MATERNAL HIGH-FAT DIET DURING PREGNANCY AND LACTATION HAD GENDER DIFFERENCE EFFECT ON ADIPONECTIN IN RAT OFFSPRING
INTRODUCTION
One emerging issue of great concern in reproductive health and obesity is the intergenerational implications of obesity, as well as consumption of fat diet during pregnancy. There is evidence from epidemiological and animal studies suggesting that the intrauterine environment in obese women can impact on both female and male offspring. It is becoming apparent, therefore, that a woman's nutritional and metabolic status during pregnancy can programme her daughter or son, and in the case of the daughter when she reaches childbearing age, she can in turn impose an effect on the next generation (1). Previous research has shown that too much or too little nutrition during fetal life resulted in profound and permanent changes in their development - including alterations in the structure of the liver, brain and pancreas - that increase their susceptibility to developing various diseases later in life, including obesity, diabetes and cardiovascular disease. Krasnow et al. (2), using a mouse model to examine how consumption of a HF diet during pregnancy affects body composition in the newborn, showed that on the day of birth, babies born to females who had consumed HF food had more body fat, less lean mass, and smaller livers than the newborns of females that consumed low-fat food.
Adipose tissue is an important source of hormones that influence both metabolism and reproduction (3). It is well known that both obesity and excessive leanness are associated with reproductive dysfunction. Recent investigations have yielded new information on the endocrine role of white adipose tissue (WAT) via the expression of protein messengers known as adipokines (4). Although adipose tissue secretes a variety of factors, only leptin and adiponectin (and possibly resistin, adipsin, and visfatin) are primarily produced by adipocytes and can therefore be properly classified as adipokines. Wronska et al., (5) observed that metabolic effects of short-term calorie restriction with subsequent refeeding in WAT depots of young and old Wistar rats was associated with increase in serum leptin concentration. Adiponectin secretion is related to the abundance of the adipose tissue and is regulated by autocrine and endocrine factors, predominately by gonadal steroids (6, 7). The production and secretion of adiponectin is inversely correlated to the severity of obesity. Women display higher circulating adiponectin levels than men, and weight loss in both is characterized by increases in adiponectin levels in the plasma (8). Adiponectin was first described in 1995 as adipocyte complement-related protein of 30 kDa (Acrp30) (9). Adiponectin mediates its action in the periphery principally through two receptors, AdipoR1 and AdipoR2 (9). The effects of adipokines on the process of ovulation and ovarian steroidogenesis have not been extensively studied, however there are data concerning their action on female reproduction (10-11). Chabrolle et al. (13) found that AdipoR1 and AdipoR2 mRNA was present in theca and granulosa cells, and that adiponectin receptors were detected in human granulosa cells and mediated adiponectin's action of increasing production of progesterone (P4) and oestradiol (E2) via insulin-like growth factor (IGF) I. Smolinska et al., (14) found that adiponectin regulates the expression of StAR, CYP11A1 and HSD3B1 genes and secretion of P4 and andrones by the porcine endometrial and myometrial tissue explants during early pregnancy and the oestrous cycle.
Whereas most studies of obesity and infertility focus on the female partner, evidence suggests a significant effect of obesity upon male reproductive function (15). Based on the role of adiponectin in the ovary, there are data showing expression of adiponectin in the testis. Caminos et al. (16) found adiponectin mRNA expression in Leydig cells, whose levels were marginally regulated by pituitary gonadotropins. Furthermore, Ocon-Grove et al. (17) reported expression of AdipoR1 and AdipoR2 mRNA in chicken testis, adding that sexual maturation was likely associated with an up-regulation of testicular AdipoR1 and AdipoR2, and consequent influences on steroidogenesis, spermatogenesis, Sertoli cell function, and spermatozoa motility. Page et al. (6), as well as Lanfranco et al. (18), showed that increased testosterone (T) levels in humans were inversely correlated with circulating adiponectin levels.
In the presented study, we set out to answer the question of how a HF diet fed to adult female Wistar rats during pregnancy and lactation: 1) influences plasma adiponectin and steroid hormone (T and E2) levels in dams and both sexes of offspring; 2) influences adiponectin and its receptors AdipoR1 and AdipoR2 protein expression in dams and both sexes of offspring gonads.
MATERIAL AND METHODS
Reagents and antibodies
Tris, Na-deoxycholate, Nonidet NP-40, sodium dodecyl sulfate (SDS), protease inhibitors (EDTA-free), dithiothreitol (DTT), Tween 20, bromophenol blue, 1 bromo-3-chloro-propane, and Western blotting luminol reagent (cat. # sc-2048) were obtained from Sigma-Aldrich (St. Louis, MO, USA). A Bradford protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA, USA). Polyvinylidene difluoride (PVDF) membrane was purchased from Merck Millipore (Darmstadt, Germany). Antibodies against adiponectin (cat. # sc-26496), AdipoR1 (cat.# sc-46749), and AdipoR2 (cat. # sc-46751), as well as horseradish peroxidase-conjugated antibody (cat. # sc-2020), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β-actin antibody (cat. # A5316) was obtained from Sigma-Aldrich.
