ALL GENES ENCODING ENZYMES PARTICIPATING IN MELATONIN BIOSYNTHESIS IN THE CHICKEN PINEAL GLAND ARE TRANSCRIBED RHYTHMICALLY
INTRODUCTION
Biological rhythms (i.e., daily changes in biological parameters) are fundamental properties of nearly all of the living organisms studied to date (1-3). Almost all daily changes in the physiological state (also referred to as circadian rhythms) are generated by endogenous oscillators that are active for nearly 24 hours, even when organisms are placed in an unchanging environment, such as constant darkness (D:D). These endogenous oscillators are synchronized or entrained to the local time via the detection of an ambient cue or time giver (ZT, Zeitgeber time); thus, the endogenous phase corresponds reliably to the environmental phase. The dominant ZT for most species is the light:dark (L:D) cycle, and specialized photoreceptive and phototransduction mechanisms have evolved in biological clock systems. The stable entrained oscillator, or population of oscillators in avian species, in turn regulates multiple downstream processes, conferring pacemaker properties to the system. This activity is regulated by clock genes and transcriptional-translational feedback loops. These clock genes are highly conserved among species; Per and Cry encode negative transcriptional regulators, while Bmal, Clock and Npas2 encode positive regulators (3). Positive regulators are capable of mediating the transcriptional regulation of genes containing elements of enhancer box (E-box) sequences in their promoters. The latter group of genes encode transcription factors (TFs) that may regulate the cyclic expression of additional clock-controlled genes (CCGs) as well as genes encoding the PER and CRY proteins via a negative feedback loop. CCG-dependent and negative feedback loop regulation occurs through the binding of different TFs to promoter consensus sequences other than E-box, such as DBP/E4BP4 binding (D-box) elements and REV-ERBα/ROR binding (RRE) elements (4, 5).
The input mechanism for ZT detection, together with the pacemakers and their outputs constitute the biological clock. In vertebrates, the regulation centers of circadian rhythms reside in specialized structures such as the pineal gland, the retina and the hypothalamic suprachiasmatic nuclei (SCN) (6). The pineal gland synthesizes the indoleamine hormone melatonin (MEL) rhythmically throughout the L:D cycle, with a peak occurring at night, while a low basal level is maintained during the day. This hormone is not stored in the pineal gland but is released immediately into the peripheral circulation; thus, the elevation of its level in the blood corresponds to the night period. Consequently, MEL conveys information about external lighting conditions and the activity state of the central oscillator (7).
The biosynthesis of MEL occurs through a well-characterized sequence of enzymatic reactions, starting with the active uptake of the essential amino acid tryptophan (TRP) from the bloodstream into the mitochondria of pineal parenchymal cells (8). In the mitochondria, TRP is transformed into 5-hydroxytryptophan (5-HTP) via tryptophan hydroxylase (TPH; E.C.1.14.16.4), which is encoded by the tryptophan hydroxylase 1 gene (Tph1) in chickens (9). In the cytosol, aromatic L-amino acid decarboxylase (AADC; E.C.4.1.1.28), encoded by the dopa decarboxylase gene (Ddc), subsequently converts 5-HTP into 5-hydroxytryptamine (serotonin, 5-HT) (10). Serotonin is then transformed into N-acetylserotonin (NAS) via arylalkylamine-N-acetyltransferase (AANAT; E.C.2.3.1.87), which is encoded by the arylalkylamine-N-acetyltransferase gene (Aanat) (11). Finally, hydroxyindole-O-methyltransferase (HIOMT; E.C.2.1.1.4), encoded by the acetylserotonin O-methyltransferase gene (Asmt), converts NAS into MEL (12).
The molecular details of the regulation of rhythmic MEL biosynthesis vary among species (6, 13). The genes encoding each of the enzymes participating in the MEL biosynthetic pathway have been identified in several species and have been cloned and sequenced. It was found that in chickens, at least three of these genes (Tph1, Aanat and Asmt) are indirectly regulated by the molecular clock within pinealocytes and directly regulated by light at the transcriptional, translational and post-translational levels (14). Thus, pineal MEL biosynthesis is a rhythmic dynamic process. In contrast, Ddc was thought to be an arrhythmic gene. However, our recent research has indicated that the pineal gland of the chicken undergoes post-embryonic maturation dependent on the hatching season, and the circadian rhythm of MEL biosynthesis is already established by the second day of postembryonic life. Our data further indicated that in 2-day-old birds maintained under a standard photoperiod (L:D, 12:12), both the Aanat and Asmt genes are expressed by this time (15), regardless of the season, whereas the Tph1 gene is expressed rhythmically in 2- and 9-day-old animals only in the summer (16). Moreover, we found that in 9-day-old individuals hatched in the summer season, Ddc gene expression is also rhythmic. However, the daily changes in Ddc mRNA levels are relatively small (16).
Taken together, these findings led us to hypothesize that all genes encoding enzymes participating in MEL biosynthesis are expressed rhythmically in the chicken pineal gland and that their expression may be regulated by the molecular clock. To verify these hypotheses, we examined daily changes in the transcription of Tph1, Ddc, Aanat and Asmt in (1) pineal glands isolated from 16-day-old chickens maintained under L:D to verify whether these genes are transcribed rhythmically; (2) pineal glands isolated from 16-day-old chickens maintained under D:D conditions to verify whether the rhythm is maintained without external light cues; and (3) pinealocyte cultures under both L:D and D:D conditions to verify whether the endogenous rhythm of gene transcription persists in vitro. Additionally, we performed the Cosinor analysis of the results in order to determine the rhythmicity of the investigated genes expression. Moreover, to examine the possibility that these genes might be CCGs, we scanned the promoter regions of all four genes in silico for putative binding sequences of transcriptional factors involved in the molecular mechanism of circadian expression.
