THE EFFECT OF THE ANGIOTENSIN II RECEPTOR, TYPE 1 RECEPTOR ANTAGONISTS, LOSARTAN AND TELMISARTAN, ON THIOACETAMIDE-INDUCED LIVER FIBROSIS IN RATS
INTRODUCTION
Angiotensin II (Ang II) is the main effector molecule of the renin-angiotensin-aldosterone (RAA) system. While Ang II, angiotensin-converting enzyme (ACE), and angiotensin II receptor type 1, AT1 receptor, are expressed in healthy liver tissue under normal physiological conditions, Ang II receptor density on rat liver increases significantly during experimentally-induced fibrosis, implying a potential role for AT1 receptor in liver fibrosis (1, 2).
Bataller et al. demonstrated that the increased expression of AT1 receptors during liver fibrosis was due to activation of hepatic stellate cells (HSCs), which are thought to be the main source of extracellular matrix (ECM) synthesis in fibrotic liver (3). Moreover, the authors found that stimulation of Ang II receptors resulted in increased production of reactive oxygen species (ROS) and enhanced secretion of cytokines that accelerate liver fibrosis and inflammation (3, 4). The above observations are supported by in vitro findings reported by Ramalho et al. (5) and data obtained from in vivo studies carried out by Yoshiji et al. (6). A putative function for AT1 receptor in liver fibrosis is clinically significant, as RAA blocking drugs such as angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), are increasingly being utilized not only for the treatment of patients with hypertension, heart failure, or nephropathy, but also with fibrotic lesions (7, 8).
In this regard, the anti-fibrotic effects of ACEI and ARB have been shown in both animal models and also in a clinical study. In this regard, patients with hepatitis C and hypertension had more severe symptoms of fibrosis compared to hepatitis C patients without hypertension. Moreover, hypertensive patients treated with ARB showed less advanced fibrosis versus patients who did not receive ARB therapy (9). Interestingly, in high-risk patients with fibrosis, the therapeutic affects of ARB treatment exceeded the effects of ACEI as an anti-fibrotic agent (10). In addition, the administration of ARB considerably improved the condition of fibrotic livers in patients with non-alcoholic steatohepatitis (NASH) and reduced the risk of cirrhosis in patients with HCV following liver transplantation (9, 11). Also beneficial activity in patients with NASH reveal administration of melatonin and tryptophan (12).
Knowledge of the function of the RAA system has increased considerably in recent years (13). In addition to the serum RAA system, tissue homologues have been identified in the liver, myocardium, blood vessels, kidneys, and adrenal glands. Following the identification of an RAA system outside of serum, an 'alternative' pathway of the RAA system was characterized. This 'alternative' RAA system is composed of a structural analogue of ACE (ACE2), angiotensin (1-7), and Mas receptor (MasR). Notably, the activity of the 'alternate' RAA system is converse to that mediated by the so-called classical RAA pathway (14-16). Interestingly, the activity and expression of RAA components have been reported to be gender-related. In this regard, an important role for MasR in pressure natriuresis and diuresis was observed in men when AT1 receptor and AT2 receptor were blocked (17). In addition, in an experimental model of liver fibrosis induced by bile duct ligation, Ang (1-7) was demonstrated to decrease the concentrations of type I collagen and hydroxyproline, and to inhibit secretion of α-actin in smooth muscle cells. On the other hand, MasR blockade intensified the extent of liver fibrosis (18). The above findings suggest that the Ang (1-7)-ACE2-MasR axis can be an important pathway by which to block liver fibrosis.
Among the RAA system blockers possessing anti-fibrotic activity, sartans, angiotensin II receptor antagonists, are of particular interest. These drugs effectively block the RAA system and have no adverse effects, such as cough or vasomotor edema rhinitis, which are typical of ACEI. Due to the conflicting opinions regarding the effectiveness of angiotensin convertase inhibitors and AT1 receptor antagonists as drugs that can be used to alleviate the process of fibrosis, we decided to undertake the current investigation.
The aim of this study was to evaluate the anti-fibrotic effects of the AT1 receptor antagonists, losartan and telmisartan, in an animal model of liver fibrosis induced by thioacetamide (TAA) (19-21). The liver lesions in this model are comparable to the fibrogenic changes seen in human liver disease (22, 23). The anti-fibrotic effects of losartan and telmisartan were evaluated on the basis of their impact upon the activity of the liver enzymes ALT, AST, and AP, and production of pro-inflammatory cytokines such as TNF-α, TGF-β1, PDGF-AB, IL-6, and IL-1β. In addition, inflammatory lesions and extent of liver fibrosis were assessed histopathologically. Markers of oxidative stress were also examined including the concentration of reduced glutathione (GSH) versus oxidized glutathione (GSSG), and the activity of paraoxonase1 (PON1), an enzyme with anti-oxidant activity.
MATERIAL AND METHODS
Animals and treatments
This study was carried out with male Wistar rats that each weighed 200 – 220 g. The rats were kept under constant environmental conditions (22°C, 12-hour day/night cycle), received a standard diet (LSM granulated chow, Agropol, Motycz n/Lublin), and had free access to water. All reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
The animals were randomly assigned to six groups: a control group (n = 6) and 5 treatment groups (n = 8 in each group). Experimental groups in this study included: 1) rats given water ad libitum for 12 weeks (control group); 2) rats treated with 300 mg/L TAA ad libitum for 12 weeks (TAA group); 3) rats treated daily with 30 mg/kg losartan intraperitoneally (i.p.) for 4 weeks (L group); 4) rats treated daily with 10 mg/kg telmisartan i.p. for 4 weeks (T group); 5) rats treated with both TAA and losartan (TAA + L group); and 6) rats treated with both TAA and telmisartan (TAA + T group).
Losartan was dissolved in 0.9% saline. Telmisartan was dissolved in two drops of Tween and diluted in 0.9% saline. TAA was dissolved in water. At the end of the experiment, the animals were euthanized and decapitated. Blood was sampled for enzymatic evaluation and liver sections were collected for histhopathology and enzymatic tests.
Biochemistry
Serum alanine (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (AP) activity were determined by the routine kinetic method in an ADVIA 1800 analyzer (Siemens). ALT and AST were measured by means of NADH and Tris buffer (according to IFCC), whereas AP was measured using p-nitrophenyl phosphate and AMP buffer. Activity of ALT, AST, and AP are expressed as U/l.
