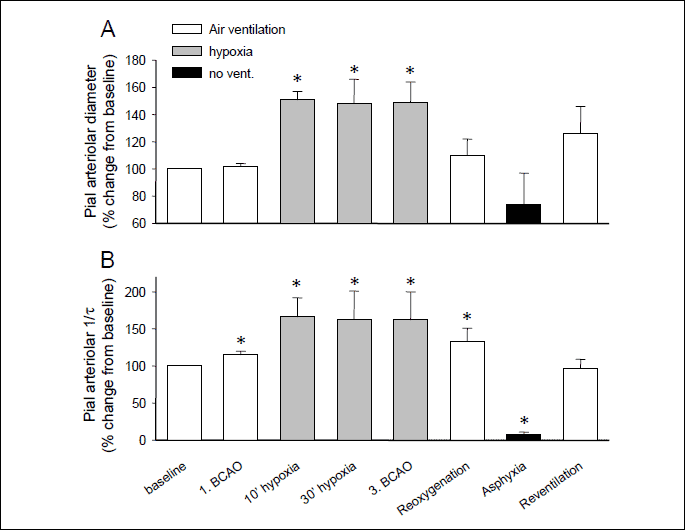
BCAO did not significantly affect pial arteriolar diameters, but it elicited a small increase in the velocity of arteriolar flow (Fig. 4A, 4B). In contrast to the minor effect of BCAO on arterioles, in the animals that maintained/increased MABP to hypoxia, hypoxia elicited a robust sustained vasodilation and a parallel increase in arteriolar flow velocity (Fig. 4A, 4B). In the two animals responding with hypotension, hypoxic vasodilation was less prominent, and arteriolar 1/τ values were also reduced to 56% and 57% of baseline values (at 10th min of hypoxia), respectively. Asphyxia elicited a ~90% but upon ventilation reversible decrease in arteriolar flow velocities again corresponding well with the ischemia/reperfusion observed in the cortical parenchyma.
DISCUSSION
The major novel finding of the present study is that BCAO does not significantly reduce cerebrocortical perfusion, and it does not even reduce hypoxia-induced hyperemia in the cortical microcirculation. We also demonstrated that asphyxia indeed elicits severe cerebrocortical ischemia.
Choosing an appropriate animal model is of vital importance for successful translational research. In rodents, unilateral carotid artery occlusion combined with hypoxia produces robust, unilateral infarction that has been widely utilized (14-16). In contrast to the neonatal rodents with premature lyssencephalic brains, the newborn pig has long been recognized as one of the best relatively easily available large animal model of the term human neonate to study HIE (17). This statement, however, refers primarily to the piglet’s brain, but not to its supplying arteries. In addition to differences in the anatomy of the origin of the common carotid arteries, there is a so-called rete mirabile at the base of the brain providing extensive anastomoses among all major extracranial arteries that serve the brain. The compensatory capacity of the rete mirabile in newborn pig HIE models remained undetermined, although in adult swine it has become an important neurosurgery model of arteriovenous malformations (18). Haaland et al. published a detailed angiographic study of the piglet extracerebral vasculature, in which they carefully proposed that ligation of any extracranial arteries likely would not induce severe focal cerebral ischemia (19). In agreement with this suggestion, unilateral carotid artery occlusion was indeed demonstrated not to affect cerebral perfusion (20), but the efficacy of BCAO to elicit cerebral ischemia augmenting the insult of simultaneous hypoxic ventilation was not questioned previously. Even very recently, a series of very important high-impact studies have been published using hypoxic ventilation with BCAO in the piglet (21-24), however, in these studies cerebrocortical blood flow was not assessed.
In our present paper, we utilized LSI/LASCA, a very sensitive novel method to detect cerebrocortical perfusion changes triggered by BCAO, hypoxia, hypoxia + BCAO, and asphyxia. We demonstrated previously that LSI/LASCA could record cerebrocortical parenchymal perfusion increases or decreases to a number of vasoactive stimuli that match blood flow changes measured by other methods (12). The present study yields novel insight on the unexpected inability of BCAO to reduce cerebrocortical perfusion, although it is well-known that in rats BCAO leads to chronic cerebral hypoperfusion (25, 26). In fact, BCAO did not even blunt the hyperemic response to hypoxia. In this study, we performed relatively short duration transient BCAOs, not as long ones used to induce HIE. However, this is clearly not a limitation, since our purpose was to test the microcirculatory effect of a sudden drop in perfusion pressure induced by BCAO. The ischemia induced by such vascular occlusion is expected to take place immediately and reach its maximum within a few seconds before the perfusion would start to recover due to the activation of autoregulation mechanisms (27). However, we saw no signs of this sudden decrease in any of our experiments. We repeated the BCAO challenge after 30 min of hypoxia, where conceivably due to the hypoxic vasodilation the compensatory capacity of the microcirculatory bed can be impaired to withstand a drop in perfusion pressure, however, BCAO was also remarkably ineffective in this condition as well. This intriguing independence of the piglet cerebral circulation from the patency of the common carotid arteries likely stems principally from the rete mirabile anastomotic network, but also the extracranial branches of the common carotid/internal carotid arteries (more specifically the external carotid, occipital and condylic arteries) can retrogradely fill the internal carotid artery after BCAO that is typically performed at the level of the 4th cervical vertebra (22) thus proximal from these branches (19). Although it might be possible to obtain a “modified” carotid artery by ligating all these branches (19), this would require quite extensive surgery and have not been utilized in any piglet HIE models yet. Our present findings in the piglet are consistent with those demonstrated in other ungulates, specifically ruminants: sheep and calves (28). These species also have a rete mirabile and an extensive extracranial (occipitovertebral) anastomotic network, and BCAO was shown to be compensated by flow increases in remaining patent arteries (28). Importantly, in fetal sheep HIE models, the ligation of occipitovertebral anastomoses is routinely used to enable BCAO to elicit brain ischemia (29).