MATERIALS AND METHODS
Animals and experimental design
The experiments were performed using 928 male Hy-Line chickens (Gallus gallus domesticus L.). The animals were transported from a commercial hatchery to the animal facility of the Faculty of Biology of the University of Warsaw on the day of hatching and maintained under a strictly controlled cycle of 12 h light and 12 h dark (L:D 12:12), using strip lighting with a 250 lux intensity. The light was switched on at 6:00 A.M. The temperature was 32 ± 2°C during first week and then gradually decreased to 24 ± 2°C. The birds were provided free access to standard food and water. For the in vivo experiments, 15-day-old chickens were divided into two groups. The birds in the first group (L:D) remained in the same light conditions previously described, whereas those in the second group (D:D) were moved to constant darkness. On the next day, starting from ZT 2 (Zeitgeber Time) or CT 2 (Circadian Time) in the D:D group, animals from each group were sacrificed every 2 hours over a 24-h period. The pineal glands of chickens from the L:D group were isolated under strip lighting at a 250 lux intensity during the day and under dim red light (< 10 lux intensity) at night, whereas the pineal glands of birds from the D:D group were isolated only under dim red light. The isolated pineal glands were immediately frozen in liquid nitrogen and stored at –80°C until further analysis. The experiments were performed twice in the summer season.
In the in vitro experiments, 16-day-old chickens were sacrificed at ZT 6, and their pineal glands were isolated under strip lighting at a 250 lux intensity, then kept in chilled (4°C) buffer containing 120 mM NaCl, 25 mM NaHCO3, 5 mM KCl, 1.2 mM KH2PO4, 86.6 mM glucose and 0.1% bovine serum albumin (Gibco/Invitrogen, Carlsbad, CA). Pineal cells were dispersed in trypsin (0.25%, Sigma Aldrich, MO, USA) through a 30 min incubation at 37°C and subsequently plated in modified McCoy’s 5A medium (Gibco/Invitrogen, Carlsbad, CA) containing an antibiotic and antimycotic solution (1%, Gibco/Invitrogen, Carlsbad, CA) and 10% heat-inactivated fetal bovine serum (Biochrome/Merck, Berlin, Germany). Cells from up to 160 glands were seeded in 6-well plates (Primaria, Corning, NY, USA) at a concentration of 1.4 × 106 cells per well. The cultures were maintained in a humidified incubator at 41°C with 5% CO2 and fed through the exchange of medium at least daily. Serum-containing media were used throughout the full three days of culture. Cells were maintained under L:D 12:12 for the duration of culture. Pinealocyte cultures in the L:D group remained under L:D 12:12 until the end of the experiment, whereas those in the D:D group were moved to constant darkness after two full days of culture. The next day, starting from ZT 2 or CT 2 in the D:D group, pinealocytes from each group were collected every 2 hours over a 24-h period from 6 wells per time point. The pinealocytes in the L:D group were collected and treated under strip lighting at a 250 lux intensity during the day and under dim red light (< 10 lux intensity) at night, whereas the pinealocytes in the D:D group were always isolated and treated under dim red light. The first step in the isolation of mRNA from pinealocytes was to wash the cells twice with PBS and then add 400 µl of cell lysis buffer RI to each well (AxyPrep Multisource Total RNA Miniprep Kit, Axygen Bioscences, CA, USA). The lysates were immediately frozen in liquid nitrogen and stored at –80°C until further analysis. The experiments were performed twice in the summer season.
All procedures were performed in accordance with the regulations of the Polish Ethical Council for the Care and Use of Laboratory Animals and the European Community Directive for the Ethical Use of Experimental Animals. The protocol was approved by the First Local Ethical Council in Warsaw (Permit No 227/2011 and No 477/2013).
Isolation and quantification of mRNA
In the in vivo as well as in in vitro experiments, total RNA was isolated from the pineal glands of the animals and from the pinealocyte cultures using the AxyPrep Multisource Total RNA Miniprep Kit (Axygen Bioscences, CA, USA). The concentration of RNA was assessed using a spectrophotometer (NanoDrop), and its quality was assessed via gel electrophoresis. In addition, DNase treatment was performed (RQ1-RNase-Free DNase, Promega, WI, USA) following the instructions of the manufacturer. The reaction mixtures for reverse transcription (RT) contained 1,000 ng of total RNA, 2 µl of Maxima Enzyme Mix, 4 µl of 5 × Maxima Reaction Mix (Maxima First Strand cDNA Synthesis Kit, Thermo Scientific, MA, USA) and nuclease-free water (NFH2O, Thermo Scientific, MA, USA) for a total volume of 20 µl. RT reactions were performed in a thermal cycler (C1000 Touch, Bio-Rad, CA, USA) employing the following incubation temperature profile: 25°C for 10 min, 50°C for 30 min and finally 85°C for 5 min. The RT products were subsequently used in quantitative real-time PCR assays (RT-qPCR) performed in 48-well transparent plates (MicroAmp Fast Optical 48-Well Reaction Plate, Applied Biosystems/Thermo Scientific, MA, USA). Each RT-qPCR mixture contained a cDNA template (10% of the RT product in the in vivo experiments and 20% of the RT product in the in vitro experiments), 5 µl of 2 × SYBR Green I PCR master mix (Kapa SybrFast Universal qPCR Kit, KapaBiosystem, MA, USA), the gene-specific forward and reverse primers at 0.75 µM (Table 1) and NF-H2O added to a total volume to 12.5 µl. The reactions were performed in a thermal cycler (StepOneTM Real-Time PCR System, Applied Biosystems/Thermo Scientific, MA, USA) using the following conditions: 95°C for 20 s, followed by 40 cycles of denaturation (95°C for 3 s), and annealing with extension (58/63°C for 30 s). Fragments of the Tph1, Ddc, Aanat, Asmt and Tbp (TATA-binding protein, reference gene) cDNAs were cleaned and used as quantification standards (108 – 101) for qPCR. Transcript-level quantification was performed using Applied Biosystems software. Each sample was assayed in duplicate. The results were normalized to the level of the Tbp transcript and expressed as the number of mRNA copies per 100 copies of Tbp mRNA. Pulled cDNA with a well-known level of gene transcription was used as an internal control between the plates. The isolation of RNA and RT-qPCR was carried out no later than 1 month after pineal gland or pinealocyte collection.
