PLACENTAL VASCULAR ENDOTHELIAL GROWTH FACTOR EXPRESSION
IN PREGNANCIES COMPLICATED BY TYPE 1 DIABETES
INTRODUCTION
The vascular endothelial growth factor (VEGF) family and its receptors have multifunctional activities besides angiogenesis, and production of these molecules is induced by hypoxia/ischemia (1). These molecules are known to be expressed in the human trophoblast and placenta, but little is known about their involvement in pathologic conditions (2, 3). Ahmed et al. (4) demonstrated that placental VEGF expression is regulated by oxygen concentration. The hypoxemic environment may develop in such pathological conditions like even mild hyperglycaemia, overt diabetes and pre-eclampsia (5, 6). It was found that impaired glucose tolerance during pregnancy has strong impact on the expression of VEGF receptors in human placenta (7). These findings indicate a placental response to altered glycaemia, what may have an important consequences for the foetus. The change in the placental VEGF/VEGFR expression ratio in mild hyperglycaemia and overt diabetes may favour angiogenesis in placental tissue and could explain the hypercapillarization of villi and may determine the foetal development in the course of pregnancy (5). Placental VEGF expression is increased in women with diabetes, intrauterine growth retardation and pre-eclampsia (8). Congenital malformation, yolk sac and vascular abnormalities are related to VEGF mutations (9).
The VEGF gene is highly polymorphic. There are more than 80 SNPs in this region of the human genome (10). Some of these SNPs which are localized in the promoter region of VEGF genes (VEGF G-405C and VEGF C-2578A) may have an impact on VEGF production in peripheral blood and in other tissues (10).
The potential role of VEGF SNPs was evaluated in terms of spontaneous abortion, recurrent pregnancy loss, preterm labour and pre-eclampsia (11, 12). Samli et al. (13) determined the possible role of 2578 C/A, 460 C/T, 936 C/T polymorphisms frequencies in women with recurrent pregnancy loss. Only the prevalence of the 1154 G/A polymorphism A/A genotype was found to be significantly more frequent in the recurrent pregnancy loss group. Some of the VEGF SNPs are related to more frequent pre-term labour occurrence (14, 15).
It was found that for the VEGF-2578C/A polymorphism a significant difference was observed between the control and pre-eclampsia groups. The allele A was more frequent in the control group, suggesting the lower susceptibility to develop pre-eclampsia (16). The prevalence of some VEGF SNPs is related to severe perinatal complication in low birth weight (LBW) infants such as necrotizing colitis (NEC) and acute renal failure (ARF) (17).
There are no studies determining the potential role of placental VEGF expression and VEGF SNPs in foetal development in the course of T1DM pregnancy.
The aim of the present study was to determine the expression of vascular endothelial growth factor (VEGF) in term placentas from T1DM women and its relation to neonatal birth weight (NBW) and glycaemic control. We also attempted to determine the role of placental VEGF expression and VEGF 2 single nucleotide polymorphisms (SNPs) in foetal development in the course of T1DM pregnancy.
MATERIAL AND METHODS
Patients
Sixty seven normotensive, non-smoking, Caucasian T1DM subjects were enrolled into the study, hospitalized in tertiary level perinatal care unit in the Department of Obstetrics and Women’s Diseases between the 2009 and 2013. All participants gave an informed consent, and the study protocol obtained approval from the Ethics Committee of Poznan University of Medical Sciences. We also affirm that original studies have been carried out in accordance with the Declaration of Helsinki.
All women were offered a routine follow-up including at least three admissions to the Department (11–14 weeks, 18–24 weeks, 28–32 weeks and close to delivery) and regular check-ups in our outpatient clinic, according to Polish recommendations (18, 19). First trimester crown-rump length (CRL) ultrasound measurement was used to confirm gestational age. Moreover, all pregnant women were re-educated in intensive insulin therapy, optimal diet and blood glucose self-control. For all subjects, we collected data from general, obstetrical and diabetic history, including age at onset, duration and the presence of the vascular complications before pregnancy. During each visit in the Department or in the outpatient clinic (at least every two weeks), we collected data on glycaemic/lipid profile and blood pressure. HbA1C concentration was estimated every 6 weeks. Once a trimester, all subjects had a retinal examination done and renal function checked.