Animals and experimental design
The experiments and treatments were conducted in compliance with European Union regulations concerning the protection of experimental animals. The Local Animal Ethics Committee, located in Warsaw, approved the study protocol. Adult female and male Wistar Han rats (11 weeks old) were obtained from the Center of Experimental Medicine at the Medical University of Bialystok, Poland. After two weeks of acclimatisation, the females were examined with a vaginal impedance checker (Muromachi Kikai Co., Ltd . Tokyo, Japan) for the precise determination of the stage of estrus for mating time. Based upon these measurements, female rats at the appropriate point in the estrous cycle were selected for breeding. The mating day was also the day when the animals were randomly allocated to either an high fat (HF; 30% fat; 4.7 kcal/g) or standard breeding diet (BD; 5% fat; 3.1 kcal/g). Diets were purchased from Wytwornia Pasz Morawski (Kcynia, Poland) (Table 1). Porcine lard was used as the source of fat in the HF diet (19, 20). The following morning, mated females were examined for the presence of a vaginal plug, and the day the plug was observed was considered to be day 1 of gestation. On day 14 of gestation, the females were separated from the males. Only females who gave birth 21 ± 1 days after mating were chosen for the experiment. On the first day of lactation, the litters were standardized to six pups. After 21 days of lactation, adult females (n = 6 for each died), and three pups from each litter were taken to further experiments. Ovaries, testes, periovarian WAT and epididymal WAT were immediately removed, frozen in liquid nitrogen, and stored at –80°C for protein expression analysis. Additional, ovarian tissue from adult females, ovarian, and testicular samples from its offspring, were subjected to immunohistochemical analysis. Blood samplings for plasma collection were done on day 21 of lactation from both groups of dams and offspring, were withdrawn on EDTA and aprotinin (0.6 TIU/mL of blood) and immediately centrifuged at 1600 × g for 15 min at 4°C. Blood plasma was harvested, distributed into Eppendorf tubes, deep frozen (–80°C), and stored until analysis.
Determination of plasma adiponectin levels
A commercially available rat adiponectin enzyme-linked immunosorbent assay (ELISA) (cat. #E091-R, Mediagnost, Reutlingen, Germany) was used to quantify total adiponectin concentrations in plasma. The sensitivity of the adiponectin assay was < 0.081 ng/ml and the inter- and intra-experimental coefficients of variation were 15% and 10%, respectively. Samples were run in triplicate within the same assay.
Determination of plasma steroids hormone levels
Steroid hormone (i.e., T and E2) levels were determined in plasma using commercially available ELISA kits (cat. #s EIA-1559 and EIA-2693, DRG MedTek Sp. z o.o, Warsaw, Poland). The sensitivity of each assay was 0.083 ng/ml for T and 9.714 pg/ml for E2, with ranges of 0 – 16 ng/ml, and 0 – 2000 pg/ml, respectively. The inter- and intra-experiment coefficients of variation were 6.71% and 3.28%, respectively, for T; and 6.72% and 2.71%, respectively, for E2. All samples were assayed in duplicate in the same assay.
Immunohistochemistry
To optimize immunohistochemical staining, the sections were immersed in 10 mM citrate buffer (pH 6.0) and heated in a microwave oven (2 × 5 min, 700 W). Thereafter, sections were immersed sequentially in H2O2 (3% v/v) for 10 min, and normal goat serum (5% v/v) for 15 min, which were used as blocking solutions. After overnight incubation with goat polyclonal antibodies against either adiponectin (1:50), AdipoR1 (1:50) or AdipoR2 (1:100) at 4°C, biotinylated antibody (anti-goat IgG; 1:400; Vector, Burlingame CA, USA) and avidin-biotinylated horseradish peroxidase complex (ABC/HRP; 1:100; Dako, Glostrup, Denmark) were applied in succession. Bound antibody was visualized using 3,3'-diaminobenzidine (0.05% v/v; Sigma-Aldrich) as the chromogenic substrate. Nuclei were counterstained with Mayer's haematoxylin (Sigma-Aldrich). Control sections included the omission of primary antibody and substitution by the appriopriate IgG. The whole procedure has been described in detail elsewhere (21). Experiments were repeated three times. The sections were examined with a Leica DMR microscope (Wetzlar, Germany).
To evaluate the intensity of immunohistochemical reaction quantitatively, digital color images were obtained using a CCD Video Camera (KY-F55, JVC) mounted on an optical microscope (Microphot, Nikon, Japan) and connected to a video capture card (PV-BT878P, Prolink, Taiwan) installed on a personal computer. Images of the ovaries and testes were captured using a 20 × objective as described previously (22). Image processing and analyses were performed using the public domain ImageJ software (National Institute of Health, Bethesda, MD, USA). The intensity of the immunohistochemical reaction was expressed as relative optical density (ROD) of diaminobenzidine brown reaction product and calculated using the formula described by Smolen (23). A total number of 90 tissue sections (n = 5 per tissue) were subjected to image analysis. Results were expressed as mean ± S.E.M.
Western blot analysis
Tissue preparation, lysis, Western blotting and quantification were performed as a standard procedure (24). In brief, the samples were separated by 10% SDS-PAGE (BioRad Mini-Protean II Electrophoresis Cell) for adiponectin (30 kDa), AdipoR1 (49 kDa) and AdipoR2 (44 kDa). Proteins (60 µg) were transferred to PVDF membranes and incubated with antibodies diluted to 1:200 at 4°C overnight. Then, the membranes were incubated with a horseradish peroxidase-conjugated antibody diluted to 1:500. Signals were detected by chemiluminescence using the Western blotting luminol reagent and visualized using a ChemiDoc-It Imaging System (UVP, LLC. Cambridge, UK). All bands visualized by chemiluminescence were quantified using ImageJ analysis software (US National Institutes of Health, Bethesda, MD, USA). The blots were then stripped and probed for anti-β-actin (42 kDa).
Statistical analyses
A two-way analysis of variance (ANOVA) was used for multiple comparisons involving more than two treatment groups. The Tukey honest significant difference (HSD) multiple range test was performed post hoc (GraphPad PRISM v. 4.0; GraphPad Software, Inc., San Diego, CA, USA). All data are expressed as the mean ± S.E.M. Statistical significance is indicated by different letters with a > b > c (P < 0.05) or by *(P < 0.05), **(P < 0.01), and ***(P < 0.001).