Cytokine ELISA
The concentrations of cytokines in serum were determined using commercially available enzyme immunoassay ELISA kits and were carried out according to the manufacturer's instructions. The following ELISA kits were purchased from Bender MedSystems GmbH (eBioscience, San Diego, CA): Rat TNF-α Immunoassay Kit, Rat IL-6 Immunoassay Kit, Rat TGF-β1 Immunoassay Kit, Rats IL-1b Immunoassay Kit. The Rat PDGF-AB Immunoassay Kit was purchased from R&D Systems Europe Ltd. (Abingdon, UK). Briefly, serum samples were added to a 96-well microtiter plate coated with monoclonal antibodies against TNF-α, IL-6, TGF-β1, IL-1β, or PDGF-AB. Subsequently, any unbound cytokine was washed away and anti-cytokine antibodies were added to the wells. The wells were washed to remove unbound antibody followed by the addition of antibodies conjugated to a specific enzyme. The wells were washed to remove any unbound conjugated antibody and enzyme substrate added to wells. Absorbance was read at 450 nm using the Reader Victor (Perkin Elmer, USA). Work Out 2 software was used to plot the calibration curve and to calculate the cytokine concentrations in samples. Concentrations are expressed in pg/mL.
Determination of glutathione (GSH) and oxidized glutathione (GSSG) concentrations in liver homogenates
The concentrations of GSH and GSSG were measured in homogenized liver tissue using the colorimetric assay, according to the manufacturer's protocol (BIOXYTEC Research, USA). The results were expressed in nmol/g tissue.
Measurement of paraoxonase 1 (PON1) activity in blood serum
Paraoxonase 1 activity in serum was measured using two synthetic substrates: diethyl-p-nitrophenyl phosphate (paraoxon) or phenyl acetate. The activity towards paraoxon was determined by measuring the initial rate of substrate hydrolysis to p-nitrophenol. A total of 20 µl of serum was added to 800 µl of the reaction buffer containing 50 mM Tris-HCL (pH 8.0), 2 mM paraoxon, and 2 mM CaCl2. An increase in absorbance at 412 nm was monitored for 2 minutes. The blank sample containing incubation mixture without serum was run simultaneously to correct for substrate degradation. Enzymatic activity was calculated from E412 of p-nitrophenol (18.290 M/cm–1) and was expressed in U/mL; 1 U of enzyme hydrolyzes 1 nmol of paraoxon per minute (24).
Paraoxonase 1 activity towards phenyl acetate was determined by measuring the initial rate of substrate hydrolysis in assay mixture (3 ml) containing 2 mM substrate, 2 mM CaCl2, and 10 µl serum in 50 mM Tris-HCl buffer (pH 8.0). Absorbance was monitored for 1 minute at 270 nm. The increase in absorbance of a blank sample without serum was subtracted and the activity was calculated by means of E270 = 1310 M/cm–1. The results are expressed in U/ml; 1 U of enzyme hydrolyzes 1 µmol of phenyl acetate per minute (16). Absorbance was measured using a UV-Vis Shimadzu 1610 spectrophotometer.
Measurement of paraoxonase 1 (PON1) activity in liver homogenates
Slices of the livers were homogenized in 10 volumes of 50 mM Tris-HCL buffer (pH 8.0) containing 2 mM CaCl2 and the homogenates were centrifuged at 10,000 × g for 15 minutes. Then, supernatants were incubated for 15 minutes in the presence of 10 µM paraoxon to inhibit B-type esterases that could interfere with the PON1 assay (16). PON1 activity toward phenyl acetate in supernatants was assayed by the same method as described above for measurement of PON1 activity in the serum. The volume of supernatant used for the assay and the time of absorbance measurement were chosen within the linear range of the relationships between these variables and the measured activity (liver 10 µl, 2 minutes). We also measured PON1 activity toward paraoxon in the liver. Ten µl of liver homogenate were mixed with 300 µl of reaction buffer containing 50 mM Tris-HCl (pH 8.0), 2 mM paraoxon, and 2 mM CaCl2. The increase in absorbance at 405 nm was monitored for 5 minutes in a microplate reader (Elx800; BioTek Instruments). Enzyme activity in the tissues was expressed in U/mg of protein. concentration was assessed by means of the Lowry method (25).
Histology
Liver sections for histopathology were collected from the right lobe and were placed in 10% buffered formalin (pH 7.4). The samples were embedded in paraffin blocks. Sections were cut on the microtome and stained with haematoxylin and eosin (H + E) and Masson's Trichrome. The slides were evaluated under an Olympus BX45 light microscope by an experienced pathologist. Sections were evaluated for the presence and severity of inflammation. Furthermore, the presence and nature of necrosis ('piecemeal' or 'bridging'), the extent of proliferation of Browicz-Kupffer cells, the presence of degenerative lesions including vacuolar degeneration, the presence of 'balloon' cells, micro- and macro-vesicular steatosis, and bile stasis in hepatocytes were also evaluated. To assess the intensity of inflammation and fibrosis the 5-point Scheuer's scale was applied (26).
Statistical analysis
The differences between the control group and groups receiving TAA, losartan, and/or telmisartan were compared. The values of parameters were presented using a mean, standard deviation, median, and range of significance. The Shapiro-Wilk test was applied first to check the distribution of results. Since the normal distribution was not found, the results were compared using the Mann-Whitney test assessing the significance of differences of medians between 2 groups, which was formulated based upon the hypothesis that Me1 = Me2, as compared to the alternative hypothesis that medians are different.
The 5% error risk was accepted. In other words, the hypothesis is rejected when P > 0.005 and the alternative hypothesis is true (denoted '-'). When P < 0.005, the intergroup differences are significant. When the conclusion was accepted at P < 0.05 the differences were considered as significant (*), at P < 0.01 as more significant (**), and at P < 0.001 as highly significant (***). Calculations were performed by means of Statistica 8.0 Software. The cumulative statistical analysis was presented in Tables 1-5.
The correlation between variables was evaluated using Pearson's coefficient. P < 0.05 was considered statistically significant. Calculations were performed by means of Statistica 9.1 Software.
RESULTS
Treatment with losartan or telmisartan reduces liver enzyme activity
To determine the effect that inhibition of AT1 receptor would have on development of liver fibrosis, we first measured the activity of the liver enzymes AST, ALT, and AP in serum from control rats, rats treated with losartan only (L group) or telmisartan only (T group), and rats induced to develop liver fibrosis (TAA group) in the presence of losartan (TAA + L) or in the presence of telmisartan (TAA + T group) (Table 1). We observed statistically significant increases in median serum ALT (93.50 U/l), AST (404.00 U/l), and AP (475.50 U/l) activity in the TAA group compared to the control group (ALT (61.50 U/l), AST (175.00 U/l), AP (208.50 U/l) (Table 1).