All participants were treated with human insulin and/or insulin analogues following a basal-bolus protocol. We adjusted the doses according to glucose levels measured by way of self-control. Target glucose values were set at 3.34–5.0 mmol/l for fasting glycaemia and less than 6.67 mmol/l for 2 hours postprandial glucose level. All biochemical parameters were analyzed in Central Laboratory of the University Hospital.
Exclusion criteria at baseline (10–14weeks) were: chronic hypertension, multiple pregnancy, also women who developed gestational hypertension or preeclampsia were excluded from the study group.
We examined 67 placentas from normotensive Caucasian T1DM subjects. Placental tissue from 67 T1DM women (age 18–40 years; at 37–40 weeks of gestation) was obtained immediately after placenta delivery, cleaned from amniotic membranes and maternal deciduae, rinsed in saline, snap frozen in liquid nitrogen and stored at –80°C until assayed.
To examine the role of placental VEGF (VEGF-A) in foetal development, the study group was divided into 3 subgroups according to NBW: 1 subgroup - neonatal birth weight <10 percentile (SGA - small for gestational age), 2 subgroup - neonatal birth weight between 10–90 percentile (AGA - appropriate for gestational age), 3 subgroup - neonatal birth weight exceeding 90 percentile (LGA - large for gestational age, macrosomic). We also examined the possible role of maternal VEGF SNPs –2578C>A and 2549 I/D in foetal development.
RNA extraction and cDNA synthesis
Total cellular RNA was isolated from the placenta tissue using TriPure Isolation Reagent (Roche, Germany) according to the manufacturer’s protocol. The concentrations and the purity of RNA were determined by measuring the absorbance at 260 and 280 nm in a spectrophotometer (BioPhotometer Eppendorf, USA). RNA samples were stored at –80°C. Complementary DNA was synthesized from 2 µg of total RNA in a total volume of 20 µl using the SuperScriptTM III First-Strand Synthesis System (Invitrogen, USA). The obtained transcripts were stored at –20°C or used directly for the real-time quantitative PCR (RT-PCR).
Real-time PCR
The level of mRNA expression was analyzed using RT-PCR. The primers and RT-PCR conditions used for VEGF and GAPDH amplifications were as follows: VEGF forward - CGG CGA AGA GAA GAG ACA CA, VEGF reverse - GCA GGA GGAAGG TCAACCACTCA, 40 cycles, denaturation 95°C, 8 s; annealing 53°C, 6 s; extension 72°C, 8 s; GADPH forward - GAA GGT GAA GGT CGG AGT C, GADPH reverse - GAA GAT GGT GAT GGG ATT TC, 40 cycles, denaturation 95°C, 8 s; annealing 54°C, 6 s; extension 72°C, 8 s. All oligonucleotide sequences were synthesized by TIB Molbiol (Poland). Amplicon size and reaction specificity were confirmed by agarose gel electrophoresis and melting curve analysis. RT-PCR was carried out using a LightCycler TM Instrument (Roche, Germany) and a LightCycler DNA Master SYBR Green I kit (Roche, Germany) according to the manufacturer’s protocol. GAPDH was used as a housekeeping gene for normalization (endogenous internal standard). The PCR program was initiated with an activation at 95°C for 10 min. Each PCR cycle comprised a denaturation step at 95°C, an annealing step at a specific temperature and an extension step at 72°C. The quantitative PCR was monitored by measuring the increase in fluorescence by the binding of SYBR Green I dye to the generated double-stranded cDNA. The data were evaluated with the Roche LightCycler Run 5.32 software. RT-PCR procedures were performed in the Institute of Natural Fibers and Medicinal Plants, Poznan, Poland.