RESULTS
Plasma levels of adiponectin and steroid hormones in high-fat (HF) and standard breeding (BD) diet females during pregnancy and lactation, and their offspring
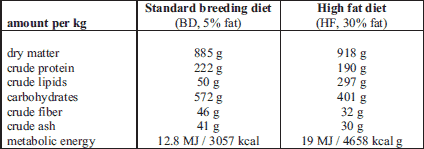
In females on the HF diet plasma adiponectin levels was 5-fold lower compared to females on the BD diet (0.397 µg/ml ± 0.09 vs. 4.749 µg/ml ± 1.8, respectively). In babies born to females who had consumed the high-fat diet, similar results to those seen in the mother were observed in female offspring (0.875 µg/ml ± 0.5 vs. 2.784 µg/ml ± 0.9, respectively, in HF offspring vs. BD offspring), while conversely, 2-fold higher plasma adiponectin levels were noted in male offspring (4.945 µg/ml ± 0.9 vs. 2.012 µg/ml ± 0.2, respectively, in HF offspring vs. BD offspring) (P < 0.05) (Fig. 1A).
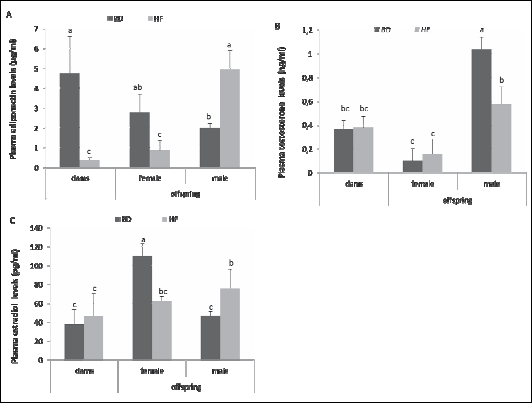 |
Fig. 1. Plasma adiponectin (A), testosterone (B) and oestradiol (C) levels in Wistar rats fed high-fat diet (HF) or standard breeding diet (BD) during pregnancy and lactation, and their female and male offspring. Different letters indicate significant differences among the diets BD and HF (P < 0.05). |
Measurement of plasma steroid hormone levels showed no difference in HF females compared to BD female with regard to T (0.379 ng/ml ± 0.09 vs. 0.364 ng/ml ± 0.06, respectively) and E2 (46.422 pg/ml ± 23.4 vs. 38.044 pg/ml ± 15.3, respectively) levels (Fig. 1B and 1C). In female offspring from HF dams, there were significantly lower plasma E2 levels compared to BD (62.513 pg/ml ± 4.7 vs. 110.262 pg/ml ± 12.5, respectively), with no change in plasma T levels. In male offspring from HF dams, plasma T levels were significantly lower (0.577 ng/ml ± 0.1 vs. 1.037 ng/ml ± 0.1, respectively), while plasma E2 levels were higher (75.469 pg/ml ± 20.1 vs. 46.773 pg/ml ± 4.6, respectively) compared to BD female offspring (Fig. 1B and 1C).
Immunolocalisation of adiponectin and its receptors AdipoR1 and AdipoR2 in the ovary of high-fat and standard breeding diet females during pregnancy and lactation
Immunohistochemical analysis of paraffin-embedded sections of ovaries revealed high levels of expression of adiponectin and adiponectin receptors AdipoR1 and AdipoR2 in the oocytes of all follicle stages and in corpora lutea, whereas in granulosa and theca cells, weak signals were detected (Fig. 2A-2F). In corpora lutea of HF females, adiponectin (Fig. 2A' and 2B'), AdipoR1 (Fig. 2C' and 2D') and AdipoR2 (Fig. 2E' and 2F') signals were reduced when compared to BD females. Increase in the intensity of the AdipoR2 signal was observed in oocytes of HF compared to BD dams (Fig. 2E and 2F). No positive signal was observed when sections were incubated with non-immune serum instead of the respective primary antibody.
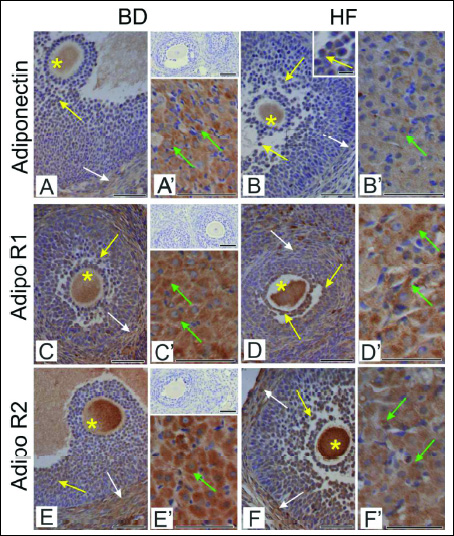 |
Fig. 2. Immunohistochemical localisation of adiponectin (A, A', B, B'), AdipoR1 (C, C', D, D') and AdipoR2 (E, E', F, F') in the ovaries of females fed with BD and HF diet during pregnancy and lactation. Adiponectin (A, B), AdipoR1 (C, D) and AdipoR2 (E, F) are localized to oocytes (asterisks), granulosa (yellow arrows) and theca cells (white arrows) of ovarian follicles. Negative controls included sections incubated with non-immune serum (upper inserts in A’, C’, E’). |
Qualitative analysis of adiponectin and adiponectin receptors AdipoR1 and AdipoR2 immunoreactivity in the ovaries of females fed with BD and HF diet during pregnancy and lactation (Fig. 2) was expressed as relative optical density of diaminobenzidine brown reaction product (Fig. 3). Statistically significant reduction of adiponectin and adiponectin receptors AdipoR1 and AdipoR2 (P < 0.001) was found in corpora lutea of HF females compared to BD dams. Adiponectin immunoexpression was decreased also in antral follicles (P < 0.01) (Fig. 3A), whereas AdipoR1 and AdipoR2 were reduced in primordial and growing follicles (P < 0.01, P < 0.001) (Fig. 3B and 3C).