Experimental groups: Control, TAA (thioacetamide-treated rats), Losartan-treated rats, Telmisartan-treated rats, TAA+L (thioacetamide-treated rats followed losartan), TAA+T (thioacetamide-treated rats followed telmisartan).
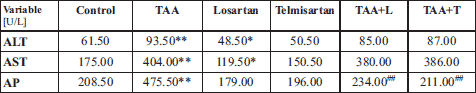
Notably, a decrease in enzymatic activity was observed in animals receiving only losartan or telmisartan compared to controls. For example, ALT and AST activity in rats that were administered losartan were 48.50 U/l and 119.50 U/l, respectively, as compared to the control group which showed ALT activity of 61.50 U/l and AST activity of 175.00 U/l. Treatment with telmisartan alone showed AST, AST, or AP activity that was comparable to the control group.
In rats induced to develop liver fibrosis (TAA group), treatment with either losartan (TAA + L) or with telmisartan (TAA + T) resulted in diminished activity of ALT, AST, and AP as compared to rats in the TAA group. Moreover, a statistically significant difference in AP activity was found between the groups treated with losartan and telmisartan. In this regard, AP activity was two-fold lower (234.00 U/l) in the TAA + L group as compared with the TAA group. A similar difference was observed in the TAA + T group which showed AP activity of 211.00 U/l.
A comparison of the control group versus TAA + L group and the TAA + T group revealed a statistically significant increase in both ALT and AST activities but not in AP activity. Thus, the median activity measured was 85.00 U/l for ALT and 380.00 U/l for AST in the TAA + L group, and 87.00 U/l for ALT and 386.00 U/l for AST in the TAA + T group versus 61.50 U/l for ALT and 175.00 U/l for AST in control rats.
Taken together, our results show that treatment of rats with losartan or telmisartan decreases function of liver enzyme and suggest that inhibition of AT1 receptor is a viable treatment for the amelioration of the symptoms of liver fibrosis.
Reduced production of inflammatory cytokines in response to losartan or telmisartan treatment
We next examined what effect AT1 receptor inhibition would have on the production of the pro-inflammatory cytokines TNF-α, IL-6, TGF-β1, IL-1β, and PDGF-AB (Table 2). In this regard, while the concentrations of serum TNF-α, IL-6, PDGF-AB, and TGF-β1 in rats from the TAA group were increased as compared to rats in the control group, the concentration of IL-1β was comparable between the TAA group and the control group (Table 2). The median cytokine concentrations in the TAA group were: 67.79 pg/mL (TNF-α), 60.36 pg/mL (IL-6), 521.35 pg/mL (PDGF-AB), and 67.66 ng/mL (TGF-β1), as compared to the values in the control group: 20.87 pg/mL (TNF-α), 22.73 pg/mL (IL-6), 232.75 pg/mL (PDGF-AB), 51.20 ng/mL (TGF-β1).
Experimental groups: Control, TAA (thioacetamide-treated rats), Losartan-treated rats, Telmisartan-treated rats, TAA+L (thioacetamide-treated rats followed losartan), TAA + T (thioacetamide-treated rats followed telmisartan).
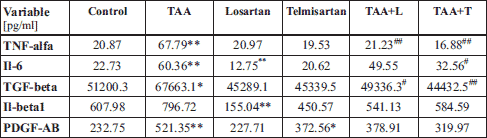
Treatment with losartan resulted in a significant decrease in the concentrations of IL-6 and IL-1β in relation to the control group. In this regard, the median concentrations of IL-6 and IL-1β in the L group were 12.75 pg/mL and 155.04 pg/mL, respectively, while the median concentrations in control rats were 22.73 pg/mL (IL-6) and 607.98 pg/mL (IL-1β). Of note, losartan treatment had no effect on concentrations of TNF-α, PDGF-AB or TGF-β1.
The administration of telmisartan caused a significant difference in the level of PDGF-AB. Thus, the median concentration of PDGF-AB in the T group was 372.56 pg/mL, which was significantly greater than the concentration of PDGF-AB measured in rats in the control group (232.75 pg/mL).
A significant decrease in the median concentrations of TNF-α and TGF-β1 was observed in the TAA + L group compared to the TAA group. The median concentrations of TNF-α and TGF-β1 in the TAA + L group were 21.23 pg/mL and 49.33 ng/mL, respectively, as compared to the median concentration of TNF-α and TGF-β1 in the TAA group, which were 67.79 pg/mL and 67.66 ng/mL, respectively. There were no significant differences observed for the concentrations of PDGF-AB, IL-6, or IL-1β.
Similarly, a significant decrease in the concentrations of TNF-α, TGF-β1, and IL-6 was found in rats with liver fibrosis treated with telmisartan (TAA + T) as compared to the TAA only group. The median concentrations of TNF-α (16.88 pg/mL), TGF-β1 (44.43 ng/mL), and IL-6 (32.56 pg/mL) were lower compared to the TAA group, which had values of 67.79 pg/mL (TNF-α, 67.66 ng/mL (TGF-β1), and 60.36 pg/mL (IL-6).
Together, these results indicate that treatment of mice with liver fibrosis with the AT1 receptor antagonists, losartan or telmisartan, results in attenuation of inflammatory cytokine production.
Reduced parameters of oxidative stress following losartan and telmisartan treatment
Treatment of rats with losartan or telmisartan resulted in decreased liver enzyme activity and attenuation of pro-inflammatory cytokine production. Consequently, we hypothesized that losartan and telmisartan treatment would also reduce oxidative stress in rats with liver fibrosis. To this end, we measured concentrations of reduced glutathione (GSH) and oxidized glutathione (GSSG) in liver homogenates from control rats and rats treated with losartan and telmisartan (Table 3).
Experimental groups: Control, TAA (thioacetamide-treated rats), Losartan-treated rats, Telmisartan-treated rats, TAA+L (thioacetamide-treated rats followed losartan), TAA + T (thioacetamide-treated rats followed telmisartan).
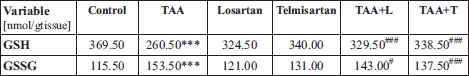
We first observed that the median GSH concentration in rat liver homogenates from the TAA group (260.50 nmol/g tissue) was significantly lower than the concentration of GSH in the control group (369.50 nmol/g tissue, P < 0.001). Moreover, statistically significant differences in GSH concentration were also measured in animals from the TAA + L (329.50 nmol/g tissue) and TAA + T (338.50 nmol/g tissue) groups as compared to the TAA group.