Vascular endothelial growth factor single nucleotide polymorphisms assessment
VEGF SNPs were determined by PCR/RFLP assay. For amplification PCR starter sequence for VEGF –2578C>A was as follows: F5`-ggA Tgg ggC TgA CTA ggT AAg C -3` and R5`-AgC CCC CTT TTC CTC CAA C-3`. 326 bp PCR product size was digested by PsuI (BstYI) (Fermentas, Lithuania) restriction enzyme all night at 37°C. After enzyme inactivation at 65°C (20 minutes), a buffer was added and samples were conducted on 2% agarose gel. The genotypes were identified after ethidium bromide dye was added: CC 326 bp, CA 326, 203, 123 bp and AA 203bp, 123bp.
The PCR starter sequence for VEGF 2549 I/D was as follows: 5’-GGCCTTAGGACACCATACC-3’ and 5’-CACAGCTTCTCCCCTATCC-3’. The PCR product was conducted on 3% agarose gel and identified at insertion when the product size was 456bp and deletion when the product size was 438bp. PCR/RFLP assays were conducted in Molecular Biology Laboratory, Department of Perinatology and Women’s Diseases, Poznan University of Medical Sciences, Poznan, Poland.
Statistical analysis
We performed a statistical analysis using Statistica 8.0 for Windows. All data are presented as mean ± standard error of mean (S.E.M.). The normality of data distribution was checked with the Lillefors test. As the distribution of variables met the criteria for normal distribution, we compared the placental VEGF gene expression using analysis of variance (ANOVA) for more than two groups, and a post-hoc test (Fisher LSD - least significant difference) to specify the groups in which the differences are significant. Multiple regression models (MRMs) analysis were developed to evaluate the influence of the specified variables on neonatal birth weight and placental VEGF expression in selected groups. The Spearman correlation rank was used to determine the concordance between selected metabolic parameters and placental VEGF. The frequency of VEGF SNPs genotypes were calculated (Arlequin software http://cmpg.unibe.ch/software/arlequin3/) and met Hardy-Weinberg equilibria in all T1DM group. The differences in SNPs genotypes between specified subgroups were checked with the Chi-squared test. Statistical significance was set at P<0.05 for all comparisons.
RESULTS
The maternal mean triglycerides level at booking was significantly highest in women who delivered T1DM SGA neonates, and the percentage of HbA1C at booking was significantly highest in women who delivered T1DM LGA neonates. No significant differences were found for maternal diabetic (disease onset and duration) and general history (age). The above data is presented in Table 1.
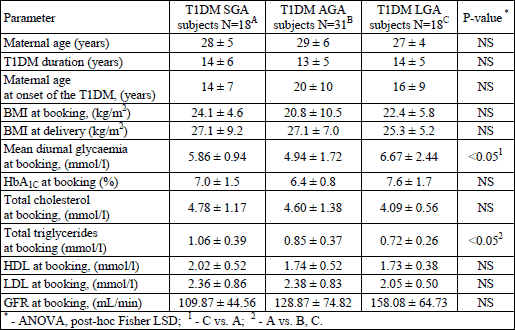
T1DM women who delivered T1DM LGA neonates were characterized by the significantly poorest glycaemic control close to delivery, defined as mean diurnal glycaemia and HbA1C percentage at delivery. Moreover, in this group we also found the greatest placental VEGF expression accompanied by the placental mass. There was also a significantly highest GFR in this subgroup. The above data is presented in Table 2.