In silico analysis
The in silico analysis of short promoter regions (–1,000/+100 from the transcription start site) from the chicken Tph1 (Gene ID: 395799, NC_006092.3), Ddc (Gene ID: 420947, NC_006089), Aanat (Gene ID: 396066, NC_006105.3) and Asmt (Gene ID: 396286, NC_006088.3) genes was performed using the Biobase Transfac® program, according to the standard protocol (17). The first translation start codon was verified for the transcription start site (TSS) in all genes analyzed, which was ATG in Tph1 and Ddc, GCA in Aanat (9) and GCC in Asmt (18), at positions 1,583 (chromosome 5, gi|358485507:c11849927-11839352), 24,558 (chromosome 2, gi|358485510:c80780541-80708806), 4,352,229 (chromosome 18) and 128,389,030 (chromosome 1), respectively.
Statistical analysis
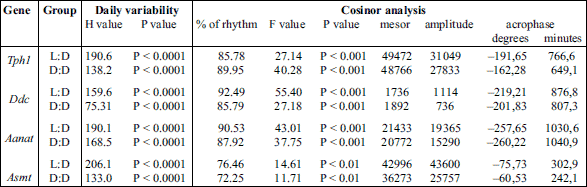
The data, presented as the mean values ± S.E.M., were compared through nonparametric statistical analysis. Significance was assessed using the Kruskal-Wallis test, followed by Dunn’s multiple-comparison post hoc test. The differences were considered significant at P < 0.05. Statistical analysis were performed using Statistica 10 PL software (StatSoft/Dell, TX, USA). In addition, the data were analyzed using the Cosinor method as previously described (15). The observed circadian variability is presented in Figs. 1-4 as a cosine curve with approximations of the cosine function. The midline estimation statistics of the rhythm and the rhythm percentage were computed, and the data are presented in Tables 2 and 3. Rejection of the zero-amplitude assumption for the approximating function at P < 0.05 was considered to demonstrate the significance of rhythmicity.
RESULTS
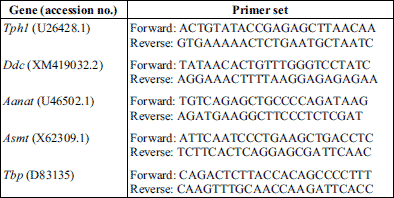
Pineal Tph1 mRNA levels
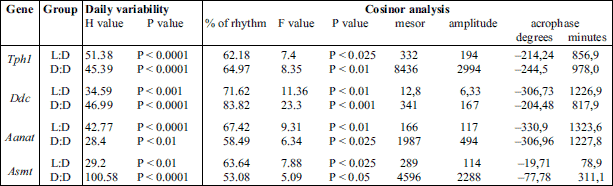
The Kruskal-Wallis test indicated significant daily variability in pineal Tph1 gene transcription (Table 2), both in chicks maintained under L:D 12:12 conditions and those maintained under constant darkness (Fig. 1A and 2A). Dunn’s multiple-comparison post hoc test revealed that in birds maintained under L:D 12:12 conditions, the transcription rate began to increase at ZT 6 (P < 0.01) and reached a plateau at the highest level at ZT 8 (P < 0.00001), then finally began to decrease at ZT 20 (P < 0.01). Similarly, in animals maintained under D:D conditions, transcription began to increase at CT 6
(P < 0.01), reached a plateau at the highest level at CT 8 (P < 0.05), but began to decrease earlier than in the L:D animals, at CT 18 (P < 0.05). The Cosinor analysis revealed significant daily rhythmicity of pineal Tph1 mRNA levels, regardless of the experimental light conditions, with the acrophase occurring during the transition from light to darkness (Table 2). However, in the birds that were reared in constant darkness, the amplitude of the mRNA rhythm was lower than in those maintained under L:D 12:12 conditions (Fig. 1A and 2A, Table 2).
 |
Fig. 1. Daily changes in the mRNA levels of Tph1, Ddc, Aanat and Asmt in chicken pineal glands in vivo. The mRNA levels were determined using pineal glands harvested from 16-day-old chicks reared under L:D conditions (n = 12). |
Pineal Ddc mRNA levels
The Kruskal-Wallis test revealed that pineal Ddc gene transcription exhibited significant daily variability (Table 2) both in animals reared in L:D 12:12 conditions and in those reared in constant darkness (Fig. 1B and 2B). Dunn’s multiple-comparison post hoc test indicated that in chicks maintained under L:D 12:12 conditions, transcription began to increase at ZT 8 (P < 0.01), reached its highest level at ZT 16 (P < 0.000001) and then began to decrease at ZT 20 (P < 0.001). In contrast, in birds reared in constant darkness, although transcription also began to increase at CT 8 (P < 0.05), it had already reached its highest level at CT 12 and 14 (P < 0.05) and then decreased rapidly at CT 24 (P < 0.05). The Cosinor analysis demonstrated significant daily rhythmicity of pineal Ddc mRNA levels, regardless of the experimental light conditions (Table 2), with the acrophase occurring during the transition from light to darkness. Nevertheless, in chickens maintained under constant darkness, the amplitude of the mRNA rhythm was lower than in those maintained under L:D 12:12 conditions (Fig. 1B and 2B, Table 2).