In contrast, the median GSSG concentration in liver homogenates in animals receiving TAA (153.50 nmol/g tissue) was greater than in liver homogenates from rats in the control group (115.50 nmol/g tissue, P < 0.001). In addition, as compared to rats in the TAA group, statistically significant increases in GSSG concentrations were observed in animals in the TAA + L group (143.00 nmol/g tissue, P < 0.05) and in the TAA + T (137.50 nmol/g tissue, P < 0.01) as compared to control animals.
Serum paraoxonase 1 (PON1) activity is diminished in the presence of angiotensin II receptor, type 1 (AT1) receptor antagonists
We also examined PON1 activity in the serum as a measure of oxidative stress in rats with liver fibrosis (Table 4). We observed a significant decrease in PON1 activity towards phenyl acetate (104.06 U/ml) as well as towards paraoxon (149.70 U/ml) in the TAA group in as compared to rats in the control group (171.02 U/ml, P < 0.01 and 240.46 U/ml, P < 0.05, respectively).
Experimental groups: Control, TAA (thioacetamide-treated rats), losartan -treated rats, telmisartan-treated rats, TAA+L (thioacetamide-treated rats followed losartan), TAA + T (thioacetamide-treated rats followed telmisartan).
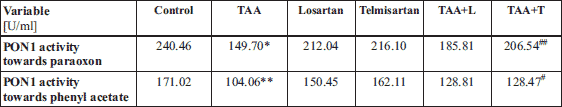
In contrast, PON1 activity towards phenyl acetate and paraoxon were decreased in rats from the TAA + L groups and the TAA + T group versus rats from the TAA group. In this regard, PON1 enzymatic activity towards phenyl acetate (128.47 U/ml, P < 0.05) and paraoxon (104.06 U/ml, P < 0.01) in the TAA + T group was reduced in liver homogenates as compared to rats in the TAA group which showed PON1 activity towards phenyl acetate at 206.54 U/ml and towards paraoxon as 149.70 U/ml.
Paraoxonase 1 (PON1) activity in liver homogenates is decreased during liver fibrosis
In addition to measuring PON1 activity in the serum, we also evaluated PON1 activity in liver homogenates from control rats and rats treated with TAA in the presence or absence of losartan or telisartan (Table 5). We observed decreased PON1 activity towards phenyl acetate (2274.44 U/mg) in the TAA group as compared to the control group (3196.08 U/mg, P < 0.01). In addition, a significant decrease in PON1 enzyme activity towards paraoxon (1038.33 U/mg) was measured in TAA-treated rats compared to the control group (1313.80 U/mg, P < 0.01).
Experimental groups: Control, TAA (thioacetamide-treated rats), Losartan-treated rats, Telmisartan-treated rats, TAA+L (thioacetamide-treated rats followed losartan ), TAA+T (thioacetamide-treated rats followed telmisartan).

Importantly, we did not observe any significant differences in the PON1 activity towards phenyl acetate or paraoxon in liver homogenates of rats with TAA-induced liver fibrosis given losartan (TAA + L) or telmisartan (TAA + T), as compared to the TAA group (Table 5). Taken together, these data show that in the serum, PON1 activity is diminished in response to AT1 receptor antagonism but is unchanged in the fibrotic liver.
Positive correlation between serum paraoxonase 1 (PON1) activity and parameters of liver damage and inflammation
As shown in Fig. 1, serum PON1 activity towards paraoxon showed a significant positive correlation with assessed parameters such as ALT (r = 0.338, P = 0.055), AST (r = 0.325 P = 0.065), and AP (r = 0.456 P < 0.008) activity, as well as production of TNF-α (r = 0.585, P < 0.01), TGF-β1 (r = 0.405 P < 0.019), and IL-lβ (r = 0.538 P = 0.001). In addition, we found a positive correlation between serum PON1 activity towards phenyl acetate with activity of ALT (r = 0.534, P = 0.001), AST (r = 0.640, P < .001), and AP (r = 0.532, P = 0.001) activity, and with secretion of TNF-α (r = 0.508, P = 0.002), TGF-β1 (r = 0.399, P = 0.019), and IL-6 (r = 0.659. P < 0.001) also was observed (Fig. 2A-2F).
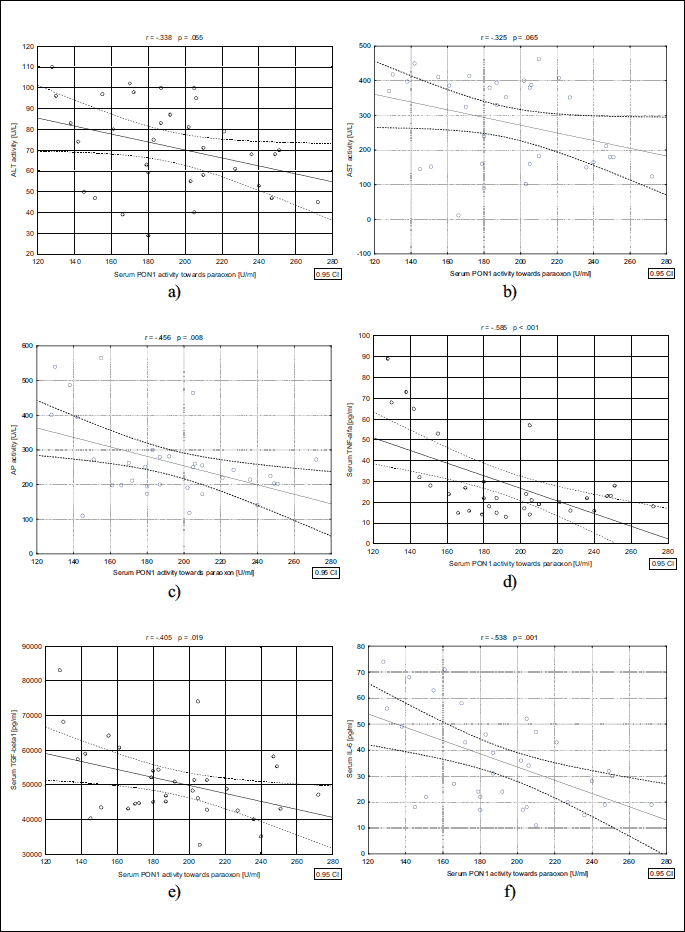
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase; TNF-alfa, tumour necrosis factor alpha; TGF-beta1, transforming growth factor beta1; IL-6, interleukin 6.