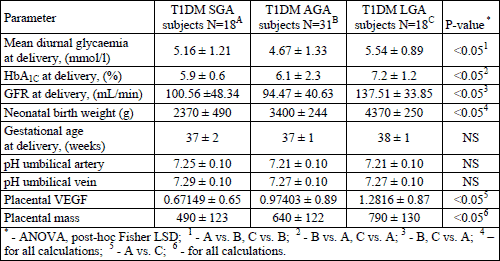
Since we discovered that T1DM women who delivered T1DM LGA newborns were characterized by the highest placental mass and VEGF expression, in the next step we attempted to analyze the possible influence of these factors as well as mean diurnal glycaemia and HbA1C percentage for neonatal birth weight. We also identified which of the maternal factors may affect placental VEGF expression. We developed multiple regression model (MRM) analyses where the above identified factors were used as explanatory variables. The analyses were performed for the entire T1DM group as well as for T1DM LGA subjects.
In the best-fitted model for T1DM LGA - (R - 0.78473465 for the model), we found an influence of placental VEGF (positive correlation, B-317.4884, P-value 0.019), and placental mass (positive correlation, B-3.4016 P-value 0.000) on NBW. Different MRMs for T1DM LGA subjects, including first and third trimester metabolic parameters (adjusted for mean diurnal glycaemia at booking and delivery, HbA1C at delivery, GFR at delivery), revealed no influence on NBW. The same MRMs performed for all T1DM group, T1DM SGA and AGA subjects also revealed no influence on NBW (Table 3).

Second MRM in T1DM LGA subjects (best-fitted model), adjusted for placental VEGF expression, including maternal features describing metabolic status - whole pregnancy mean diurnal glycaemia, HbA1C percentage, lipid profile, maternal BMI as explanatory variables, revealed significant impact of 3rd trimester mean blood glucose and HbA1C on placental VEGF expression. We also revealed significant correlations between these variables. No 1st and 2nd trimester parameters were found to have any role in stimulating placental VEGF expression. The above data are presented in Table 4, Figs. 1 and 2.
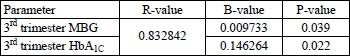
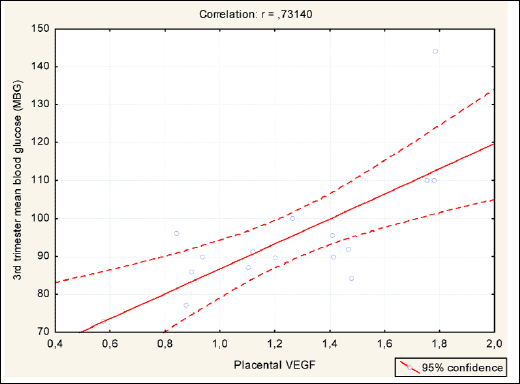 |
Fig. 1. Significant correlation between 3rd trimester MBG and placental VEGF in T1DM LGA subjects. |
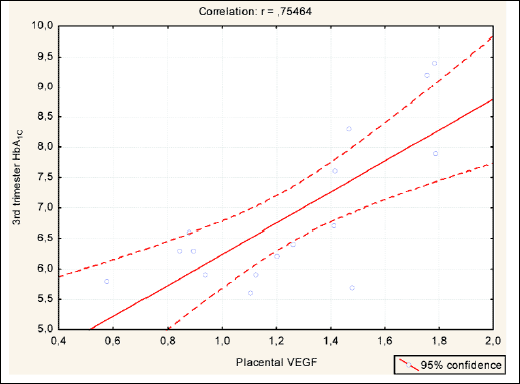 |
Fig. 2. Significant correlation between 3rd trimester HbA1C and placental VEGF in T1DM LGA subjects. |
We also attempted to analyze the possible maternal susceptibility to increased placental VEGF expression, defined as possibly disrupted frequency of VEGF –2578C>A and 2549 I/D SNPs. In our study groups, we found a statistically significant difference in homozygous and heterozygous frequency variants of VEGF SNPs between T1DM SGA and T1DM AGA subjects. The above data is presented in Table 5.
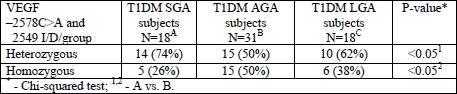
In the next step, the authors will attempt to confirm these results on larger study groups and to find out whether there is any difference in placental VEGF expression, metabolic control, the course of pregnancy and perinatal outcome in these groups, as divided according to VEGF SNPs variants.