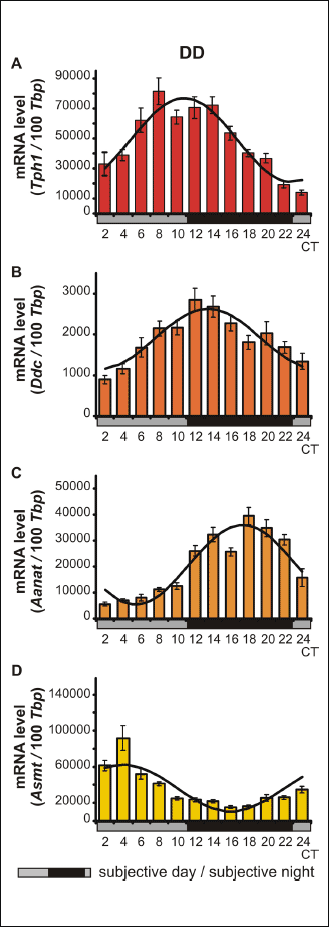 |
Fig. 2. Daily changes in the mRNA levels of Tph1, Ddc, Aanat and Asmt in the chicken pineal glands in vivo. The mRNA levels were determined using pineal glands harvested from 16-day-old chicks reared in constant darkness D:D (n = 12). |
Pineal Aanat mRNA levels
The Kruskal-Wallis test revealed significant daily variability in pineal Aanat gene transcription (Table 2) in birds maintained under both L:D 12:12 conditions and constant darkness (Fig. 1C and 2C). Dunn’s multiple-comparison post hoc test revealed that in animals reared under L:D 12:12 conditions, transcription increased rapidly at ZT 12 (P < 0.001), remained at this elevated level until reaching a peak at ZT 20 (P < 0.001), and finally decreased rapidly at ZT 24 (P < 0.001). Similarly, in chicks hatched in constant darkness, gene transcription increased rapidly at CT 12 (P < 0.05), then remained at an elevated level until reaching its peak at CT 20 (P < 0.001), and finally decreased rapidly at CT 24 (P < 0.05). The Cosinor analysis indicated significant daily rhythmicity of pineal Aanat mRNA levels, regardless of the experimental light conditions (Table 2), with the acrophase occurring in the middle of the scotophase. In birds kept in constant darkness, however, the amplitude of the mRNA rhythm was lower than in those maintained under L:D 12:12 conditions (Fig. 1C and 2C, Table 2).
Pineal Asmt mRNA levels
The Kruskal-Wallis test revealed that pineal Asmt gene transcription exhibited significant daily variability (Table 2) in chicks reared under both L:D 12:12 conditions and in constant darkness (Fig. 1D and 2D). Dunn’s multiple-comparison post hoc test showed that in animals hatched in L:D 12:12 conditions, transcription began to increase at ZT 24 (P < 0.01), reached its maximum at ZT 4 (P < 0.01) and then decreased gradually until ZT 14 (P < 0.01). On the contrary, in birds kept in constant darkness, transcription began to increase at CT 2 (P < 0.001), reached its maximum at CT 4 (P < 0.01) and then decreased gradually until CT 10 (P < 0.05). The Cosinor analysis demonstrated significant daily rhythmicity of pineal Asmt mRNA levels, regardless of the experimental light conditions (Table 2), with the acrophase occurring in the photophase. In animals reared in constant darkness, however, the amplitude of the mRNA rhythm was lower than in those maintained under L:D 12:12 conditions (Fig. 1D and 2D, Table 2).
in vitro Tph1 mRNA levels
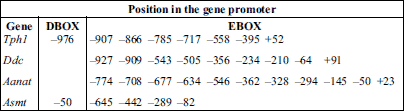
The Kruskal-Wallis test revealed significant daily variability in Tph1 gene transcription in vitro (Table 3), in both pinealocytes maintained under L:D 12:12 conditions and those kept in constant darkness (Fig. 3A and 4A). Dunn’s multiple-comparison post hoc test indicated that in cells cultured in L:D 12:12 conditions, the transcription rate remained at a low level during the day and rapidly increased at ZT 12 (P < 0.01) to reach its highest level, then rapidly decreased at ZT 18 (P < 0.001). Similarly, in pinealocytes maintained under D:D conditions, transcription remained at a low level during the subjective day and rapidly increased at CT 14 (P < 0.01), reaching its highest level at CT 16 (P < 0.0001), then began to decrease at CT 18 (P < 0.05). Interestingly, in pinealocytes kept in constant darkness, transcription also increased at CT 24 (P < 0.01). The Cosinor analysis demonstrated significant daily rhythmicity of in vitro Tph1 mRNA levels, regardless of the experimental light conditions (Table 3), with the acrophase occurring during the transition from light to darkness. However, in the pinealocytes that were kept in constant darkness, the amplitude of the mRNA rhythm was higher than in those maintained under L:D 12:12 conditions (Fig. 3A and 4A, Table 3).
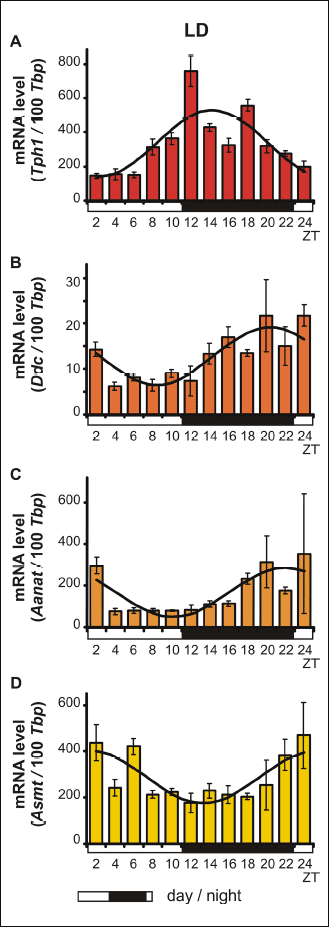 |
Fig. 3. Daily changes in the mRNA levels of Tph1, Ddc, Aanat and Asmt in chicken pinealocyte cultures in vitro. The mRNA levels were determined using pinealocytes maintained under L:D conditions (n = 12). |
in vitro Ddc mRNA levels
The Kruskal-Wallis test indicated that the in vitro transcription of the Ddc gene exhibited significant daily variability (Table 3) both in pinealocytes cultured in L:D 12:12 conditions and those reared in constant darkness (Fig. 3B and 4B). Dunn’s multiple-comparison post hoc test revealed that in pinealocytes maintained under L:D 12:12 conditions, transcription significantly increased only at ZT 16 (P < 0.05). In contrast, in pinealocytes cultured in constant darkness, transcription increased rapidly at CT 14 (P < 0.0001), reaching its maximum level at this point, then remained at a high level at CT 16 (P < 0.01), and finally began to decrease rapidly at CT 20 (P < 0.05). The Cosinor analysis revealed significant daily rhythmicity of in vitro Ddc mRNA levels, regardless of the experimental light conditions (Table 3), with the acrophase occurring during the transition from light to darkness. Nevertheless, in cells cultured in constant darkness, the amplitude of the mRNA rhythm was higher than in those maintained under L:D 12:12 conditions (Fig. 3B and 4B, Table 3).