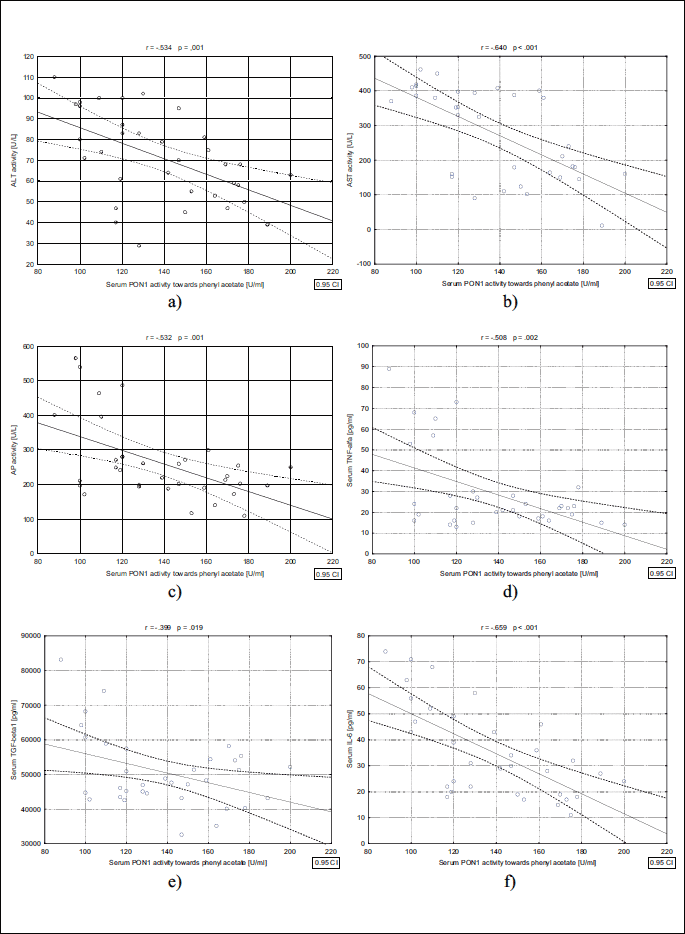
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase; TNF-alfa, tumour necrosis factor alpha; TGF-beta1, transforming growth factor beta1, IL-6, interleukin 6.
Improvement in liver histopathology following losartan or telmisartan treatment
In addition to measuring activity of liver enzymes, production of inflammatory cytokines, and level of oxidative stress, we also determined the effect of AT1 receptor antagonism on liver microanatomy. As expected, the trabecular arrangement of hepatocytes in the lobules and their microscopic appearance were preserved, and there were no inflammatory cells or signs of fibrosis noted in livers from control rats. Moreover, in control livers, the Browicz-Kupffer cell count in the hepatic sinuses was not increased and the central veins of the lobules showed a slight passive congestion (Fig. 3A and 3B). Notably, pathology of livers from animals receiving either losartan or telmisartan was not markedly different from those from the control group (Fig. 4A and 4B). Consistent with this, Masson's trichrome staining did not reveal fibrosis in the portal tract.
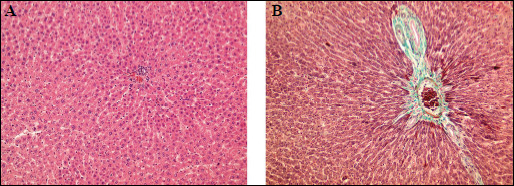 |
Fig. 3. Light microscopy. Control group. (A): The normal architecture of liver lobule.H + E × 400. (B): Portal tract with minimal amount of fibrous tissue. Masson Trichrome × 400. |
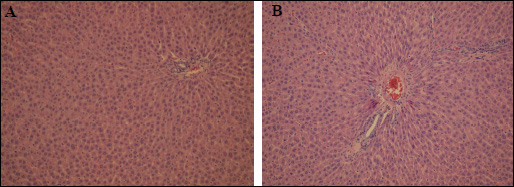 |
Fig. 4. Light microscopy. (A): Losartan group. Liver morphology within normal limits. H + E × 400. (B): Telmisartan group. Portal tract with mild passive hyperaemia. H + E × 400. |
In striking contrast to control livers, livers from the TAA-treated rats showed substantial inflammation, degeneration and necrosis of hepatocytes, and fibrosis. In this regard, livers from rats in the TAA group were assessed as having stage 3/4 inflammation (Fig. 5) and fibrosis (Fig. 6). Specifically, necrotic changes including 'piecemeal' necrosis involving hepatocytes in the region of the border plate of the portal tracts and 'bridging' necrosis involving hepatocytes inside the lobules were both observed in livers from the TAA group (Fig. 7A and 7B). Degenerative 'vacuolar' changes were also seen in the cytoplasm of hepatocytes. TAA livers also contained groups of 'ballon' cells with marked swelling of the cytoplasm and steatosis. Features of hepatocyte regeneration (in the form of enlarged nuclei and binuclear hepatocytes) were noted in the foci of intensified inflammation in the TAA-treated rat livers.
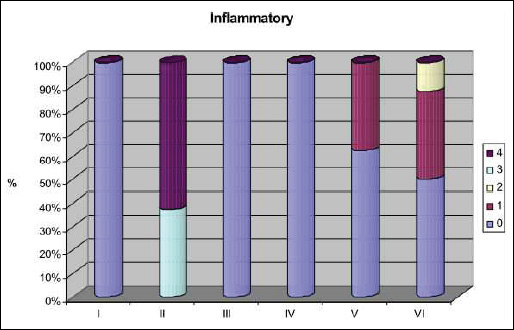 |
Fig. 5. Activity of inflammation according to Scheuer scale (0 - no portal inflammation; 1 - mild portal inflammation; 2 - mild piecemeal necrosis and inflammation; 3 - moderate necrosis and inflammation; 4 - bridging necrosis and inflammation. I - control group; II - TAA group; III - losartan group; IV - telmisatran group; V - TAA + losartan; VI - TAA + telmisartan. |
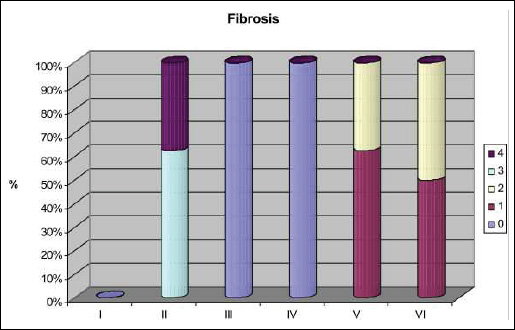 |
Fig. 6. Fibrosis staged according to Scheuer scale: 0 - no fibrosis; 1 - no portal/periportal fibrosis; 2 - septal fibrosis; 3 - bridging fibrosis with architectural distortion; 4 - cirrhosis. I - control group; II - TAA group; III - losartan group; IV - telmisartan group; V - TAA + losartan group; VI - TAA + telmisartan group. |
The intensity of microanatomic changes was reduced in TAA + L and in the TAA + T livers compared to livers from rats in the TAA group. Specifically, the structure of the hepatic lobules was preserved in the majority of animals in the TAA + L and TAA + T rats and there were mild inflammatory infiltrates of mononuclear cells in the portal tracts. The presence of 'piecemeal' necrosis accompanied by inflammatory infiltration was observed in border plate hepatocytes (Fig. 8A). In TAA-treated animals given losartan or telmisartan, liver fibrosis was found to be located mainly within and around the portal tracts. Moreover, 'bridging' necrosis was observed as stage 1 in 62.5% (5/8) and as stage 2 in 37.5% (3/8) of the animals in the TAA + L group, and as stage 1 in 50% (4/8) and as stage 2 in 50% (4/8) of those in the TAA + T group (Fig. 8B).
