CRATAEGUS SPECIAL EXTRACT WS 1442:
UP-TO-DATE REVIEW OF EXPERIMENTAL AND CLINICAL EXPERIENCES
INTRODUCTION
Hawthorn (Crataegus spp.) extracts have long lasting and well established position as cardiotonic and cardioprotective remedies used in traditional medicine since ancient times. First descriptions of Hawthorn beneficial action on the heart come from first century A.D. and since then products evaluated from its berries, leaves and flowers gained growing popularity among herbalists and medics around the world (1, 2). Nowadays Hawthorn preparations are widely available in various forms (from tinctures to extracts) as prescription drugs or over-the-counter medications. Extracts are produced from the plant material using predominating hydroalcoholic solvent equivalent in strength to a minimum of 45% ethanol. The most studied and popular among them are WS 1442 and LI 132, both derived from leaves and flowers of the plant.
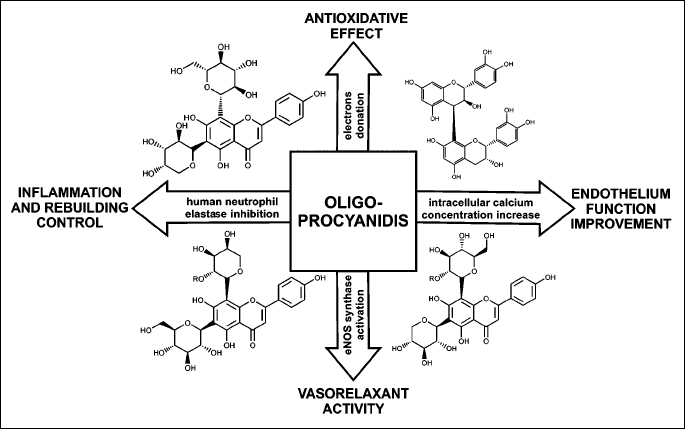
Crataegus special extract WS 1442 is standardized aqueous alcoholic (45% ethanol) Hawthorn extract containing 18.75% oligomeric procyanidins (OPC) which are thought to be responsible for its beneficial actions (Fig. 1). While many other extracts are insufficiently characterized, the pharmacological properties of WS 1442 have been repeatedly investigated in in vitro experiments, animal studies, and in clinical trials. Moreover, outcomes of mentioned observations resulted in specific therapeutic recommendations including use of Hawthorn in some countries (3).
Data for this review were obtained through PubMed searches, and private manuscript collection. Conflict of interests with the manufacturer of WS-1442 was not declared.
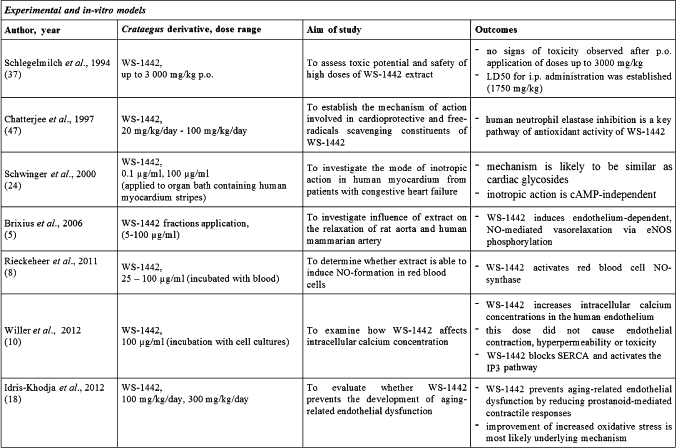
Crataegus special extract WS 1442 pharmacological activity and recent experiences in experimental models (Table 1)
OPC contained in Crataegus special extract WS 1442 act as free radicals scavengers. They are products of the ‘plant aromatic pathway’, which consists of three main sections: the shikimate segment that produces the aromatic amino acids phenylalanine, tyrosine and tryptophan, the phenylpropanoid segment that produces the cinnamic acid derivatives that are precursors of flavonoids and lignans, and the flavonoid route that produces the diverse flavonoid compounds.