 |
Fig. 3. A histogram of adiponectin (A), AdipoR1 (B) and AdipoR2 (C) staining intensity in the ovaries of females fed with BD and HF diet during pregnancy and lactation, expressed as ROD of diaminobenzidine brown reaction product. Values are means ± S.E.M. Asterisks indicate significant differences between the BD versus HF group, **P < 0.01 and ***P < 0.001 (n = 5 each group). |
Protein expression of adiponectin and its receptors AdipoR1 and AdipoR2 in the ovary and fat tissue of high-fat and standard breeding diet female diets during pregnancy and lactation
Western blot and densitometry analysis documented that protein expression of adiponectin (P < 0.001), and its receptors AdipoR1 (P < 0.05) and AdipoR2 (P < 0.001) was significantly lower in ovaries collected from HF females compared to BD females (Fig. 4). In periovarian WAT, adiponectin protein expression was significantly lower in HF dams, with no change in AdipoR1 and AdipoR2 expression (Fig. 4).
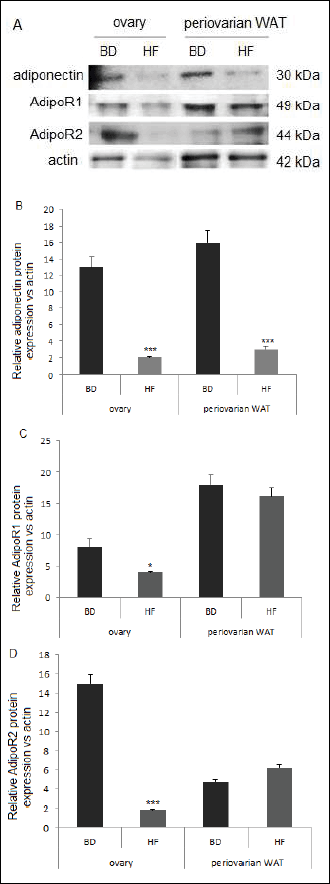 |
Fig. 4. Protein expression of adiponectin, AdipoR1 and AdipoR2 in ovary and periovarian WAT in Wistar rats fed HF and BD diets during pregnancy and lactation. Representative blots of three BD and HF dams (n = 3) are shown. Signal intensity was expressed in arbitrary units. The data are plotted as the mean ± S.E.M. Significance between HF and BD is indicated by *P < 0.05 and ***P < 0.001. |
Immunolocalization of adiponectin and its receptors AdipoR1 and AdipoR2 in the ovary and testis of offspring
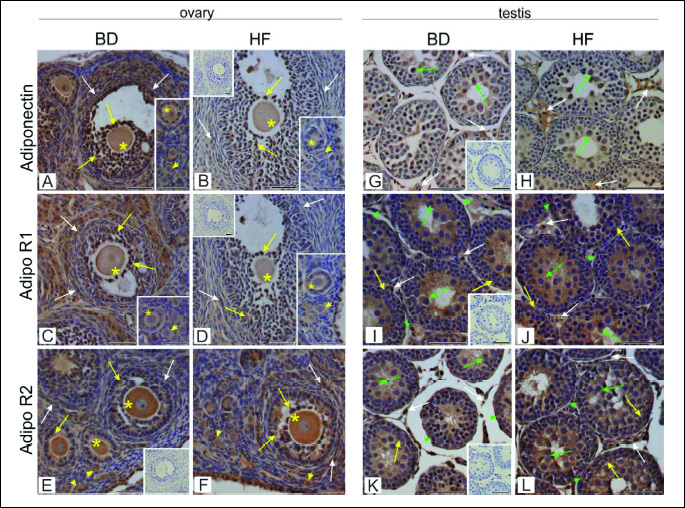
Immunohistochemistry revealed adiponectin localisation in all preantral (primordial, primary and secondary) and antral follicles in the ovaries of both HF and BD groups (Fig. 5A and 5B). The signal was most abundant in the granulosa cells of antral follicles, whereas moderate stain intensity was found in oocytes and theca cells. In granulosa and theca cells of the offspring of HF dams, signal intensity was reduced when compared to the BD dams (Fig. 5B). Distribution of AdipoR1 in the ovaries of offspring was similar to the distribution of adiponectin. In the offspring of HF dams lower signal was observed mainly in ovarian somatic cells, when compared to the BD dams (Figs. 5C and 5D). AdipoR2 was expressed in preantral and antral follicles, and displayed the strongest immunoexpression in oocytes. Weaker signal was localised to granulosa and theca cells. No apparent difference in signal localisation was observed between BD and HF offspring (Fig. 5E and 5F). Quantitative analysis revealed statistically significant decrease (P < 0.01, P < 0.001) of adiponectin and AdipoR1 immunoreactivity in primordial, primary and secondary follicles (Figs. 6A and 6B), while the signal for AdipoR2 was markedly increased in primordial follicles (P < 0.001) (Fig. 6C).