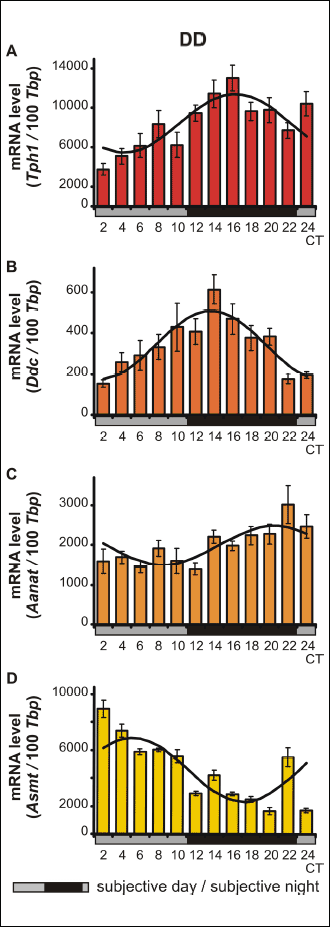 |
Fig. 4. Daily changes in the mRNA levels of Tph1, Ddc, Aanat and Asmt in chicken pinealocyte cultures in vitro. The mRNA levels were determined using pinealocytes kept in constant darkness, D:D (n = 12). |
in vitro Aanat mRNA levels
The Kruskal-Wallis test indicated that the in vitro transcription of the Aanat gene exhibited significant daily variability (Table 3) in pinealocytes maintained under both L:D 12:12 conditions and in constant darkness (Fig. 3C and 4C). Dunn’s multiple-comparison post hoc test demonstrated that in cells cultured in L:D 12:12 conditions, Aanat gene transcription remained at the lowest level during the day (P < 0.05), then began to increase to reach a peak at ZT 18 (P < 0.05), and finally decreased rapidly at ZT 4 (P < 0.01). In pinealocytes cultured in constant darkness, gene transcription remained at a relatively high level, with a peak being observed at CT 22 (P < 0.05). However, the Cosinor analysis revealed that the in vitro daily rhythmicity of the Aanat mRNA level was significant, regardless of the experimental light conditions (Table 3), with the acrophase occurring in scotophase. Nevertheless, in cells kept in constant darkness, the amplitude of the mRNA rhythm was higher than in those maintained under L:D 12:12 conditions (Fig. 3C and 4C, Table 3).
in vitro Asmt mRNA levels
The Kruskal-Wallis test revealed significant daily variability of the transcription of the Asmt gene in vitro (Table 3) in pinealocytes cultured under both L:D 12:12 conditions and constant darkness (Fig. 3D and 4D). Dunn’s multiple-comparison post hoc test demonstrated that in cells maintained under L:D 12:12 conditions, transcription remained at the relatively low level with a nadir at ZT 12 (P < 0.05). On the contrary, in pinealocytes kept in constant darkness, transcription remained at its highest level during the subjective day, with a peak being observed at CT 2 (P < 0.0001). Transcription then decreased gradually from CT 12 (P < 0.01) until reaching its lowest level at CT 20 and 24 (P < 0.0000001). The Cosinor analysis indicated significant daily rhythmicity of in vitro Asmt mRNA levels, regardless of the experimental light conditions (Table 3), with the acrophase occurring during the transition from light to darkness in L:D conditions and in the photophase in D:D conditions. In cells kept in constant darkness, however, the amplitude of the mRNA rhythm was higher than in those maintained under L:D 12:12 conditions (Fig. 3D and 4D, Table 3).
In silico analysis
The in silico analysis of short promoter regions (–100/+100 from the transcription start site) from the chicken Tph1, Ddc, Aanat and Asmt genes indicated that all of these genes may potentially be regulated transcriptionally by the molecular clock mechanism. The analysis predicted 7 enhancer box sequences (E-box) in the Tph1 promoter, 9 sequences in the Ddc promoter, 11 sequences in the Aanat promoter and 4 sequences in the Asmt gene promoter (Table 4). In addition, DBP/E4BP4 binding elements (D-box) were demonstrated to be present in the promoters of Tph1 and Asmt.
DISCUSSION
It is generally accepted that genes that remain under the control of the molecular biological clock are expressed rhythmically under L:D conditions as well as under constant conditions of either darkness (D:D) or dim light (dim L:L) (14), both in vivo and in vitro. Therefore, the aim of the present study was to verify whether all of the genes encoding enzymes participating in MEL biosynthesis in the pinealocytes of chickens are transcribed rhythmically under these conditions. Moreover, we sought to examine whether all of these genes may be defined as clock-controlled genes (CCGs). CCGs should contain E-box elements in their promoters; therefore, we performed an in silico analysis of short promoter regions. The results of this study clearly confirmed that all of these genes exhibit a circadian rhythm in the pineal glands (in vivo) as well as isolated pinealocytes of 16-day-old chicks (in vitro) under L:D 12:12 light conditions. Moreover, rhythmicity was sustained when the animals or pinealocytes were transferred to D:D conditions. We found that the expression of the Aanat gene was at its highest level during the scotophase, whereas the maximum expression of Asmt occurred during the photophase. Additionally, the Tph1 and Ddc genes were expressed at their highest levels during the transition from light to darkness. We also found possible E-box and D-box binding sites in the promoters of the investigated genes.