Their anticipated interaction with biological systems originates primarily from their characteristically ability to form complexes, both with metal ions and with macromolecules such as proteins and polysaccharides, and from their anti-oxidative scavenging properties. All those interactions at the basis of their physiological and pharmacological interactions, are in principle directly derived from the physical and chemical properties of the polyphenolic skeleton.
The main, and best known effect of OPCs administration is free radical scavenging. They participate in the process of scavenging as donors of electrons of hydroxyl groups to form stable forms of radicals, instead of free radicals. They transfer an unpaired electron by reacting with other antioxidants or by binding metals. The antioxidative effect depends on local environment and the concentration and composition of the antioxidant extract.
They are also proven to inhibit human neutrophil elastase which is released from accumulated and activated leukocytes after blood flow restoration in previously ischemic myocardium (4). This action could be one of important mechanisms explaining cardioprotective properties of WS 1442 extract, along with well described endothelium function improvement causing increase of coronary flow.
Vasorelaxant effect is induced by increased release of nitric oxide (NO) from vascular endothelium and it was previously suggested that this increase is due to activation of endothelial nitric oxide synthase (eNOS) in isolated rat heart (5). It has been described that certain plant extracts reveal vasodilatory action mediated by NO and Akt cellular signalling in vivo as well as in vitro (6, 7). More recent studies confirmed that indeed WS 1442 Crataegus oxycantha special extract induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at Serine 1177 also in isolated human mammary artery (8). Interestingly, new findings suggest that the intracellular formation of superoxide anions is an trigger-factor which begins the cascade of events leading to an enhanced endothelial formation of NO by increasing the phosphorylation of eNOS via the Src/PI3-kinase/Akt pathway (9). It could be hypothesized that endothelium-mediated action is not the sole participation of WS 1442 extract in NO-formation, as latest studies revealed its influence on recently discovered NO synthase of red blood cells (rbcNOS) (10). It was shown that Crataegus extract activates rbcNOS and causes NO-formation in red blood cells without altering their deformability (11).
WS 1442 has also positive effect on endothelial permeability dysfunction, protecting from endothelial hyperpermeability which is crucial in edema formation and inflammatory processes in heart failure (12). This action is mediated by affecting endothelial calcium signaling. The most recent studies revealed that Crataegus extract WS 1442 increases intracellular calcium concentrations by inhibiting the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) and by activating the inositol 1,4,5-trisphosphate (IP(3)) pathway. Importantly, WS 1442 did not induce store-operated calcium entry (SOCE), but even irreversibly prevented histamine-induced SOCE, preventing intracellular calcium concentration associated cytotoxicity (13). This observations additionally explain mechanisms underlying positive influence of WS 1442 in treating heart failure.
Interestingly, there are suggestions that postulated positive effect of Hawthorn extracts on endothelium may be more complex. Apart from important role in NO-synthesis increase, positive role of WS 1442 may be due to stimulation of releasing endothelium-derived hyperpolarizing factor (EDHF). EDHF is an important factor taking part in regulation of vascular homeostasis and angiorelaxation (14). Exact mechanism of action of EDHF is not fully understood. However, its role in endothelial regulation is undisputable and confirmed in many experimental studies with different species of animals as well as in humans (15). EDHF takes part in vasodilatation pathway which is independent from NO release as well as prostacyclin (16-18). Dysfunction of this mechanism is strictly connected with age-related endothelial dysfunction (19, 20). Idris-Kodhja et al. proved protective effect of WS 1442 on endothelial dysfunction related to age as well as oxidative stress (21).
Moreover, protective action of WS 1442 on endothelial layer surface (ELS) was proposed. ELS plays pivotal role in maintaining endothelial homeostasis, by regulating vascular permeability as well as inactivating pro-coagulative and pro-inflammatory processes in subendothelium (22-24). It was shown, that high sodium levels damages glycocalyx of endothelium (25). Peters et al. demonstrated, that WS-1442 standardized extract diminishes permeability for sodium ions in ELS. This effect is provided by WS-1442 ability to perform conformational alterations in ELS what results in transition from densely packed to the loosely packed state of ESL. As known fact, functional parameters of ESL e.g. the sodium permeability, strongly depend on the state of ESL (26). Also WS-1442 have been reported to cause endothelium protection (26).