DISCUSSION
Over the last decades a significant improvement in the management, diagnostic and therapeutic approaches of diabetes in pregnancy was achieved. Despite these facts, it still constitutes a serious therapeutic problem for women health, since diabetes-related complications, such as microangiopathy or hypertension, remain the major risk factors for poor perinatal outcome and morbidity (20, 21).
As we previously reported, in diabetic pregnancy several factors influencing foetal birth weight have been discovered - glycaemic control and lipid profile, maternal nutritional status and hormones, weight gain, energy intake and the presence of concomitant disorders influencing placental function, like hypertension or preeclampsia (22-25). It was also widely reported that even in well-controlled diabetic pregnant women, abnormalities in foetal growth may occur (26). It suggests features different than maternal ones, which may be involved in foetal development.
VEGF constitutes a major angiogenic regulator of endothelial cell proliferation (27). VEGF receptors (VEGFR) were discovered in the majority of human tissues, suggesting its multipotential role in human development (26). It was widely reported that VEGFR are located in human trophoblast and placenta, stimulating its vasculature proliferation and development. Abnormal VEGF expression during pregnancy is related to several pathological conditions (i.e. diabetes, pregnancy-induced hypertension, pre-eclampsia) that may affect VEGF expression in placenta, determining the potential consequences for the foetus (28).
Confusing data is available on maternal VEGF SNPs in placental VEGF expression regulation. One study by Chedraui et al, (29) documented a significant trend towards lower umbilical vein VEGF levels in pre-eclamptic carriers of –2578 CC and –1154 AG genotypes.
In our study, the potential role of maternal VEGF -2578C>A and 2549 I/D SNPs was not found, neither in VEGF placental expression, nor in the course of diabetic pregnancy in terms of glycaemic/metabolic control, foetal development and the outcome defined as NBW. These analyses were conducted in two ways: first, we established all possible genotypes of VEGF –2578C>A and 2549 I/D SNPs, and checked for any significant differences in foetal/maternal variables. Second, we documented significant differences in the frequency of VEGF –2578C>A and 2549 I/D SNPs homozygotic/heterezygotic variants, as adjusted to NBW. Since our groups were unequal, we conclude that these results need to be re-analysed on larger/equal groups to confirm frequency differences. Furthermore, the possible role of VEGF –2578C>A and 2549 I/D SNPs must be re-evaluated in terms of VEGF placental expression, the course of pregnancy and perinatal outcome.
In the course of diabetic pregnancy, the perinatal outcome defined as NBW is the major prognostic factor for neonatal mortality and morbidity. Macrosomic newborns are in the risk group for increased delivery complications of long-term consequences (20, 30). In our study, we discovered that the greatest placental VEGF expression was present in pregnancies where macrosomic neonates were delivered. There were also specific disturbances in maternal features (mean daily glycaemia, HbA1C) in this group, both at booking and delivery. According to our developed MRMs models, NBW in this group depends on placental VEGF and mass (R-0.78473465, B-317.4884 and 3.4016, respectively) rather than on maternal features. These findings suggest that the placental response for hyperglycaemia was reflected by increased VEGF expression and prolonged hyperglycaemia defined as altered HbA1C induces placental VEGF up-regulation (28). We also confirmed that the altered daily glycaemia, defined as increased mean diurnal glycaemic profile, up-regulates placental VEGF expression and leads to foetal macrosomia. In the study of Pietro et al. (5), under hyperglycaemic conditions, the increased placental VEGF/VEGFR expression and villi hypercapillarization were documented as the response to hyperglycaemia. Another study by Marini et al. (31) demonstrated the effect of impaired glucose tolerance during pregnancy on the increased expression of VEGF receptors in human placenta. Since we discovered the increased placental VEGF expression in poorly controlled pregnant women, who delivered macrosomic infants, in the next step we checked, which factors may contribute to these effects. In the study of Zhao et al. (32) up-regulation of VEGF in response to maternal hyperglycaemia was reported. VEGF together with hyperglycaemia up-regulates the expression of placental growth factor.