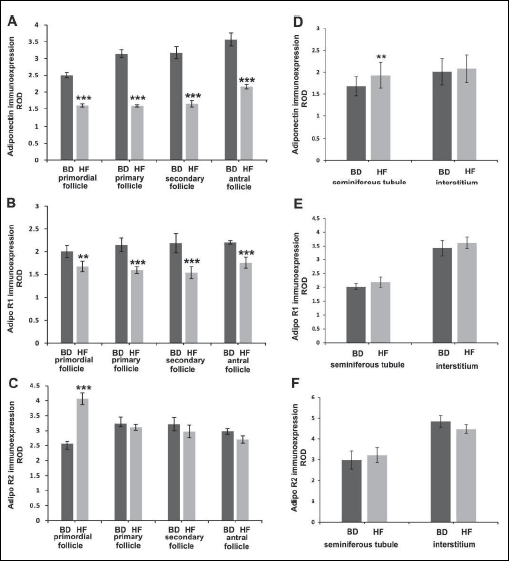 |
Fig. 6. A histogram of adiponectin (A, D), AdipoR1 (B, E) and AdipoR2 (C, F) staining intensity in the ovaries (A-C) and testes (D-F) of offspring of mothers fed with BD or HF diet during pregnancy and lactation, expressed as ROD of diaminobenzidine brown reaction product. Values are means ± S.E.M. Asterisks indicate significant differences between the BD versus HF group, **P < 0.01 (n = 5 each group). |
In the testis, immunoexpression of adiponectin, AdipoR1, and AdipoR2 was detected in both interstitial tissue and seminiferous tubules (Fig. 5). Specifically, adiponectin signal was found predominantly in the Leydig cells and spermatocytes of BD and HF male offspring (Fig. 5G and 5H). Both AdipoR1 and AdipoR2 were abundantly expressed in Leydig cells and Sertoli cells, as well as in germ cells (spermatogonia and spermatocytes). Signal intensity and localisation of the receptors were not affected by the HF diet (Fig. 5I - 5L). No positive signal was observed when sections were incubated with non-immune serum instead of the respective primary antibody.
Quantitative analysis revealed statistically significant increase (P < 0.01, P < 0.001) of adiponectin immunoreactivity in seminiferous tubules (Fig. 6D). No statistically significant differences between HF and BD groups in AdipoR1 and AdipoR2 immunoexpression neither in seminiferous tubules nor Leydig cells were found (Fig. 6E and 6F).
Protein expression of adiponectin and its receptors AdipoR1 and AdipoR2 in the ovary and testis of offspring
Adiponectin and AdipoR1 protein expression was significantly lower both in the ovary and periovarian WAT of female offspring born to HF compared to BD dams (P < 0.01; P < 0.001). There were no differences in AdipoR2 expression in ovarian tissue, while a decrease in expression was observed in the periovarian WAT of HF compared to BD offspring (P < 0.05) (Fig. 7).
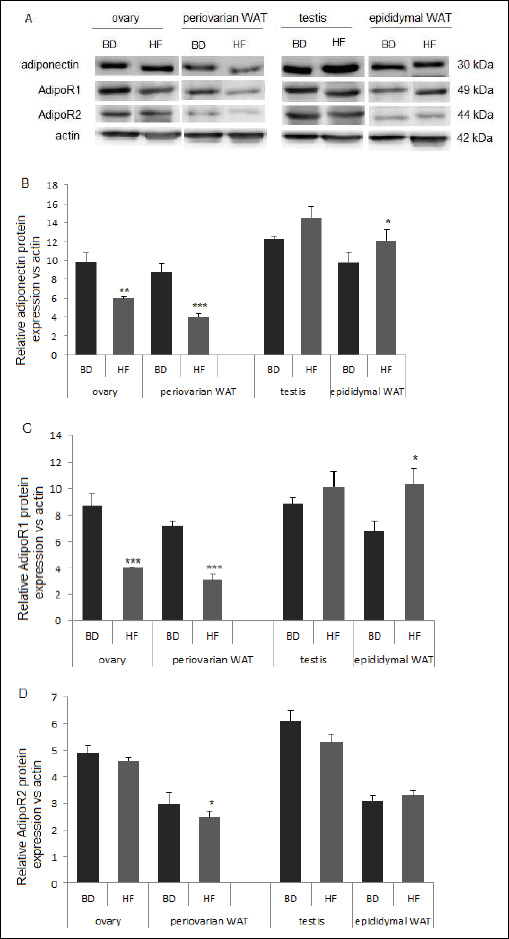 |
Fig. 7. Protein expression of adiponectin, AdipoR1 and AdipoR2 in ovary and periovarian WAT from female and testis and epididymal WAT from male offspring collected from Wistar rats fed HF or BD diets during pregnancy. Representative blots of three BD and HF female and male offspring (n = 3) are shown. Signal intensity was expressed in arbitrary units. The data are plotted as the mean ± S.E.M. Significance between HF and BD is indicated by *P < 0.05, *P < 0.01 and ***P < 0.001. |
In the testis, increased adiponection expression and no effect on AdipoR1, and AdipoR2 was noted in HF compared to BD offspring. Increased expression of adiponection and AdipoR1, but not AdipoR2, was observed in the epididymal WAT of HF compared to BD male offspring (P < 0.05) (Fig. 7).