When it comes to direct effects on myocardium, Crataegus special extract is proven to have positive inotropic effect, resulting in increase of heart contractile force. It was also shown that mechanism of this action is cAMP-independent (27). In models of isolated cardiomyocytes and hearts, a prolongation of the refractory period was observed after treatment with Crataegus extracts, and those observations suggested antiarrhythmic potential (28, 29). Moreover, in in vivo model oral treatment of rats with WS 1442 for 7 days effectively reduced reperfusion induced arrhythmias and mortality as well as hypotensive crises resulting from occlusion of the left coronary artery (30).
Mentioned effects were confirmed in outcomes of study conducted in our Chair and Department of Pharmacology (31). Analyzed data coming from model of early reperfusion-induced arrhythmias in vivo in rats revealed dose- and time-dependent, cardioprotective effect of standardized WS-1442 extract. It was expressed by mortality index reduction, limitation in incidence and duration of severe ventricular arrhythmias as well as decrease in the total amount of heart injury biochemical markers.
More recent observations confirmed also reduction of the extent of ST segment elevation in the electrocardiogram, as well as the incidence of ventricular arrhythmias, the size of the infarction zone, and mortality in animals pretreated with WS 1442 (32). Described cardioprotective action seems to be connected to mentioned above strong antioxidative properties of OPC contained in extract (4). What is more, extract from Crataegus was also proven to inhibit thromboxane A2 biosynthesis in vitro, by reducing arachidonic acid metabolism, cyclooxygenase pathway inhibition and inhibition of Ca2+ dependent protein kinase C isoforms (33, 34), however following observations did not confirm antiplatelet effect in healthy volunteers (35, 36).
There are also observations concluding that Crataegus special extract inhibits development of cardiac hypertrophy which leads to irreversible cardiac insufficiency. It has been demonstrated, that WS-1442 inhibits cardiac hypertrophy in experimentally induced cardiac hypertrophy animal model developed by aortic constriction or treatment with deoxycorticosterone and NaCl/KCl supplementation (37). It is worth to be emphasized, that mentioned study revealed also no influence on blood pressure in control rats, what could suggest that WS 1442 positive cardioprotective action is triggered by pathologies accompanying cardiac hypertrophy. Study published by Rodriguez et al. indicated that the mechanism of cardiac activity of Hawthorn is via the Na(+),K(+)-ATPase and intracellular calcium concentrations are also influenced (38). What is more, further studies on effects of Crataegus extract on the progression of heart failure in rat model confirmed that treatment with WS 1442 has beneficial effects on cardiac remodeling and function also during long-term, pressure overload-induced heart dysfunction (39).
Another aspect of Crataegus cardioprotective properties is influence on lowering blood cholesterol, and several mechanisms of this action have been proposed. Tinctures prepared from its berries reduced total cholesterol as well as all cholesterol fractions (VLDL, LDL, HDL) by increasing cholesterol liver uptake, degradation and decreasing synthesis (40). Outcomes of more recent studies on explaining mechanisms of those beneficial action brought up to light significant decrease of intestinal CoA: cholesterol acyltransferase (ACAT) accompanied with increased expression of liver cholesterol-7-α-hydroxylase in animals fed with high cholesterol diet (41). Promising results coming from animal models encouraged researchers to study effects of Crataegus extracts on lipid metabolism in human cell cultures. Indeed, significant increase in LDL receptors as well as decrease of apolipoprotein B100 were found in human HepG2 hepatocytes cells line treated with WS 1442 (42). These particular molecules play a key role in systemic lipid homeostasis, remaining important targets in treatment of hyperlipidemia.