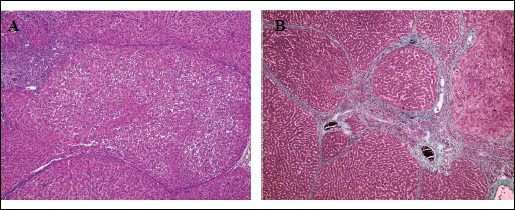 |
Fig. 7. Light microscopy. TAA group. (A): Nodular appearance of the liver lobule. Bridging necrosis with accompanied mononuclear inflammatory cells. Sign of vacuolar degeneration of hepatocytes with ballon cells visible, focal steatosis. Focal proliferation of biliary ductules within the periportal tracts. H + E × 200. (B): Cirrhosis. Intense fibrosis dividing the liver lobule into the nodules. Massone Trichrome × 200. |
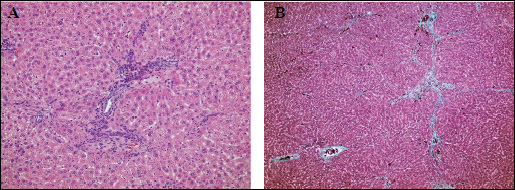 |
Fig. 8. Light microscopy. TAA + Losartan group. (A): Chronic inflammatory infiltrate within the periportal tract entering focally the liver lobule. Vacuolar degeneration of single hepatocytes. H + E × 400. TAA + Telmisartan group. (B): Mild fibrosis within the periportal tract entering focally the liver lobule. Massone Trichrome × 200. |
DISCUSSION
Recently, many novel diagnostic tools, such as elastometry, and therapies are being utilized for the diagnosis and treatment of liver diseases. These tools allow for reversion of symptoms and improvement in liver function and anatomy even in cases of advanced fibrosis or cirrhosis. An important new therapeutic option involves RAA system blockade using angiotensin convertase inhibitors (ACEI) or AT1 receptor antagonists (ARB). The primary aim of the present study was to evaluate the anti-fibrotic effects of losartan and telmisartan in the livers of rats with TAA-induced fibrosis. To this end, we examined biochemical parameters (AST, ALT, and AP enzymatic activity), production of pro-inflammatory cytokines (TNF-α, TGF-β1, PDGF-AB, IL-6, IL-1β), markers of oxidative stress (GSH and GSSG) and PON1 activity, and pathology of the microanatomy of the liver.
The enzymatic activity of aminotransferases and alkaline phosphatase are commonly evaluated as markers of liver damage. In our present study, the enzymatic activities of AST and AP were statistically higher in rats with TAA-induced liver fibrosis compared to control animals. In addition, we observed inflammation, as well as degeneration and necrosis of hepatocytes ('piecemeal' and 'bridging') in the liver tissue of TAA-treated animals.
The administration of losartan or telmisartan after TAA treatment improved the biochemical parameters although the statistical significance in the TAA + L and TAA + T groups was only found for alkaline phosphatase activity compared to the TAA group. Histological examination of liver sections revealed markedly smaller microscopic changes in both TAA + L and TAA + T rats as compared to rats treated with TAA only. In this regard, inflammatory infiltrations were of minor intensity and fibrosis, mainly found in the portal tracts, and were assessed as stage 1 and 2 in both TAA + L and TAA-T groups (Fig. 6).
The above results acquired significance when taken in combination with the measurement of pro-inflammatory cytokines in serum, which were correlated with the extent of inflammation and subsequent liver fibrosis. Thus, fibrosis is preceded by inflammation that is initiated by the injury of the hepatocytes. Local inflammatory infiltrates are formed by lymphocytes, plasma cells, and blood platelets and the activation of these cells results in the release of considerable amounts of cytokines and growth factors (TNF-α, TGF-β1, PDGF-AB, IL-6, IL-1β), which activate stellate cells and modulate the the balance in the extracellular matrix (ECM).
TNF-α is a pro-inflammatory cytokine that stimulates fibrogenesis and induces apoptosis during fibrosis. Elevated serum levels of TNF-α were measured in patients with advanced inflammatory lesions in the liver and cirrhosis (27). During chronic hepatitis, the prolonged stimulation of hepatic stellate cells results in the release of pro-fibrogenic factors such as TGF-β1 and leads to the development of liver cirrhosis (28). PDGF-AB belongs to the group of cytokines that profoundly enhances proliferation and chemotaxis of hepatic stellate cells. In the cirrhotic liver, the expression of PDGF genes and their receptors increases dynamically (29). IL-6 is considered to exert both pro- and anti-inflammatory effects. Initially, IL-6 was suggested to inhibit oxidative stress and prevent mitochondrial dysfunction (30), however, further studies demonstrated that a long-term increase in IL-6 expression injured hepatocytes and induced apoptosis (31). Like IL-6, IL-1β facilitates the development of inflammation acting as a chemoattractant for neutrophils and monocytes, and stimulating the production of IL-6. In our study, losartan and telmisartan acted as anti-inflammatory agents decreasing serum levels of TNF-α and TGF-β1 (TAA + L group) and of IL-6 (TAA + T group), as compared to the TAA group.
The mechanism of anti-inflammatory action of losartan or telmisartan was manifested as decreased synthesis of pro-inflammatory cytokines, chemokines, and adhesive molecules. The beneficial effects of sartans can be explained not only via antagonism of the AT1 receptor, but also by compensatory stimulation of angiotensin receptors (AT2, AT3, AT4, and AT1-7) mediated by circulating angiotensin II, a mechanism that occurs during chronic use of sartans. Notably, the role of angiotensin receptors other than AT1 receptors in the inflammatory fibrotic process is poorly known. The observed anti-inflammatory action of telmisartan may be related to its ability to activate PPARγ or other cellular signaling pathways including NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) or MAP kinase (mitogen-activated protein kinase), which are directly involved in the synthesis of pro-inflammatory cytokines.
