THERAPEUTIC EFFECT OF EXOGENOUS GHRELIN IN THE HEALING OF GINGIVAL ULCERS IS MEDIATED BY THE RELEASE OF ENDOGENOUS GROWTH HORMONE AND INSULIN-LIKE GROWTH FACTOR-1
INTRODUCTION
Ghrelin, an acylated 28-amino acid polypeptide, was primary isolated from the human and rat stomach, and the stomach is a main source of circulating ghrelin (1, 2). It exist in two main molecular forms, as an acylated ghrelin (acyl-ghrelin) and non-acylated ghrelin (des-acyl ghrelin (3, 4). Acyl-ghrelin, which mainly occurs as octaynolated ghrelin is an active form of this peptide because it is an endogenous ligand of ghrelin receptor, formerly known as the growth hormone secretagogue receptor 1a (GHS-R) (3-5). GHS-Rs are mainly expressed in the pituitary gland and hypothalamus; however their presence was also shown in other central and peripheral tissues (6). Activation of ghrelin receptor strongly and dose dependently stimulates release of growth hormone from the anterior pituitary (1). Apart from the release of growth hormone, ghrelin stimulates food intake and fat deposition (7-10), as well as exhibits protective and therapeutic effect in numerous organs, including the gut (3). Treatment with ghrelin protects gastric mucosa against damage evoked by ethanol (11), stress (12) or alendronate (13), as well as accelerates healing of gastric and duodenal ulcers (14-16). Also, experimental data indicate that ghrelin reduces the severity and accelerates the healing of colitis (17-20). In the pancreas, pretreatment with ghrelin inhibits the development of cerulein- and ischemia/reperfusion-induced acute pancreatitis (21, 22), as well as, accelerates the recovery in these models of acute pancreatitis (23, 24). Furthermore, ghrelin protects the liver, pancreas and remote organs against oxidative injury evoked by pancreatobiliary obstruction (25).
In the oral cavity, administration of ghrelin was shown to accelerate recovery in the course of oral ulcers and this effect was observed in rats with intact salivary glands, as well as in sialoadenectomized rats (26). This observation taken together with findings that ghrelin is present in saliva in a similar or even higher concentration than in serum or plasma (27-29), suggest that healing effect of ghrelin in oral mucosa damage is related to direct impact of ghrelin on oral epithelial cells. This concept is additionally support by observation that human oral epithelial cells express mRNA for ghrelin and ghrelin receptor, and ghrelin protein is present in gingival epithelium and fibroblasts located in the lamina propria (29).
On the other hand, ghrelin strongly stimulates release of growth hormone and insulin-like growth factor-1 (IGF-1) (1, 30). Growth hormone and IGF-1 are anabolic hormones and they promote cell survival, proliferation and differentiation (31, 32). The presence of growth hormone receptors and IGF-1 receptors was found in the oral cavity (33-37). These data suggest that therapeutic effect of ghrelin in the healing of oral mucosa lesions may be related to the ghrelin-induced release of endogenous growth factor and IGF-1.
The aim of the present study was to determine the role of endogenous growth hormone and IGF-1 in therapeutic effects of exogenous ghrelin in the healing of gingival ulcers. For this reason, our research was performed in pituitary-intact and hypophysectomized rats.
MATERIALS AND METHODS
Animals and treatment
Studies were performed on 120 male Wistar rats weighing 150 – 170 g. Experimental protocol was approved by the Jagiellonian University Ethical Committee for Research and Animal Ethic. During experiments, the animals were kept in cages placed in a room temperature with 12 h light-darkness cycle.
Studies were conducted on pituitary-intact or hypophysectomized rats. For this reason, before start of the main experiment, rats were randomly divided into two groups, anesthetized with ketamine (50 mg/kg i.p., Bioketan, Biowet, Gorzow Wielkopolski, Poland) and sham-operated or hypophysectomized via the transauricular approach according to a method described by Falconi and Rossi (38).