These results are consistent with the available data concerning the diurnal profiles of Tph1, Aanat and Asmt gene transcription in the pineal glands of chickens. The first day/night changes in Asmt gene expression were observed in pineal glands collected from chicks between day 8 of embryonic life and day 31 post-hatching (19). The first gene to be recognized as rhythmic, both in pineal glands and chicken pineal cultures, was Tph1 (20). The authors showed that the circadian rhythm of Tph1 not only persisted under L:D 12:12 conditions, but was also sustained in constant darkness. In a later study, circadian rhythm of Aanat expression was demonstrated in chicken pineal cells cultured under both L:D 12:12 and D:D conditions (11). Moreover, exposure to light during the first 6 h of the night was shown to inhibit the nocturnal increase in the mRNA level of Aanat. The same authors confirmed day/night changes in the expression of the Tph1, Aanat and Asmt genes in both pineal glands and chicken pineal cultures. They also found day/night changes in the expression of the Tph1 and Aanat genes in the chicken retina (21). Interestingly, it was observed that in contrast to the pineal gland, exposure to light at night does not suppress Aanat gene expression in the chicken retina (11). In light of these results, the Ddc gene was thought to be arrhythmic. Moreover, this gene was generally believed to be constitutively expressed at a high level (22, 23), and it has therefore been disregarded and generally not even tested alongside the other parameters.
Two new methodological approaches have been introduced in recent years: 1) characterization of microRNAs (miRNA) as new players in the regulation of gene expression; and 2) microarray transcriptome analysis. Profiling of the pineal miRNA population in mammals (24) indicated that it exerts limited influence on the expression of genes encoding enzymes involved in MEL biosynthesis. Microarray analysis has been applied to the study of both avian (25, 26) and mammalian (27-30) pineal physiology. Bailey and co-workers investigated the mRNAs encoding the enzymes of the MEL biosynthesis pathway in chicken pineal glands under L:D conditions. They found that the highest levels of Tph1 and Ddc mRNA occurred in the early evening, while for the Aanat gene, the levels were highest during the night. In contrast, the level of Asmt mRNA peaks during the day (25). However, these researchers detected significant daily oscillations in the level of pineal Ddc mRNA in chickens maintained under L:D 12:12 conditions, with a lack of rhythmicity being observed under D:D conditions. Interestingly, these authors also identified orthologues of some mammalian clock genes in the chicken pineal gland and suggested that the expression of Tph1, Aanat and Asmt remains under clock control. Our results are in partial agreement with the above-mentioned studies. Comparison of our results with other published data indicated the same diurnal changes in the mRNA levels of the Tph1, Aanat and Asmt genes in chickens (11, 19, 21). However, our results clearly indicated that the Ddc gene is transcribed in vivo in a rhythmic fashion, with a 6-fold increase being observed during the first half of the night. This inconsistency may be due to differences in the experimental procedures adopted. Bernard and colleagues used 1- or 2-day-old animals in their in vitro experiments, whereas the in vivo experiments were conducted on older birds (11, 21). Moreover, in the aforementioned studies, the season of hatching was not taken into consideration. Our recent studies indicate that the chicken pineal gland matures between the 1st and 16th day of life, and the duration of the maturation may vary between seasons (15, 16). Some processes that occur in this period are arrhythmic in the youngest birds, and rhythmicity develops in older ones. Additionally, we were able to employ chronobiological cosine analysis because of the frequent and regular (every 2 hours over a 24-h period) collection of the pineal glands and mRNA measurements performed in the present work. Moreover, the expression of Ddc in chicken pineal glands as well in pinealocyte cultures is quite low in comparison with expression of other pineal genes. Thus, the previously applied methods might have been insufficient to measure Ddc expression.
Although most of our knowledge concerning the vertebrate molecular clock has been derived from studies in rodents, several orthologues of the mammalian clock genes have been identified in birds (31). These genes encode positive proteins, such as CLOCK, MOP4 (NPAS2), BMAL1 and BMAL2; negative proteins, such as CRY1, CRY2 and PER2, PER3; and regulatory elements, such as E4BP4. Rhythmic changes in clock gene expression are responsible for the measurement of biological time in animals and influence their physiology through the expression of CCGs containing E-box enhancer elements (32). In addition to the clock genes described above, auxiliary transcriptional-translational feedback loops have also been identified. In mammals, two other promoter elements, the DBP/E4BP4 binding element (D-box) and the REV-ERBα/ROR binding element (RRE), were also found to participate in cellular clock functions. REV-ERBα, an orphan nuclear receptor, negatively regulates the activity of CLOCK:BMAL1. The same mechanism controlling Per and Cry gene transcription also regulates the transcription of REV-ERBα. Similarly, the transcription factor DBP is positively regulated by the CLOCK:BMAL1 complex and acts as an important output mechanism, driving the rhythmic transcription of other output genes via a PAR basic leucine zipper factor (PARbZIP) (33).