In our study, the increased placental VEGF expression was associated with abnormal glycaemic control in the course of pregnancy and foetal macrosomia, what was also confirmed by MRMs model, which revealed a significant impact of these variables on placental VEGF expression. There was also a significant correlation between these variables in the 3rd trimester of pregnancy. What is confusing is that no other correlations were found between the 1st and 2nd trimester maternal factors, neither in T1DM LGA subjects nor the entire T1DM group. We conclude that the number of subjects in these subgroups should be increased (important limitation factor MRM models) and re-analyzed. Another small study (macrosomic foetuses N=4) by Lash et at (33), indicated that there was no difference in the expression of either VEGF isoforms between the healthy IUGR or macrosomic groups and the normal controls.
Another confusing issue in this study is the fact that little is known on insulin impact on the level of placental VEGF expression in diabetic population. There are no studies demonstrating the possible role of exogenous insulin on placental VEGF expression in terms of dosage and demand. There is one study of Loukovaara et al. (34) that demonstrates an increased level of placental growth factor (PlGF) in foetal cord blood in women with insulin-treated diabetes. In this case, the level of PlGF may indirectly reflect an increased expression of placental VEGF, since PlGF/VEGF pathways are linked in placental metabolism.
It is also known that VEGF family members (VEGF-B, VEGF-C, VEGF-D) are involved and promote both endothelial and lymphatic vascularization during embryonic development (35). The expression of members of the vascular endothelial growth factor (VEGF) family (VEGF-A, VEGF-C, VEGF-D and their receptors VEGF-R1, VEGF-R2 and VEGF-R3) in the placental bed was found throughout normal and pre-eclamptic human pregnancy. These findings suggest that VEGF family members may play a role in the process of spiral artery remodelling in these conditions (36). The most recent data from Lappas (37) demonstrated the increased expression of VEGF-A in omental tissue but not in placenta in pregnant women with pre-existing gestational diabetes and obesity. Another recent study revealed increased placental VEGF-A expression in type 1 diabetic placenta in comparison to gestational diabetes placentas (38). The similar data regarding gestational diabetes were documented by the Chinese investigators (39).
To conclude, our data demonstrate that the placental VEGF expression, together with poor glycaemic control are related to foetal macrosomia in T1DM pregnancy. The factors influencing placental VEGF expression in this subgroup are related to the 3rd trimester glycaemic control (MBG, HbA1C). Other 1st and 2nd trimester maternal factors as well as maternal VEGF SNPs need to be evaluated in larger study group.
In our previous studies, we demonstrated that placental leptin and leptin receptor are involved in the course of T1DM pregnancy and perinatal outcome (40). This study, as well as the recent findings regarding VEGF, are the part of larger project focused on assessment of possible placental and feto-maternal factors affecting the course of T1DM pregnancy. In the upcoming papers, we’ll present our data on the role of placental visfatin, resistin and angiotensin converting enzyme inhibitor (ACE-I) in the normotensive, T1DM pregnant women.
Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial rrowth factor receptor; T1DM, type 1 diabetes mellitus; SNP, single nucleotide polymorphisms; NBW, neonatal birth weight; LBW, low birth weight; SGA, small for gestational age; LGA, large for gestational age; MRM, multiple regression model; PlGF, placental growth factor; MBG, mean blood blucose
Acknowledgements: This study was supported by the scientific grant from Polish Ministry of Science awarded to Prof. Ewa Wender-Ozegowska. The authors are indebted to Mr Piotr Iciek for his linguistic assistance.
Conflict of interests: None decared.
REFERENCES
- Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol 2002; 33: 1069-1077. Erratum: 2002; 33: 1244.