Effects of Crataegus special extract WS 1442 in clinical studies
Mentioned above outcomes on cardioprotective, positive influence observed in pre-clinical studies are combined with particularly good tolerance of Crataegus special extract WS 1442 (Table 2). No signs of toxicity were observed after oral application of doses up to 3000 mg/kg and LD50 for intraperitoneal administration was described as 1750 mg/kg in animal studies (43). Data coming from previous experimental studies finally led to introducing WS 1442 also into number of trials exploring clinical potential of Crataegus special extract.
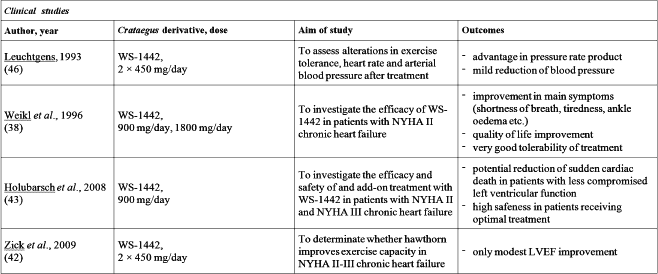
Positive results of Crataegus treatment such as reduced shortness of breath, ankle edema etc. combined with considerably better quality of life for the patient, in particular with respect to mental well-being were observed in a multicenter, placebo-controlled double-blind study in patients with NYHA II class patients (44). Also in study comparing the three month therapy of patient in NYHA II class heart failure with WS 1442 special extract vs placebo, notable decrease of secondary end-point which was double product (heart rate × systolic blood pressure × 10–2) was observed in WS 1442 group (45). Investigations on Crataegus extract in patients with more advanced congestive heart failure (NYHA III class) were also made (46). What is particularly important, in this study it was emphasized that the efficacy of WS 1442 was dose dependent.
Higher dose of 1800 mg was demonstrated to cause statistically significant improvement in the maximal workload tolerated versus placebo group. Mentioned above studies, along with other randomized, placebo controlled, double-blind, clinical studies were analyzed and published in Cohrane Collaboration review (47). This document assessed the benefits and harms coming from metaanalysis of studies on treatment with WS 1442 special extract, concluding that there is a significant improvement in symptom control and beneficial outcomes from Hawthorn extract as an adjunctive therapy for chronic heart failure.
Two more recent clinical trials were proceeded to evaluate addition of WS 1442 to standard therapy for NYHA II/III heart failure (48, 49). The HERB CHF trial has tested the efficiency of treatment with Crataegus special extract 450 mg twice daily on submaximal exercise capacity at 6 months as determined by the 6 min walk test. Secondary outcomes investigated in this trial were quality of life measures, peak oxygen consumption and anaerobic threshold during maximal treadmill exercise testing, NYHA classification, left ventricular ejection fraction (LVEF), neurohormones, and measures of oxidative stress and inflammation.
Significant improvement of LVEF was noted in WS 1442 treated patients. On the other hand, due to no positive influence on secondary outcomes, authors concluded that Crataegus special extract provides no clinical benefit as addition to standard therapy, at the dosing regimen evaluated. The SPICE trial was the largest, randomized, double-blind, placebo-controlled multicenter study of WS 1442 effects in adults with NYHA class II/III heart failure and reduced left ventricular ejection fraction (LVEF ≤ 35%). Also this trial a dose of 900 mg/day of Crataegus special extract was used. Investigators referred that WS 1442 can potentially reduce the incidence of sudden cardiac death in patients with LVEF ≥ 25%, and can be safely used as an addition to standard optimal therapy.
Most recent summary of data coming from clinical trials investigating WS 1442 was made by Eggeling et al. (50). In their pooled analysis of previous studies, authors concluded that Crataegus special extract treatment effects on physiologic outcomes and typical symptoms are modulated by baseline severity. The strongest effects were described in patients with more impaired baseline conditions.