DISCUSSION
Our study related the effect of HF diets during pregnancy and lactation in Wistar rats to plasma concentrations of adiponectin and protein expression of adiponectin and its receptors in the gonads and gonadal adipose tissue, correlated with steroids levels in dams and their female and male offspring. We reported that adiponectin protein expression, both in the ovary as well as periovarian WAT, parallel with plasma adiponectin levels in females fed with a HF diet during pregnancy and lactation was significantly lower compared to a standard BD suggesting that lower plasma adiponectin levels could be affected by secretion from both adipose tissue and reproductive tissue. Immunohistochemical studies demonstrated high expression of adiponectin and adiponectin receptors AdipoR1 and AdipoR2 in oocytes at all follicular stages and in corpora lutea, whereas there was only a weak signal in granulosa and theca cells. Our results are consistent with past studies demonstrating that the mammalian ovary and, in particular, the ovarian follicle, expresses both adiponectin and AdipoR1 and AdipoR2 (13, 25, 26), indicating local production and action of adiponectin in theca, granulosa and luteal cells of the ovary. Interestingly, lower protein expression of adiponectin and both receptors in HF dams was observed only in corpora lutea. Kurzynska et al., (27) suggest that peroxisome proliferator-activated receptors (PPARs) agonists, which regulates adiponectin secretion and action, reduced P4 secretion secretion by the corpus luteum during pregnancy. Earlier published data described that lower adiponectin levels are correlated with obesity. Lower blood levels of adiponectin in obese people compared to normal weight individuals have been mentioned by Madeira et al. (28). Furthermore, it has been shown that reduction of obesity increases adiponectin levels (29, 30).
Our research indicates that HF diet during pregnancy and lactation affects the level of adiponectin but does not affect the secretion of steroid hormones by the ovaries. Independently of diet, no differences between T and E2 plasma levels were observed. Previous studies of the E2-adiponectin relationship reported an inverse relationship (31, 32, 33), or no association (34). Data published by Laughlin et al. (7) assessing the sex-specific association of adiponectin with multiple factors thought to influence its levels. Bezpalko et al., (35) suggested that maternal stress evoked in pregnant female rats fed with high fat diet contribute significantly to regulation of liver defense and inflammation. Changes in fat tissue in offsprings increase in the leptin/adiponectin index and increased production of pro-inflammatory mediators lead to exacerbation of liver damage. Tworoger et al. (36) examined the correlations between adiponectin and sex hormones in women and showed that the inverse correlations of E2, oestrone, and oestrone sulphate with adiponectin were substantially attenuated after adjustment for body mass index, suggesting that the relationship between oestrogens and adiponectin is mediated primarily by body fat. The changes we observed in adiponectin blood levels at 21 days of lactation were not correlated with the body mass of dams, however an increase in the weight of offspring (P < 0.01) without differences between male and female (unpublished data). A lack of correlation between body weight of HF dams and body weight of offspring with other metabolic hormones (ghrelin and obestatin) has been showed by Slupecka et al. (19). This is in agreement with other data showing no effect of HF diet on dam body weight during gestation and lactation (37, 38, 39).
Interestingly, gender differences in plasma adiponectin and steroid levels and expression of adiponectin and its receptors, were evident. Female offspring opposite to male offspring born to HF dams had lower plasma adiponectin levels, reduced intensity of adiponectin and AdipoR1 receptor in the ovary, and decreased E2 in parallel with increased T plasma levels. The ratio of E2 to T toward T secretion is characteristic of atretic follicles. Similarly, gender differences in adiponectin mRNA expression in gonadal white adipose tissue have been observed by Amengual-Cladera et al. (40) expression increased significantly in epididymal WAT in male rats, while it decreased in periovarian WAT in female rats of 10 weeks of age fed with HF diet for 26 weeks. Immunohistochemical study showed reduced signal intensity for adiponectin and AdipoR1, but not for AdipoR2 in preantral and antral follicles in the offspring of HF mothers, and observed changed morphology of granulosa cells on typical for atretic follicles confirmed that of protein expression. This is in line with very recent report by Tsoulis et al. (20) who showed increase in the number of atretic follicles in HF prepubertal offspring. Moreover, according to their results in this offspring primordial, primary, secondary, and antral follicle numbers were similar to that of control. It seems likely therefore that reduced level of adiponectin and AdipoR1 in HF offspring ovaries resulted rather from decreased expression of both proteins in particular follicle stages than from loss of the follicles. Of note, significant increase in AdipoR2 immunoexpression in HF primordial follicles may reflect their altered functioning. We assume that such disturbance may in turn result in the increase in primordial follicle recruitment during the prepubertal period as recently suggested by the above-mentioned authors (20).
It has been further suggested that locally produced adiponectin may act as a key neuromodulator of reproductive functions. It is well known that adiponectin has a direct role in ovarian cells in many species. For example, in rat granulosa cells, adiponectin had no effect on basal or FSH-induced P4 and E2 secretion, but stimulated in response to IGF-I both P4 and E2 secretion (41). Similarly, in the chicken ovary, adiponectin significantly increased P4 secretion in response to IGF-I, but blocked LH- and FSH-induced P4 secretion (26). In cultured bovine theca and granulosa cells, adiponectin decreased P4 and androstenedione secretion induced by LH and insulin (42). Also, in porcine granulosa cells, adiponectin decreased P4 secretion induced by IGF-I and insulin (43). Low adiponectin levels have also been reported in women with elevated T levels associated with the polycystic ovarian syndrome (PCOS) (44). Overweight and obese women with low adiponectin levels have early puberty and are prone to develop PCOS, gestational diabetes mellitus, and preeclampsia (45). Tsoulis et al. (20) showed that female rat offspring born to mothers fed HF diet (with lard as a source of fat) throughout pregnancy and lactation enter puberty early and display aberrant reproductive cyclicity with substantial changes in ovarian development.