- Dubova EA, Pavlov KA, Esayan RM, et al. Vascular endothelial growth factor and its receptors in the placenta of women with type 1 diabetes mellitus. Bull Exp Biol Med 2012; 152: 367-370.
- Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta 1997; 18: 657-665.
- Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen - a review. Placenta 2000; 21 (Suppl. A): S16-S24.
- Pietro L, Daher S, Rudge MV, et al. Vascular endothelial growth factor (VEGF) and VEGF-receptor expression in placenta of hyperglycemic pregnant women. Placenta 2010; 31: 770-780.
- Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod 2001; 7: 205-210.
- Madazli R, Tuten A, Calay Z, Uzun H, Uludag S, Ocak V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol Obstet Invest 2008; 65: 227-232.
- Vuorela P, Halmesmaki E. Vascular endothelial growth factor, its receptors, and the tie receptors in the placental bed of women with preeclampsia, diabetes, and intrauterine growth retardation. Am J Perinatol 2006; 23: 255-263.
- Madri JA, Enciso J, Pinter E. Maternal diabetes: effects on embryonic vascular development - a vascular endothelial growth factor-A-mediated process. Pediatr Dev Pathol 2003; 6: 334-341.
- Banyasz I, Bokodi G, Vasarhelyi B, et al. Genetic polymorphisms for vascular endothelial growth factor in perinatal complications. Eur Cytokine Netw 2006; 17: 266-270.
- Papazoglou D, Galazios G, Papatheodorou K, et al. Vascular endothelial growth factor gene polymorphisms and idiopathic recurrent pregnancy loss. Fertil Steril 2005; 83: 959-963.
- Lee HH, Hong SH, Shin SJ, Ko JJ, Oh D, Kim NK. Association study of vascular endothelial growth factor polymorphisms with the risk of recurrent spontaneous abortion. Fertil Steril 2010; 93: 1244-1247.
- Samli H, Demir BC, Ozgoz A, Atalay MA, Uncu G. Vascular endothelial growth factor gene 1154 G/A, 2578 C/A, 460 C/T, 936 C/T polymorphisms and association with recurrent pregnancy losses. Genet Mol Res 2012; 11: 4739-4745.
- Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: a HuGE review. Genet Med 2005; 7: 593-604.
- Atis A, Oruc O, Aydin Y, Cetincelik U, Goker N. Vascular endothelial growth factor gene +813CC polymorphism of foetus is associated with preterm labour but not with pre-eclampsia in Turkish pregnant women. Int J Immunogenet 2012; 39: 241-246.
- Cunha VM, Grecco RL, Paschoini MC, Silva SR, Ruiz MT, Balarin MA. Genetic polymorphisms of vascular endothelial growth factor in pre-eclampsia. Rev Bras Ginecol Obstet 2011; 33: 158-163.
- Banyasz I, Szabo S, Bokodi G, et al. Genetic polymorphisms of vascular endothelial growth factor in severe pre-eclampsia. Mol Hum Reprod 2006; 12: 233-236.
- Wender-Ozegowska E, Bomba-Opon D, Brazert J, et al. Rekomendacje Sekcji Cukrzycy, otylosci i innych zaburzen metabolicznych w ciazy. Standardy Polskiego Towarzystwa Ginekologicznego postepowania u kobiet z cukrzyca. Ginek Pol 2011; 82: 474-479.
- Pietryga M, Brazert J, Iciek R, et al. Rekomendacje Sekcji Ultrasonografii Polskiego Towarzystwa Ginekologicznego w zakresie przesiewowej diagnostyki ultrasonograficznej w ciazy o przebiegu prawidlowym (2.12.2011). Ginek Pol 2012; 83: 309-315.
- Langer O, Conway DL. Level of glycemia and perinatal outcome in pregestational diabetes. J Matern Fetal Med 2000; 9: 35-41.
- Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Molvig J, Mathiesen ER. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care 2001; 24: 1739-1744.