What is more, taking baseline differences into account, benefits were comparable in male and female patients with impaired exercise-tolerance in early chronic heart-failure. This conclusion is important in the light of suggested by other authors gender-limitations of treatment benefit with Crataegus special extract WS 1442 (51). Significant improvement of excersise tolerance as well as reduction of symptoms in groups of patients with modest cardiac insufficiency were also described by other researchers in randomized clinical trials (52).
Blood pressure lowering effect of Crataegus extracts remains controversial. However data coming from experimental studies suggested potential hypotensive cardioprotective action of Crataegus extracts (5, 8-10, 47) mediated by endothelial increase of NO release, outcomes of clinical observation published by Asher et al. did not confirm blood pressure lowering effect which could be mediated via an NO-dependent mechanism (53).
What is necessary to mention, Crataegus products are very safe to use drugs. The major adverse effects of Crataegus products are vertigo, dizziness, nausea, fatigue, sweating, palpitations, headache and epistaxis (54). The only reported contraindication for use of Crataegus products is hypersensitivity to its compounds. These products are not recommended for use during pregnancy, they can cause uterine stimulation. Also, it is not recommended for children and breastfeeding mothers. But yet, adverse effects and side effects of Crataegus products can be reported as very rare.
CONCLUSION
Data coming from experimental and clinical studies on Crataegus special extract WS 1442 suggests that it can be successfully used as an addition to optimal treatment of chronic heart failure. What is more, WS 1442 has a range of vasoactive and cardio active properties that could be possibly useful in treatment of other diseases of cardiovascular system like endothelial dysfunction, coronary disease, arrhythmias, or prevention of restenosis after endovascular treatment. More information about optimal dose regiment for those purposes still needs to be provided.
Conflict of interests: None declared.
REFERENCES
- Weihmayr T, Ernst E. Therapeutic effectiveness of Crataegus. [in German]. Fortschr Med 1996; 114: 27-29.
- Rastogi S, Pandey MM, Rawat AK. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine 2016; 15: 1082-1089.
- Blumenthal M, Goldberg A. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Boston, Integrative Medicine Communications, 1998.
- Miller AL. Botanical influences on cardiovascular disease. Altern Med Rev 1998; 3: 422-431.
- Koch E, Chatterjee SS. Crataegus extract WS®-1442 enhances coronary flow in the isolated rat heart by endothelial release of nitric oxide. Naunyn Schmiedeberg’s Arch Pharmacol 2000; 361 (Suppl.): R48.
- Sun YY, Su XH, Jin JY, et al. Rumex acetosa L. induces vasorelaxation in rat aorta via activation of PI3-kinase/Akt- AND Ca(2+)-eNOS-NO signaling in endothelial cells. J Physiol Pharmacol 2015; 66: 907-915.
- Su XH, Duan R, Sun YY, et al. Cardiovascular effects of ethanol extract of Rubus chingii Hu (Rosaceae) in rats: an in vivo and in vitro approach. J Physiol Pharmacol 2014; 65: 417-424.
- Brixius K, Willms S, Napp A, et al. Crataegus special extract WS 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc Drugs Ther 2006; 20: 177-184.
- Anselm E, Socorro VF, Dal-Ros S, Schott C, Bronner C, Schini-Kerth VB. Crataegus Special Extract WS 1442 Causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of endothelial NO synthase but not via activation of estrogen receptors. J Cardiovasc Pharmacol 2009; 53: 253-260.
- Kleinbongard P, Schulz R, Rassaf, T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006; 107: 2943-2951.
- Rieckeheer E, Schwinger R, Bloch W, Brixius K. Hawthorn special extract WS® 1442 increases red blood cell NO-formation without altering red blood cell deformability. Phytomedicine 2011; 19: 20-24.
- Furst R, Bubik M, Bihari P, et al. The hawthorn special extract WS® 1442 protects against endothelial barrier dysfunction - elucidation of the underlying molecular mechanisms. Planta Med 2010; 76 (SL 53): 1191.
- Willer EA, Malli R, Bondarenko A, et al. The vascular barrier-protecting hawthorn extract WS 1442 raises endothelial calcium levels by inhibition of SERCA and activation of the IP3 pathway. J Moll Cell Cardiol 2012; 53: 567-577.