Sartans are a group of drugs of increasing popularity as they are well tolerated and are promote hypotension effectively. As a group, however, sartans are not uniform and individual drugs differ in their chemical structure and pharmacological effects. While the main component of a majority of sartans is an imidazole ring, as in the case of losartan, the structure of some sartans, such as telmisartan, is similar to pioglitazone (thiazolidinediones). The capacity of telmisartan to activate nuclear PPARγ is a unique feature of this drug (32, 33). The ability of telmisartan to activate PPARγ is mainly associated with increased insulin sensitivity and reduced content of free fatty acids in the muscles and liver, improved lipid profiles (increased concentrations of HDL), and also inhibition of pro-inflammatory cytokine production (34). The activating effects of telmisartan on PPARγ are particularly important with respect to its action on hepatic stellate cells (HSCs). In this regard, it has been demonstrated that non-activated HSCs express PPARγ whereas telmisartan induces PPARγ expression in activated HSCs. It can be hypothesized that telmisartan modulates the function of HSCs and returns HSCs to their inactive state by activating PPARγ and blocking the pro-fibrogenic action of Ang II (34, 35).
In the present study, we also analyzed the impact of sartans on PON1 activity in the serum and in the liver. Enhanced peroxidation of lipids in chronic liver diseases (inflammation, fibrosis, cirrhosis) contributes to a distinct decrease in PON1 activity. Since the PON1 is sensitive to oxidative inactivation, oxidative stress accompanying the development of liver fibrosis is responsible for the decrease in its activity, which translates to increased PON1 requirements to balance the extent of damage in the liver (36). PON1 activity in the serum was observed to be two-fold lower in patients and a decrease in PON1 activity was proportional to the extent of organ damage (37, 38). Furthermore, according to Feingold et al., mediators of inflammation such as TNF-α and IL-1β, caused a reduction in PON1 mRNA in the liver and decreased the enzyme activity in serum (39).
The findings of our study confirmed a significant decrease in serum PON1 activity towards phenyl acetate and paraoxon in the TAA group as compared to the control group. Moreover, beneficial effects of telmisartan on serum PON1 activity in rats with TAA-induced fibrosis were observed. In contrast losartan had no effects on serum PON1 activity in these animals. Since telmisartan is a known PPARγ agonist, a noticeable increase in enzyme activity after administration of the drug most likely resulted from increased expression of the PPARγ. This hypothesis is confirmed by the data from the literature demonstrating that the PON1 expression depends upon PPARγ activation (40).
The positive effect of sartan therapy on fibrosis is also supported by clinical trials which involved patients with chronic hepatitis C. In a pilot study performed by Sokolan et al., patients with liver fibrosis that were resistant to standard treatment (interferon, ribaviryn) underwent 6-month therapy with losartan at a dose of 50 mg/kg/bw (41). After the therapy, reduced fibrosis was found in half of the patients compared to the untreated group based upon histhopathological examination of liver biopsies. Similarly, Colmenero et al. reported that prolongation of losartan use for 18 months in patients infected with HCV reduced the degree of fibrosis in 50% of patients (42). Additionally, in a study by Leung et al., administration of losartan substantially reduced the secretion of TGF-β1, most likely via a mechanism related to prevention of binding of Ang II with its receptors in Browicz-Kupffer cells (43).
The biochemical and histological analysis of cytokine concentrations in the present study was supplemented by the evaluation of the expression of oxidative stress markers GSH and GSSG. Oxidative stress, defined as the state of enhanced production of reactive oxygen species (ROS) that are insufficiently removed due to impaired anti-oxidative mechanisms, leads to dysfunction of cells (44). In addition to reacting with basic molecules of the body such as proteins, lipids, and DNA, ROS are also involved in cell transmission (ex. to growth factors or cytokines). The role of ROS in liver fibrosis is well documented. It has been reported that in liver fibrosis induced by bile duct ligation, the density of angiotensin II receptors is significantly increased, which leads to increased production of ROS and secretion of cytokines intensifying fibrosis and inflammation in the liver (45). In a study by Karimian et al., the developing symptoms of oxidative stress in liver tissue was manifested as elevated levels of malondialdehyde and GSSH as well as reduced levels of GSH (45).
In our study, biochemical evaluation of liver homogenates revealed significantly decreased GSH concentrations and increased GSSG concentrations in the TAA-treated group as compared to the control group, which might result from both GSH involvement in ROS scavenging and from impaired regeneration of GSH by glutathione reductase. In the groups receiving TAA and losartan or telmisartan, the concentration of GSH was significantly elevated, whereas the concentration of GSSG was reduced, compared to the TAA group. Our results support the notion of a favorable effect of the losartan or telmisartan on antioxidant status in the liver.
In conclusion, this study revealed that treatment with losartan or telmisartan resulted in improvements in biochemical, inflammatory, and anti-oxidant parameters as well as reduced intensity and incidence of alterations in liver morphology in rats with TAA-induced fibrosis. The results of the present study indicate that sartans can play an important anti-inflammatory, anti-fibrotic, and anti-oxidant functions in the treatment of liver diseases associated with fibrosis. The novelty of the study is the evaluation of the positive influence of telmisartan on PON1 activity.
Conflict of interests: None declared.
REFERENCES
- Paizis G, Cooper MF, Schembri JM, Tikelis C, Burrel LM, Angus PW. Up regulation of components of the renin angiotensin system in the bile duct ligated rat liver. Gastroenterology 2009; 54: 1790-1796.
- Pereira RM, dos Santos RA, da Costa Dias FL, Teixeira MM, Simoes e Silva AC. Renin angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol 2009; 15: 2579-2586.
- Bataller R, Sancho Bru P, Gines P, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 2003; 125: 117-125.
- Bataller R, Gabele E, Shoonhoven R, et al. Prolonged infusion of angiotensin II into normal rats induced stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol 2003; 285: 642-651.
- Ramalho LN, Ramalho FS, Zucoloto S, et al. Effect of losartan, an angiotensin II antagonist on secondary billiary cirrhosis. Hepatogastroenterology 2002; 49: 1499-1502.
- Yoshiji H, Yoshiji J, Ikenaka Y, et al. Inhibition of renin angiotensin system attenuates liver enzyme-altered preneoplastic lesions and fibrosis development in rats. J Hepatol 2002; 37: 22-30.