- Nomura RM, Paiva LV, Costa VN, Liao AW, Zugaib M. Influence of maternal nutritional status, weight gain and energy intake on fetal growth in high-risk pregnancies. Rev Bras Ginecol Obstet 2012; 34: 107-112.
- Catalano PM, Thomas AJ, Huston LP, Fung CM. Effect of maternal metabolism on fetal growth and body composition. Diabetes Care 1998; 21; 85-90.
- Wender-Ozegowska E, Zawiejska A, Michalowska-Wender G, Iciek R, Wender M, Brazert J. Metabolic syndrome in type 1 diabetes mellitus. Does it have any impact on the course of pregnancy? J Physiol Pharmacol 2011; 62: 567-573. Erratum in: 2012; 63: 205.
- Iciek R, Wender-Ozegowska E, Seremak-Mrozikiewicz A, et al. Leptin gene, leptin gene polymorphisms and body weight in pregnant women with diabetes mellitus type I. J Physiol Pharmacol 2008; 59 (Suppl. 4): 19-31.
- Higgins M, Mc Auliffe F. A review of maternal and fetal growth factors in diabetic pregnancy. Curr Diabetes Rev 2010; 6: 116-125.
- Galazios G, Papazoglou D, Tsikouras P, Kolios G. Vascular endothelial growth factor gene polymorphisms and pregnancy. J Matern Fetal Neonatal Med 2009; 22: 371-378.
- Pinter E, Haigh J, Nagy A, Madri JA. Hyperglycemia-induced vasculopathy in the murine conceptus is mediated via reductions of VEGF-A expression and VEGF receptor activation. Am J Pathol 2001; 158: 1199-1206.
- Chedraui P, Solis EJ, Bocci G, et al. Feto-placental nitric oxide, asymmetric dimethylarginine and vascular endothelial growth factor (VEGF) levels and VEGF gene polymorphisms in severe preeclampsia. J Matern Fetal Neonatal Med 2013; 26: 226-232.
- Sacks DA. Etiology, detection, and management of fetal macrosomia in pregnancies complicated by diabetes mellitus. Clin Obstet Gynecol 2007; 50: 980-989.
- Marini M, Vichi D, Toscano A, et al. Effect of impaired glucose tolerance during pregnancy on the expression of VEGF receptors in human placenta. Reprod Fertil Dev 2008; 20: 789-801.
- Zhao B, Cai J, Boulton M. Expression of placenta growth factor is regulated by both VEGF and hyperglycaemia via VEGFR-2. Microvasc Res 2004; 68: 239-246.
- Lash G, MacPherson A, Liu D, Smith D, Charnock-Jones S, Baker P. Abnormal fetal growth is not associated with altered chorionic villous expression of vascular endothelial growth factor mRNA. Mol Hum Reprod 2001; 7: 1093-1098.
- Loukovaara M, Leinonen P, Teramo K, Andersson S. Concentration of cord serum placenta growth factor in normal and diabetic pregnancies. BJOG 2005; 112: 75-79.
- Wathen KA, Stenman UH, Leinonen E, Andersson S, Vuorela P. Concentrations of vascular endothelial growth factor C and D in amniotic fluid and maternal plasma. Acta Obstet Gynecol Scand 2009; 88: 629-634.
- Schiessl B, Innes BA, Bulmer JN, et al. Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta 2009; 30: 79-87.
- Lappas M. Markers of endothelial cell dysfunction are increased in human omental adipose tissue from women with pre-existing maternal obesity and gestational diabetes. Metabolism 2014; 63: 860-873.
- Shchegolev AI, Dubova EA, Pavlov KA, Esayan RM, Shestakova MV, Sukhikh GT. Comparative immunohistochemical evaluation of vascular endothelial growth factor and its receptors in the placental villi in gestational diabetes mellitus and type 1 diabetes. Arkh Patol 2013; 75: 13-18.
- Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013; 6: 650-659.
- Iciek R, Wender-Ozegowska E, Zawiejska A, et al. Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol 2013; 64: 579-585.
A c c e p t e d : August 6, 2014