- Kozlowska H, Baranowska M, Gromotowicz A, Malinowska B. EDHF - srodblonkowy czynnik hiperpolaryzujacy. Znaczenie w fizjologii i chorobach naczyn krwionosnych. Postepy Hig Med Dosw 2007; 61: 555-564.
- Feletou M, Vanhoutte PM. EDHF: new therapeutic targets? Pharmacol Res 2004; 49: 565-580.
- Edwards G, Weston AH. Potassium and potassium clouds in endothelium-dependent hyperpolarizations. Pharmacol Res 2004; 49: 535-541.
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 2002; 23: 374-380.
- Nagao T, Illiano S, Vanhoutte PM. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am J Physiol 1992; 263: H1090-H1094.
- Goto K, Fujii K, Onaka, U, Abe I, Fujishima M. Angiotensin-converting enzyme inhibitor prevents age-related endothelial dysfunction. Hypertension 2000; 36: 581-587.
- Kansui Y, Fujii K, Goto K, Abe I, Iida M. Angiotensin II receptor antagonist improves age-related endothelial dysfunction. J Hypertens 2002; 20: 439-446.
- Idris-Khodja N, Auger C, Koch E, Schini-Kerth VB. Crataegus special extract WS(®)1442 prevents aging-related endothelial dysfunction. Phytomedicine 2012; 19: 699-706.
- Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 2000; 278: H285-H289.
- Jacob M, Bruegger D, Rehm M, Welsch U, Conzen P, Becker BF. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology 2006; 104: 1223-1231.
- Tovar AM, de Mattos DA, Stelling MP, Sarcinelli-Luz BS, Nazareth R, Mourao PA. Dermatan sulfate is the predominant antithrombotic glycosaminoglycan in vessel walls: implications for a possible physiological function of heparin cofactor II. Biochim Biophys Acta 2005; 1740: 45-53.
- Oberleithner H, Peters W, Kusche-Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch 2011; 462: 519-528.
- Peters W, Drueppel V, Kusche-Vihrog K, Schubert C, Oberleithner H. Nanomechanics and Sodium Permeability of Endothelial Surface Layer Modulated by Hawthorn Extract WS 1442. PLoS One 2012; 7: e29972. doi: 10.1371/journal.pone.0029972
- Schwinger RH, Pietsch M, Frank K, Brixius K. Crataegus special extract WS 1442 increases force of contraction in human myocardium cAMP independently. J Cardiovasc Pharmacol 2000; 35: 700-707.
- Poepping S, Rose H, Ionescu I, Fischer Y, Kammermeier H. Effect of a hawthorn extract on contraction and energy turnover of isolated rat cardiomyocytes. Arzneimittelforschung 1995; 45: 1157-1161.
- Joseph G, Zhao Y, Klaus W. Pharmakologisches Wirkprofil von Crataegus-Extrakt im Vergleich zu Epinephrin, Amrinon, Milrinon und Digoxin am isoliert perfundierten Meerschweinchenherzen. Arzneimittelforschung 1995; 45: 1261-1265.
- Krzeminski T, Chatterjee SS. Ischemia and reperfusion induced arrhythmias: beneficial effects of an extract of Crataegus oxyacantha L. Pharm Pharmacol Lett 1993; 3: 45-48.
- Zorniak M. Analysis of Cardioprotective Properties of WS-1442 in the Model of Early Reperfusion Arrhythmias in rats. Dissertation, Zabrze 2016.
- Veveris M, Koch E, Chatterjee SS. Crataegus special extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci 2004; 74: 1945-1955.
- Rein D, Paglieroni TG, Wun T, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr 2000; 72: 30-35.
- Mower RL, Landolfi R, Steiner M. Inhibition in vitro of platelet aggregation and arachidonic acid metabolism by flavone. Biochem Pharmacol 1984; 33: 357-363.