A certain limitation of the present study is that perfusion of subcortical structures was not assessed, however, it is well known that subcortical forebrain and brainstem structures have even better autoregulation than the cortex (30). Since the cortical blood flow change was virtually zero after BCAO, we can safely expect the same for the other brain regions as well. This conclusion is also supported by a magnetic resonance imaging study, where reduction in regional cerebral blood flow in response to hypoxic ventilation with BCAO was found not to correlate with the success of BCAO (31). Accordingly, BCAO should not be expected to worsen the neurological damage induced by hypoxic ventilation alone, and although such comparative studies are very scarce in the literature, at least one previous study found no difference between these models concluding that BCAO was not necessary (32). Our present findings fully support this notion providing hemodynamic evidence on the lack of developing ischemia during hypoxic ventilation + BCAO resulting in a H/I stress of similar severity to hypoxic ventilation alone.
In contrast to hypoxia + BCAO, asphyxia induced by suspended ventilation results in severe ischemia, perfusion decreases by 80–90% in accordance with our previous study with laser-Doppler flowmetry (LDF) (33). We would like to emphasize however, that LSI/LASCA compared to single-point LDF enabled us to select multiple and more precise parenchymal ROIs in each animals, thus resulting in less variable data while maintaining the excellent temporal resolution of the measurements. In addition LSI/LASCA also provides means to follow diameter changes of pial arterioles similar to intravital microscopy. Our present study showed that pial arteriolar diameter changes correspond well with parencymal perfusion alterations indicating an important role for this segment of resistance vessels in regulating cortical blood flow.
It is noteworthy that we created a novel LSI imager for the present study. Our relatively simple technical innovation can transform virtually any operating microscope equipped with a sufficient video camera into a LSI device. The advantages of this arrangement are twofold (in addition to the low cost): (I) the light source is stabilized with respect to the objective, so the same illumination of the cranial window is assured in every experiment; (II) the attached optical cube can be quickly mounted/dismounted thus the traditional use of the microscope remains possible.
In conclusion, BCAO does not elicit cortical ischemia making this intervention unnecessary to be included in the methodology of piglet HIE models. HIE models that combine hypoxic ventilation with systemic hypotension or induce asphyxia (11, 34), appear to better reproduce the ischemic aspect of HIE in this species.
FD and DZ-S contributed equally to this manuscript.
Acknowledgments: Financial support: National Scientific Research Fund of Hungary (OTKA, K81266, K100851). Orsolya Olah was supported through the TAMOP-4.2.2/B-10/1-2010-0012 grant. Ferenc Domoki was supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences and through the TAMOP-4.2.2/A-11/1/KONV-2010-0052 grant.
Conflict of interest: None declared.
REFERENCES
- Leffler CW, Busija DW, Beasley DG, Armstead WM, Mirro R. Postischemic cerebral microvascular responses to norepinephrine and hypotension in newborn pigs. Stroke 1989; 20: 541-546.
- Temesvari P, Joo F, Koltai M, et al. Cerebroprotective effect of dexamethasone by increasing the tolerance to hypoxia and preventing brain oedema in newborn piglets with experimental pneumothorax. Neurosci Lett 1984; 49: 87-92.
- Lorek A, Takei Y, Cady EB, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 1994; 36: 699-706.
- Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 1995; 37: 667-670.
- McCulloch KM, Raju TN, Navale S, et al. Developing a long-term surviving piglet model of neonatal hypoxic-ischemic encephalopathy. Neurol Res 2005; 27: 16-21.
- Rootwelt T, Loberg EM, Moen A, Oyasaeter S, Saugstad OD. Hypoxemia and reoxygenation with 21% or 100% oxygen in newborn pigs: changes in blood pressure, base deficit, and hypoxanthine and brain morphology. Pediatr Res 1992; 32: 107-113.
- DiGiacomo JE, Pane CR, Gwiazdowski S, Mishra OP, Delivoria-Papadopoulos M. Effect of graded hypoxia on brain cell membrane injury in newborn piglets. Biol Neonate 1992; 61: 25-32.
- Foster KA, Colditz PB, Lingwood BE, Burke C, Dunster KR, Roberts MS. An improved survival model of hypoxia/ischaemia in the piglet suitable for neuroprotection studies. Brain Res 2001; 919: 122-131.