As presented in the current work, our in silico analysis of short promoter regions (–100/+100 from TSS) of chicken genes encoding enzymes that catalyze pineal MEL biosynthesis provided additional support for our suggestion that all of the analyzed genes may potentially be regulated transcriptionally by the molecular clock mechanism. Our analyses revealed the presence of E-box sequences in the promoters of the Tph1, Ddc, Aanat and Asmt genes and D-box sequences in the promoters of Tph1 and Asmt. The transcription of Aanat and Asmt, the most frequently examined genes among the genes encoding enzymes participating in MEL biosynthesis, is regulated via numerous endogenous and exogenous factors. Noteworthy among these factors are clock genes working through E-box and D-box promoter binding sites, cAMP-responsive elements (CRE) and inducible cAMP early repressor (ICER), which interact with CRE-binding protein (CREB), mediating positive and negative transcriptional regulation. Two CRE-like sequences (CLSs) and two E-box elements were identified in the chicken Aanat gene promoter. Moreover, it was revealed that a complex of TFs (c-Fos/JunD/pCREB) may augment the E-box-activated transcription of Aanat at night through effects on the CLSs and via characteristic regulatory elements (repetitive 8 × TTATT motif) in the proximal promoter (34). Analyses of the chicken Aanat and Asmt promoters also revealed the presence of potential binding sites for the photoreceptor-specific transcription factor cone-rod homeobox-containing protein (CRX). In addition, sixteen potential E-box elements were identified in the Asmt promoter (18, 35). Prior to our study, the relative dearth of available data meant that little was known about the transcriptional regulation of pineal MEL biosynthesis in birds, and major gaps in our knowledge remain. However, a few recent studies conducted in chickens have confirmed the involvement of clock genes in the regulation of Aanat transcription via suppressing its transcription using Bmal1 antisense oligonucleotides (36) and through RNA interference (RNAi)-mediated knockdown of CLOCK and NPAS2 (37). Involvement of MAP kinases and cFos/Jun in the rhythmicity of pineal MEL biosynthesis in birds has also been noted, but the relevant results have not been consistent (38, 39).
MEL is an universal and multifunctional molecule. In vertebrates this hormone is synthesized not only in the pineal gland, but also in many peripheral tissues, and is well known to regulate many physiological processes including seasonal reproduction, locomotor activity and immunity. It was demonstrated that MEL, which exerts its biological role acting trough both G protein-coupled membrane receptors as well as cytoplasmic and nuclear receptors, may affect the function of the pituitary-adrenal cortex axis (40). Moreover, a paracrine function of C-cell-synthesized MEL within thyroid gland was reported recently (41). Additionally, it was also found that MEL is involved in thyroid function by regulating thyroglobulin gene expression (41). It was also shown that MEL may stimulate differentiation of mesenchymal stem cells into osteoblastic cell lineage as well as may inhibit proliferation and differentiation of osteoclasts (42). On the other hand the new studies demonstrate that changes of the carbon monoxide concentration may impact on the systemic MEL level (43). These numerous functions of MEL indicate that knowledge about the regulation of its biosynthesis may be crucial for understanding the numerous properties of this hormone.
In conclusion, we observed rhythmic expression of all of the genes involved in pineal MEL biosynthesis in chicks maintained under both L:D and D:D conditions and in pinealocytes cultured in comparable light conditions. Moreover, our in silico analysis suggested the existence of enhancer box sequences in the promoters of all of the examined genes and DBP/E4BP4 binding elements in the promoters of Tph1 and Asmt. Taken together, our findings suggested that the Tph1, Ddc, Aanat and Asmt genes are clock-controlled genes, which must be confirmed through further functional promoter analyses of all genes encoding enzymes participating in MEL biosynthesis.
Acknowledgements: We would like to thank Professor Krystyna Skwarlo-Sonta from our Department for inspiration and helpful discussion. We would also like to thank Ms. Magdalena Twardowska from our Department for assistance during the in vivo experiments.
This work was supported by the Polish Ministry of Science and Higher Education Grant No. N N 401 629140 and National Science Centre grant UMO-2012/07/B/NZ3/02919.
Author contributions: Dr. P.M. Majewski funded the study, designed the experimental plan, coordinated the study, evaluated the obtained results and prepared the manuscript. Dr. I. Adamska funded the study, evaluated the obtained results, performed statistical analyses, and critically revised the manuscript. K. Marhelava performed the in vitro experiments and commented on the manuscript. Dr. M. Markowska performed the Cosinor analyses and commented on the manuscript. U. Kedzierska performed the in silico analyses and commented on the manuscript. D. Walkiewicz performed the in vivo experiments and commented on the manuscript. All authors read and approved the final manuscript.
Conflict of interests: None declared.
REFERENCES
- Golden SS, Canales SR. Cyanobacterial circadian clocks - timing is everything. Nat Rev Microbil 2003; 1: 191-199.
- Schibler U. The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells. EMBO Rep 2005; 6 Spec No: S9-S13.
- Dunlap JC, Loros JJ, Liu Y, Crosthwaite SK. Eukaryotic circadian systems: cycles in common. Genes Cells 1999; 4: 1-10.
- Bellet MM, Sassone-Corsi P. Mammalian circadian clock and metabolism - the epigenetic link. J Cell Sci 2011; 123: 3837-3848.
- Ueda HR, Hayashi S, Chen W, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet 2005; 37: 187-192.
- Cassone VM. Melatonin’s role in vertebrate circadian rhythms. Chronobiol Int 1998; 15: 457-473.
- Barclay JL, Tsang AH, Oster H. Interaction of central and peripheral clocks in physiological regulation. Prog Brain Res 2012; 199: 163-181.
- Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev 2003; 55: 325-395.
- Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem 2000; 275: 32991-32998.
- Lovenberg W, Weissbach H, Udenfriend S. Aromatic L-amino acid decarboxylase. J Biol Chem 1962; 237: 89-93.
- Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem 1997; 68: 213-224.
- Voisin P, Guerlotte J, Bernard M, Collin JP, Cogne M. Molecular cloning and nucleotide sequence of a cDNA encoding hydroxyindole O-methyltransferase from chicken pineal gland. Biochem J 1992; 282: 571-576.
- Zeman M, Herichova I. Circadian melatonin production develops faster in birds than in mammals. Gen Comp Endocrinol 2011; 172: 23-30.
- Cassone VM. Avian circadian organization: a chorus of clocks. Front Neuroendocrinol 2014; 35: 76-88.
- Piesiewicz A, Kedzierska U, Podobas E, Adamska I, Zuzewicz K, Majewski PM. Season-dependent postembryonic maturation of the diurnal rhythm of melatonin biosynthesis in the chicken pineal gland. Chronobiol Int 2012; 29: 1227-1238.