- Vibes J, Lasserre B, Gleye J, Declume C. Inhibition of thromboxane A2 biosynthesis in vitro by the main components of Crataegus oxyacantha (Hawthorn) flower heads. Prostaglandins Leukot Essent Fatty Acids 1994; 50: 173-175.
- Dalli E, Valles J, Cosin-Sales J, et al. Effects of hawthorn (Crataegus laevigata) on platelet aggregation in healthy volunteers. Thromb Res 2011; 128: 398-400.
- Koch E, Sporl-Aich G. Oral treatment with the Crataegus special extract WS® 1442 inhibits cardiac hypertrophy in rats with DOCA-salt or aortic banding induced hypertension. Planta Med 2006; 72(P 265): 1061.
- Rodriguez ME, Poindexter BJ, Bick RJ, Dasgupta A. A comparison of the effects of commercially available hawthorn preparations on calcium transients of isolated cardiomyocytes. J Med Food 2008; 11: 680-686.
- Hwang HS, Boluyt MO, Converso K, Russell MW, Bleske BE. Effects on the progression of heart failure in a rat model of aortic constriction. Pharmacotherapy 2009; 29: 639-648.
- Rajendran S, Deepalakshmi PD, Parasakthy K, Devaraj H, Devaraj, SN. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis 1996; 123: 235-241.
- Zhang Z, Ho WKK, Huang Y, Chen ZY. Hypocholesterolemic activity of hawthorn fruit is mediated by regulation of cholesterol-7α-hydroxylase and acyl CoA: cholesterol acetyltransferase. Food Res Int 2002; 35: 885.
- Koch E, Lanzendorfer-Goossens H, Weibezahn C. Crataegus extract WS® 1442 inhibits apolipoprotein B100 (ApoB) secretion and increases low-density lipoprotein receptor (LDL-R) transcription in human HepG2 cells. Z Phytother 2006; 27: S25-S26.
- Schlegelmilch R, Heywood R. Toxicity of Crataegus (Hawthorn) extract (WS 1442). J Am Coll Toxicol 1994; 13: 103-111.
- Weikl A, Assmus KD, Neukum-Schmidt A, et al. Crataegus Special Extract WS 1442. Assessment of objective effectiveness in patients with heart failure (NYHA II). [in German]. Fortschr Med 1996; 114: 291-296.
- Zapfe JG. Clinical efficacy of crataegus extract WS 1442 in congestive heart failure NYHA class II. Phytomedicine 2001; 8: 262-266.
- Tauchert M. Efficacy and safety of crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am Heart J 2002; 143: 910-915.
- Pittler MH, Guo R, Ernst E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst Rev 2008; 23: CD005312.
- Zick SM, Vautaw BM, Gillespie B, Aaronson KD. Hawthorn extract randomized blinded chronic heart failure (HERB CHF) trial. Eur J Heart Fail 2009; 11: 990-999.
- Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. Survival and prognosis: investigation of Crataegus extract WS 1442 in CHF (SPICE) trial study group. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur J Heart Fail 2008; 10: 1255-1263.
- Eggeling T, Regitz-Zagrosek V, Zimmermann A, Burkart M. Baseline severity but not gender modulates quantified Crataegus extract effects in early heart failure-a pooled analysis of clinical trials. Phytomedicine 2011; 18: 1214-1219.
- Podczeck-Schweighofer A, Dornaus C. Women and heart: are there gender differences in diagnosis and treatment of heart failure? [in German]. Journal fur Kardiologie 2006 13: 352-354.
- Leuchtgens H. Crataegus Special Extract WS 1442 in NYHA II heart failure. A placebo controlled randomized double-blind study. [in German]. Fortschritte der Medizin 1993; 111: 352-354.
- Asher GN, Viera AJ, Weaver MA, Dominik R, Caughey M, Hinderliter AL. Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: a randomized, controlled cross-over trial. BMC Complement Altern Med 2012; 12: 26. doi: 10.1186/1472-6882-12-26
- Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med 2003; 114: 665-674.
A c c e p t e d : August 16, 2017