- Domoki F, Olah O, Zimmermann A, et al. Hydrogen is neuroprotective and preserves cerebrovascular reactivity in asphyxiated newborn pigs. Pediatr Res 2010; 68: 387-392.
- Martin LJ, Brambrink AM, Lehmann C, et al. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol 1997; 42: 335-348.
- Ni X, Yang ZJ, Wang B, et al. Early antioxidant treatment and delayed hypothermia after hypoxia-ischemia have no additive neuroprotection in newborn pigs. Anesth Analg 2012; 115: 627-637.
- Domoki F, Zolei D, Olah O, et al. Evaluation of laser-speckle contrast image analysis techniques in the cortical microcirculation of piglets. Microvasc Res 2012; 83:311-317.
- Rojas-Ochoa LF, Lacoste D, Lenke R, Schurtenberger P, Scheffold F. Depolarization of backscattered linearly polarized light. J Opt Soc Am A Opt Image Sci Vis 2004; 21: 1799-1804.
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131-141.
- Dragun P, Makarewicz D, Wojcik L, Ziemka-Nalecz M, Slomka M, Zalewska T. Matrix metaloproteinases activity during the evolution of hypoxic-ischemic brain damage in the immature rat. The effect of 1-methylnicotinamide (MNA). J Physiol Pharmacol 2008; 59: 441-455.
- Kristek F, Malekova M, Ondrias K, Cacanyiova S. Blood pressure-independent hypotrophy of the heart, kidneys and conduit arteries after 7-nitroindazole administration to Wistar rats from the prenatal period to adulthood. J Physiol Pharmacol 2013; 64: 35-39.
- Thoresen M, Haaland K, Loberg EM, et al. A piglet survival model of posthypoxic encephalopathy. Pediatr Res 1996; 40: 738-748.
- De Salles AA, Solberg TD, Mischel P, et al. Arteriovenous malformation animal model for radiosurgery: the rete mirabile. AJNR Am J Neuroradiol 1996; 17: 1451-1458.
- Haaland K, Orderud WJ, Thoresen M. The piglet as a model for cerebral circulation: an angiographic study. Biol Neonate 1995; 68: 75-80.
- Laptook AR, Stonestreet BS, Oh W. The effect of carotid artery ligation on brain blood flow in newborn piglets. Brain Res 1983; 276: 51-54.
- Iwata O, Iwata S, Bainbridge A, et al. Supra- and sub-baseline phosphocreatine recovery in developing brain after transient hypoxia-ischaemia: relation to baseline energetics, insult severity and outcome. Brain 2008; 131: 2220-2226.
- Faulkner S, Bainbridge A, Kato T, et al. Xenon augmented hypothermia reduces early lactate/N-acetylaspartate and cell death in perinatal asphyxia. Ann Neurol 2011; 70: 133-150.
- Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013; 136: 90-105.
- Robertson NJ, Kato T, Bainbridge A, et al. Methyl-isobutyl amiloride reduces brain Lac/NAA, cell death and microglial activation in a perinatal asphyxia model. J Neurochem 2013; 124: 645-657.
- Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 2007; 54: 162-180.
- Sakr HF, Khalil KI, Hussein AM, Zaki MS, Eid RA, Alkhateeb M. Effect of dehydroepiandrosterone (DHEA) on memory and brain derived neurotrophic factor (BDNF) in a rat model of vascular dementia. J Physiol Pharmacol 2014; 65: 41-53.
- Grant DA, Franzini C, Wild J, Eede KJ, Walker AM. Autoregulation of the cerebral circulation during sleep in newborn lambs. J Physiol 2005; 564: 923-930.
- Baldwin BA, Bell FR. Blood flow in the carotid and vertebral arteries of the sheep and calf. J Physiol 1963; 167: 448-462.
- Guan J, Bennet L, George S, et al. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab 2001; 21: 493-502.
- Laptook A, Stonestreet BS, Oh W. Autoregulation of brain blood flow in the newborn piglet: regional differences in flow reduction during hypotension. Early Hum Dev 1982; 6: 99-107.
- Munkeby BH, Lyng K, Froen JF, et al. Morphological and hemodynamic magnetic resonance assessment of early neonatal brain injury in a piglet model. J Magn Reson Imaging 2004; 20: 8-15.
- Hou XL, Zhou YX, Zhou CL, Ding HY, Ding HS. Comparison of the models of acute hypoxia and hypoxic-ischemia in newborn piglets [in Chinese]. Beijing Da Xue Xue Bao 2009; 41: 702-706.
- Domoki F, Zimmermann A, Cserni G, Bori R, Temesvari P, Bari F. Reventilation with room air or 100% oxygen after asphyxia differentially affects cerebral neuropathology in newborn pigs. Acta Paediatr 2006; 95: 1109-1115.
- Ni X, Yang ZJ, Carter EL, Martin LJ, Koehler RC. Striatal neuroprotection from neonatal hypoxia-ischemia in piglets by antioxidant treatment with EUK-134 or edaravone. Dev Neurosci 2011; 33: 299-311.
A c c e p t e d : July 22, 2014