- Piesiewicz A, Kedzierska U, Turkowska E, Adamska I, Majewski PM. Seasonal postembryonic maturation of the diurnal rhythm of serotonin in the chicken pineal gland. Chronobiol Int 2015; 32: 59-70.
- Matys V, Kel-Margoulis OV, Fricke E, et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 2006; 34 (Database issue): D108-D110.
- Grechez-Cassiau A, Bernard M, Ladjali K, Rodriguez IR, Voisin P. Structural analysis of the chicken hydroxyindole-O-methyltransferase gene. Eur J Biochem 1998; 258: 44-52.
- Grechez-Cassiau A, Greve P, Guerlotte J, Collin JP, Voisin P. Hydroxyindole-O-methyltransferase gene expression in the pineal gland of chicken embryo: development of messenger RNA levels and regulation by serum. Brain Res Dev Brain Res 1995; 88: 204-211.
- Florez JC, Seidenman KJ, Barrett RK, Sangoram AM, Takahashi JS. Molecular cloning of chick pineal tryptophan hydroxylase and circadian oscillation of its mRNA levels. Brain Res Mol Brain Res 1996; 42: 25-30.
- Bernard M, Guerlotte J, Greve P, et al. Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reprod Nutr Dev 1999; 39: 325-334.
- King TS, Steinlechner S. Pineal indolalkylamine synthesis and metabolism: kinetic considerations. Pineal Res Rev 1985; 3: 69-113.
- Huang Z, Liu T, Chattoraj A, et al. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J Pineal Res 2008; 45: 506-514.
- Clokie SJ, Lau P, Kim HH, Coon SL, Klein DC. MicroRNAs in the pineal gland: miR-483 regulates melatonin synthesis by targeting arylalkylamine N-acetyltransferase. J Biol Chem 2012; 287: 25312-25324.
- Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL, Cassone VM. Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol 2003; 17: 2084-2095.
- Karaganis SP, Kumar V, Beremand PD, Bailey MJ, Thomas TL, Cassone VM. Circadian genomics of the chick pineal gland in vitro. BMC Genomics 2008; 9: 206. doi: 10.1186/1471-2164-9-206
- Klein DC. Arylalkylamine N-acetyltransferase: ‘the Timezyme’. J Biol Chem 2007; 282: 4233-4237.
- Bailey MJ, Coon SL, Carter DA, et al. Night/day changes in pineal expression of >600 genes: central role of adrenergic/cAMP signaling. J Biol Chem 2009; 284: 7606-7622.
- Klein DC, Bailey MJ, Carter DA, et al. Pineal function: impact of microarray analysis. Mol Cell Endocrinol 2010; 314: 170-183.
- Bustos DM, Bailey MJ, Sugden D, et al. Global daily dynamics of the pineal transcriptome. Cell Tissue Res 2011; 344: 1-11.
- Bell-Pedersen D, Cassone VM, Earnest DJ, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 2005; 6: 544-556.
- Okano T, Yamamoto K, Okano K, et al. Chicken pineal clock genes: implication of BMAL2 as a bidirectional regulator in circadian clock oscillation. Genes Cells 2001; 6: 825-836.
- Williams WP, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne) 2012; 3: 60. doi: 10.3389/fendo.2012.00060
- Haque R, Chong NW, Ali F, et al. Melatonin synthesis in retina: cAMP-dependent transcriptional regulation of chicken arylalkylamine N-acetyltransferase by a CRE-like sequence and a TTATT repeat motif in the proximal promoter. J Neurochem 2011; 119: 6-17.
- Bernard M, Guerlotte J, Cogne M, Greve P, Collin JP, Voisin P. Transcriptional regulation of hydroxyindole O-methyltransferase in the chicken pineal gland: day/night changes and long-term effects of light and darkness. Biochem J 1993; 290: 661-664.
- Rekasi Z, Horvath RA, Klausz B, Nagy E, Toller GL. Suppression of serotonin N-acetyltransferase transcription and melatonin secretion from chicken pinealocytes transfected with Bmal1 antisense oligonucleotides containing locked nucleic acid in superfusion system. Mol Cell Endocrinol 2006; 249: 84-91.
- Haque R, Ali FG, Biscoglia R, et al. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem 2010; 113: 1296-1306.
- Hayashi Y, Sanada K, Hirota T, Shimizu F, Fukada Y. p38 mitogen-activated protein kinase regulates oscillation of chick pineal circadian clock. J Biol Chem 2003; 278: 25166-25171.
- Yadav G, Straume M, Heath J, Zatz M. Are changes in MAPK/ERK necessary or sufficient for entrainment in chick pineal cells? J Neurosci 2003; 23: 10021-10031.
- Juszczak M, Roszczyk M, Kowalczyk E, Stempniak B. The influence od melatonin receptors antagonists, luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT), on melatonin-dependent vasopressin and adrenocorticotropic hormone (ACTH) release from the rat hypothalamo-hypophysial system. in vitro and in vivo studies. J Physiol Pharmacol 2014; 65: 777-784.
- Garcia-Marin R, Fernandez-Santos JM, Morillo-Bernal J, et al. Melatonin in the thyroid gland: regulation by thyroid-stimulating hormone and role in thyroglobulin gene expression. J Physiol Pharmacol 2015; 66: 643-52.
- Michalowska M, Znorko B, Kaminski T, Oksztulska-Kolanek E, Pawlak D. New insights into tryptophan and its metabolites in the regulation of bone metabolism. J Physiol Pharmacol 2015; 66: 779-791.
- Romerowicz-Misielak M, Oren DA, Sowa-Kucma M, et al. Changes in melatonin synthesis parameters after carbon monoxide concentration increase in the cavernous sinus. J Physiol Pharmacol 2015; 66: 505-514.
A c c e p t e d : August 16, 2016