- El-Ashmawy NE, El-Bahrawy HA, Shamloula MM, Ibrahim AO. Antifibrotic effect of AT-1 blocker and statin in rats with hepatic fibrosis. Clin Exp Pharmacol Physiol 2015; 14: 1440-1681.
- Boryczka G, Hartleb M, Rudzki K, Janik MA. Influence of upright body position on the size of intrapulmonary blood shunts in patients with advanced liver cirrhosis. J Physiol Pharmacol 2015; 66: 855-861.
- Yokohama S, Yoneda M, Okamoto S, Okada M, Aso K. Therapeutic efficacy of on angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004; 40: 1222-1225.
- Al Khalaf MM, Thalib L, Doi SA. Cardiovascular outcomes in high-risk patients without heart failure treated with ARB: a systematic review and meta-analysis. Am J Cardiovasc Drugs 2009; 9: 29-43.
- Rimola A, Londono MC, Guevara G. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation 2004; 78: 686-689.
- Brzozowski T. Role of renin-angiotensin system and metabolites of angiotensin in the mechanism of gastric mucosal protection. Curr Opin Pharmacol 2014; 19: 90-98.
- Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease-14 months follow up. J Physiol Pharmacol 2014; 65: 75-82.
- Skipworth JR, Szabadkai G, Olde Daming SW, Leung PS, Humphries SE. Montgomery HE. Pancreatic-renin-angiotensin systems in health and disease. Aliment Pharmacol Ther 2011; 34: 840-852.
- Lubel JS, Herath CB, Burrel LM, Angus PW. Liver disease and the renin angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol 2008; 23: 1327-1338.
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin angiotensin systems. Physiol Rev 2006; 86: 747-806.
- Mansoori A, Oryan S, Nematbakhsh M. Role of Mas receptor antagonist (A779) on pressure diuresis and natriuresis and renal blood flow in the absence of angiotensin II receptors type 1 and 2 in female and male rats. J Physiol Pharmacol 2014; 65: 633-639.
- Herath CB, Warner FJ, Lubel JS, et al. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE 2) and angiotensin (1-7) levels in experimental biliary fibrosis. J Hepatol 2007; 47: 387-395.
- de Cavanagh EM, Flores I, Ferder M, Inserra F, Ferder L. Renin-angiotensin system inhibitors protect against age-related changes in rat liver mitochondrial DNA content and gene expression. Exp Gerontol 2008; 43: 919-928.
- Halici Z, Bilen H, Albayrak F, et al. Does telmisartan prevent hepatic fibrosis in rats with alloxan-induced diabetes? Eur J Pharmacol 2009; 614: 146-152.
- Czechowska G, Celinski K, Korolczuk A, et al. Protective effects of melatonin against thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol 2015; 66: 567-579.
- Muller D, Sommer M, Kretzschmar M. Lipid peroxidation on thioacetamide-induced macronodular cirrhosis. Arch Toxicol 1991; 65: 199-203.
- Perez MJ, Suarez A, Gomez-Capilla JA, Sanchez-Medina F, Gil A. Dietary nucleotide supplementation reduces thioacetamide-induced liver fibrosis in rats. J Nutr 2002; 132: 652-657.
- Ayub A, Mackness MI, Arol S, Mackness B, Patel J, Durington PN. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol 1999; 19: 330-335.
- Lowry OH, Rosenbrough NJ, Farr AJ, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991; 13: 372-374.
- Panasiuk A, Pogorzelska J, Prokopowicz D. Growth factors in chronic hepatitis C [in Polish]. Przegl Epidemiol 2007; 61: 559-566.
- Verecchia F, Mauviel A. Transforming factor-beta and fibrosis. World J Gastroenterol 2007; 13: 356-362.
- Constantin S, Capone F, Maio P, et al. Cancer biomarker profiling in patients with chronic hepatitis C virus, liver cirrhosis and hepatocellular carcinoma. Oncol Rep 2013; 29: 2163-2168.
- El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol 2004; 1: 205-211.
- Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short and long-term interleukin-6 exposure on liver injury and repair. Hepatology 2006; 43: 474-484.
- Filipiak K, Splawinski J, Opolski G. Sartany - czy wszystkie takie same? Terapia i Leki 2006; 1: 5-16.
- Kurtz TW, Pravenec M. Antidiabetic mechanisms of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists: beyond the renin-angiotensin-system. J Hypertens 2004; 22: 2253-2261.
- Benson SC, Pershadsingh HA, Ho CI. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR-gamma modulating activity. Hypertension 2004; 43: 993-1002.
- Rosen ED, Spiegelman BM. PPAR-gamma: a nuclear regulator of metabolism differentiation and cell growth. J Biol Chem 2001; 276: 37731-37734.
- Ferre N, Camps J, Prats E, et al. Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damage. Clin Chem 2002; 48: 261-268.
- Camps J, Marsillach J, Joven J. Measurement of serum paraoxonase-1 activity in the evaluation of liver function. World J Gastroenterol 2009; 15: 1929-1933.
- Siddiqua J, Ishag M, Alam JM, Hussain SMW. Paraoxonase activity in patients with chronic renal failure and hepatic insufficiency. Pak J Biochem Mol Biol 2010; 43: 54-57.
- Feingold KR, Memon RA, Moser AH, Grunfeld C. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 1998; 139: 307-315.
- Attia YM, Elalkamy EF, Hammon OA, Mahmoud SS, EL-Khatib AS. Telmisartan, an AT1 receptor blocker and PPAR-gamma activator, alleviates liver fibrosis induced experimentally by Schistosoma mansoni infection. Parasit Vectors 2013; 6: 199. doi: 10.1186/1756-3305-6-199.
- Sokolan S, Fernandez MA, Castanao G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol 2005; 11: 7760-7763.
- Colmanero J, Bataller R, Sancho-Bru P, et al. Effects of losartan on hepatic expression of nanophagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol 2009; 297: G726-G734.
- Leung PS, Suen PM, Ip SP, Yip CK, Chen G, Lai PB. Expression and localization of AT1 receptors in hepatic Kupffer cells: its potential role in regulating a fibrogenic response. Regul Pept 2003; 116: 61-69.
- Galaly SR, Ahmed OM, Mahmoud AM. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J Physiol Pharmacol 2014; 65: 823-832.
- Tiebosh M, Karimian G, Moshago H. Oxidative stress and hepatocellular injury. In: Studies on Hepatic Disorders, E Albano, M Parola (eds.). Springer International Publishing 2015, pp.99-112.
A c c e p t e d : August 18, 2016