The opposite situation was observed in male offspring. Higher plasma adiponectin levels and adiponectin protein expression was demonstrated in HF male rats. We also observed that the plasma T level was reduced in parallel with an increased plasma E2 level. Immunohistochemistry documented that signal intensity and localisation of the adiponectin and its receptors in testes were not significantly affected by the HF diet apart from increased signal for adiponectin in the seminiferous tubules (P < 0.01). Also in fat tissue surrounding the testis increased adiponectin and AdipoR1 was observed (P < 0.05). One possible explanation is that oestrogens increase adiponectin production; however, evidence supporting this hypothesis is limited and contradictory (46). There are data suggesting an adiponectin effect on the pituitary hormone levels by suppressing basal and GnRH-stimulated LH secretion (47), or acting directly at the level of the testis. Caminos et al. (16) showed significant inhibition of basal and hCG-stimulated testosterone secretion by adiponectin, suggesting that adiponectin could be considered an integral negative modifier of reproductive function in conditions of low adiposity.
The results of our study concur with this hypothesis. Increased plasma oestradiol levels in male offspring corresponded with increased adiponectin blood levels and expression in fat tissue. Higher adiponectin concentrations were shown in hypogonadal men compared to eugonadal men, despite the higher body fat associated with hypogonadism (18). The sex difference in adiponectin could also be explained by an inhibitory influence of testosterone. We have shown there to be lower adiponectin blood levels, reduced intensity of adiponectin and AdipoR1 receptors in the ovary, and increased T plasma levels in female offspring. Nishizawa et al. (48) showed that treatment of both sham-operated and castrated male mice with testosterone was accompanied by a reduction in serum adiponectin, and adiponectin secretion in cultured adipocytes. Bottner et al. (49) observed significantly reduced adiponectin levels in adolescent boys compared with girls, inversely related to testosterone and dehydroepiandrosterone sulphate serum concentrations, and this may account for the gender differences seen in adults. Lastly, data from Yarrow et al. (50) examining the effects of gonadectomy and testosterone administration on adiponectin in young and adult rodents, had proved that changes in total adiponectin were highly correlated with circulating androgen concentrations, but not with E2 concentrations.
It is worth mentioning that in males, apart from its role in the regulation of steroidogenesis (supported by the presence of adiponectin and both receptors in Leydig cells), adiponectin might be involved in the control of germ cell differentiation. Such a hypothesis is based on the widespread immunoexpression of AdipoR1 and AdipoR2 throughout the adluminal and luminal compartments of the seminiferous tubule, as demonstrated previously (16, 17) and confirmed herein. Moreover, in the present study, we detected adiponectin protein in spermatocytes, which indicates that it is likely to act locally in the seminiferous epithelium as a paracrine or autocrine factor, potentially influencing spermatogenesis. Finally, the unaltered expression pattern of adiponectin and its receptors in the seminiferous tubules of HF males suggests that in contrast to disruptive endocrine action of adiponectin, the local regulatory interactions were not affected by the mother's diet.
In summary, HF diet fed to dams during pregnancy and lactation decreases the adiponectin secretion and its protein expression in the female whereas increases in male offspring. As a consequence, there was a disruption of steroid secretion in offspring, towards testosterone in females, and oestradiol in males. In conclusion, we hypothesis that the HF diet of mothers during pregnancy and lactation is not only the cause of obesity in the offspring, but also later reproductive problems. Further studies concerning the influence of the other adipokines related to obesity are needed.
Acknowledgements: We are grateful to Ms. Karolina Szatanik and Ms. Karolina Pniok for their technical assistance with the immunohistochemistry. This work was supported by the K/ZDS/005404 and K/ZDS/005402, from the Jagiellonian Univeristy in Cracow, Poland, and by the National Science Centre Grant no. 2011/03/D/NZ9/03697.
Conflict of interests: None declared.
REFERENCES
- Ryan D. Obesity in women: a life cycle of medical risk. Int J Obes 2007; 31: 3-7.
- Krasnow SM, Nguyen ML, Marks DL. Increased maternal fat consumption during pregnancy alters body composition in neonatal mice. Am J Physiol Endocrinol Metab 2011; 301: 1243-1253.
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010; 316: 129-139.
- Fietta P, Delsante G. Focus on adipokines. Theor Biol Forum 2013; 106: 103-129.
- Wronska A, Sledzinski T, Goyke E, Lawniczak A, Wierzbicki P, Kmiec Z. Short-term calorie restriction and refeeding differently affect lipogenic enzymes in major white adipose tissue depots of young and old rats. J Physiol Pharmacol 2014; 65: 117-126.
- Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. J Androl 2005; 26: 85-92.
- Laughlin GA, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes (Lond) 2007; 31: 457-465.
- Campos DB, Palin MF, Bordignon V, Murphy BD. The 'beneficial' adipokines in reproduction and fertility. Int J Obes (London) 2008; 32: 223-231.
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995; 270: 26746-26749.
- Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288-1295.
- Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction 2005; 130: 583-597.
- Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 2006; 147: 5178-5186.
- Chabrolle C, Tosca L, Rame C, Lecomte P, Royere D, Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril 2009; 92: 1988-1996.
- Smolinska N, Dobrzyn K, Kiezun M, Szeszko K, Maleszka A, Kaminski T. Effect of adiponectin on the steroidogenic acute regulatory protein, P450 side chain cleavage enzyme and 3b-hydroxysteroid dehydrogenase gene expression, progesterone and androstenedione production by the porcine uterus during early pregnancy. J Physiol Pharmacol 2016; 67: 443-456.
- Martin LJ. Implications of adiponectin in linking metabolism to testicular function. Endocrine 2014; 46: 16-28.
- Caminos JE, Nogueiras R, Gaytaan F, et al. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology 2008; 149: 3390-3402.
- Ocon-Grove OM, Krzysik-Walker SM, Maddineni SR, Hendricks GL, Ramachandran R. Adiponectin and its receptors are expressed in the chicken testis: influence of sexual maturation on testicular ADIPOR1 and ADIPOR2 mRNA abundance. Reproduction 2008; 136: 627-638.
- Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol 2004; 60: 500-507.
- Slupecka M, Romanowicz K, Wolinski J. Maternal high-fat diet during pregnancy and lactation influences obestatin and ghrelin concentrations in milk and plasma of Wistar rat dams and their offspring. Int J Endocrinol 2016; 2016: 5739763. doi: 10.1155/2016/5739763.
- Tsoulis MW, Chang PE, Moore CJ, et al. Maternal high-fat diet-induced loss of fetal oocytes is associated with compromised follicle growth in adult rat offspring. Biol Reprod 2016; 94: 1-11.
- Hejmej A, Kopera I, Kotula-Balak M, Lydka M, Lenartowicz M, Bilinska B. Are expression and localization of tight and adherens junction proteins in testes of adult boar affected by foetal and neonatal exposure to flutamide? Int J Androl 2012; 35: 340-352.
- Kotula-Balak M, Hejmej A, Kopera I, Lydka M, Bilinska B. Prenatal and neonatal exposure to flutamide affects function of Leydig cells in adult boar. Domest Anim Endocrinol 2012; 42: 142-154.
- Smolen AJ. Image analytic techniques for quantification of immunocytochemical staining in the nervous system. In: Methods in Neurosciences, PM Conn (ed.), San Diego, CA, Academic Press, 1993; pp. 208-229.
- Rak-Mardyla A, Duda M, Gregoraszczuk EL. A role for resistin in the ovary during the estrous cycle. Horm Metab Res 2014; 47: 493-498.
- Lord E, Ledoux S, Murphy BD, Beaudry D, Palin MF. Expression of adiponectin and its receptors in swine. J Anim Sci 2005; 83: 565-578.
- Chabrolle C, Tosca L, Crochet S, Tesseraud S, Dupont J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: potential role in ovarian steroidogenesis. Domest Anim Endocrinol 2007; 33: 480-487.
- Kurzynska A, Bogacki M, Chojnowska K, Bogacka I. Peroxisome proliferator activated receptor ligands affect progesterone and 17β-estradiol secretion by porcine corpus luteum during early pregnancy. J Physiol Pharmacol 2014; 65: 709-717.
- Madeira IR, Carvalho CN, Gazolla FM, Pinto LW, Borges MA, Bordallo MA. Impact of obesity on metabolic syndrome components and adipokines in prepubertal children. J Pediatr (Rio J) 2009; 85: 261-268.
- Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003; 289: 1799-1804.
- Coughlin CC, Finck BN, Eagon JC, et al. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity (Silver Spring) 2007; 15: 640-645.
- Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 2003; 88: 4823-4831.
- Sumino H, Takahashi T, Itoh T, et al. Plasma adiponectin levels in post-menopausal women receiving hormone replacement therapy. J Int Med Res 2004; 32: 639-645.
- Im JA, Lee JW, Lee HR, Lee DC. Plasma adiponectin levels in postmenopausal women with or without long-term hormone therapy. Maturitas 2006; 54: 65-71.
- Sieminska L, Wojciechowska C, Niedziolka D, et al. Effect of postmenopause and hormone replacement therapy on serum adiponectin levels. Metabolism 2005; 54: 1610-1614.
- Bezpalko L, Gavrilyuk O, Zayachkivska O. Inflammatory response in visceral fat tissue and liver is prenatally programmed: experimental research. J Physiol Pharmacol 2015; 66: 57-64.
- Tworoger SS, Mantzoros C, Hankinson SE. Relationship of plasma adiponectin with sex hormone and insulin-like growth factor levels. Obesity 2007; 15: 2217-2224.
- Tallman DL, Taylor CG. Effects of dietary fat and zinc on adiposity, serum leptin and adipose fatty acid composition in C57BL/6J mice. J Nutr Biochem 2003; 14: 17-23.
- Mendes-da-Silva C, Giriko CA, Mennitti LV, Hosoume LF, Souto Tdos S, Silva AV. Maternal high-fat diet during pregnancy or lactation changes the somatic and neurological development of the offspring. Arq Neuropsiquiatr 2014; 72: 136-144.
- Cerf M, Herrera E. High fat diet administration during specific periods of pregnancy alters maternal fatty acid profiles in the near-term rat. Nutrients 2016; 8: E25. doi: 10.3390/nu8010025.
- Amengual-Cladera E, Llado I, Proenza AM, Gianotti M. High-fat diet feeding induces a depot-dependent response on the pro-inflammatory state and mitochondrial function of gonadal white adipose tissue. Br J Nutr 2013; 109: 413-424.
- Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007; 133: 719-731.
- Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function Mol Cell Endocrinol 2008; 284: 38-45.
- Maleszka A, Smolinska N, Nitkiewicz A, et al. Expression of adiponectin receptors 1 and 2 in the ovary and concentration of plasma adiponectin during the oestrous cycle of the pig. Acta Vet Hung. 2014; 62: 386-396.
- Orio F Jr, Palomba S, Cascella T, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88: 2619-2623.
- Palin MF, Bordignon VV, Murphy BD. Adiponectin and the control of female reproductive functions. Vitam Horm 2012; 90: 239-287.
- Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 2003; 52: 268-276.
- Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, et al. Regulation of pituitary cell function by adiponectin. Endocrinology 2007; 148: 401-410.
- Nishizawa H, Shimomura I, Kishida K, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002; 51: 2734-2741.
- Bottner A, Kratzsch J, Muller G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 2004; 89: 4053-4061.
- Yarrow JF, Beggs LA, Conover CF, McCoy SC, Beck DT, Borst SE. Influence of androgens on circulating adiponectin in male and female rodents. PLoS One 2012; 7: e47315. doi: 10.1371/journal.pone.0047315.
A c c e p t e d : August 27, 2016