After 2-week recovery rats were divided into twelve groups:
1) control pituitary-intact rats without induction of gingival ulcer and treated with saline;
2) pituitary-intact rats without induction of gingival ulcer and treated with ghrelin at a dose of 8 nmol/kg/dose;
3) pituitary-intact rats with gingival ulcers treated with saline;
4 – 6) pituitary-intact rats with gingival ulcer treated with ghrelin at a dose of 4, 8 or 16 nmol/kg/dose, respectively;
7) hypophysectomized rats without induction of gingival ulcer and treated with saline;
8) hypophysectomized rats without induction of gingival ulcer and treated with ghrelin at a dose of 8 nmol/kg/dose;
9) hypophysectomized rats with gingival ulcer treated with saline;
10 – 12) hypophysectomized rats with gingival ulcer treated with ghrelin at a dose of 4, 8 or 16 nmol/kg/dose, respectively.
Before induction of gingival ulcers, rats were fasted for 16 h and reanesthetized with ketamine. Gingival ulcers were induced by acetic acid. Briefly, about 70 µl of 100% acetic acid through the plastic tube (4 mm inner diameter) was applied for 15 s to the frontal surface of gingival mucosa of the maxilla above the incisors.
After induction of ulcers, rats were treated intraperitoneally twice a day with saline (0.9% NaCl) or ghrelin (given at the dose of 4, 8 or 16 nmol/kg/dose) for six days. Rat recombinant octanoylated ghrelin was obtained from Yanaihara Institute, Shizuoka, Japan. Also, rats without induction of gingival ulcers were treated intraperitoneally twice a day with saline or ghrelin given at a dose of 8 nmol/kg/dose for six days before the end of study. Ghrelin was diluted in saline immediately before administration. Final volume of saline or ghrelin solution was 0.3 ml.
Determination of mucosal blood flow and measurement of the gingival ulcer area
Three and six days after induction of gingival ulcers, rats were anesthetized again with ketamine. Blood flow in gingival mucosa of the maxilla was measured using laser Doppler flowmeter (PeriFlux 4001 Master monitor, Perimed AB, Järfälla, Sweden). Blood flow was measured in five areas of gingival mucosa, and mean value of these recordings was presented as percent of mucosal blood flow measured in saline-treated pituitary-intact rats and without induction of ulcers (control group).
After measurement of mucosal blood flow, the area of ulcerated mucosa was measured, using computerized planimeter (Morphomat, Carl Zeiss, Berlin, Germany) as described previously (14).
Biochemical analysis
At the end of the study, after measurement of mucosal blood flow, the abdominal cavity was opened in anesthetized rats and blood samples were collected from the abdominal aorta. Before determination of serum growth hormone and IGF-1 concentrations, serum was frozen and stored at -60°C.
One, 12 or 24 h after administration of ethanol, rats were anesthetized with ketamine (50 mg/kg i.p., Bioketan, Vetoquinol Biowet, Gorzow Wielkopolski, Poland) and the abdomen was opened by a midline incision.
Samples from gingival mucosa were taken for determination of mucosal DNA synthesis and mucosal concentration of pro-inflammatory interleukin-1β.
Serum growth hormone concentration was determined by radioimmunoassay, using a commercial Rat Growth Hormone RIA Kit (LINCO Research, St. Charles, Missouri, USA). Serum IGF-1 concentration was measured by radioimmunoassay, using a commercial Mouse/Rat IGF-I RIA Kit (Diagnostic System Laboratories, Inc., Webster, Texas, USA). Serum concentration of interleukin-1β was measured using the BioSource Cytoscreen rat IL-1β kit (BioSource International, Camarillo, California, USA) based on ELISA.
DNA synthesis, an index of cell proliferation, was determined by measurement of thymidine incorporation ([6-3H]-thymidine, 20-30 Ci/mmol, Institute for Research, Production and Application of Radioisotopes, Prague, Czech Republic) into mucosal DNA as described previously (39). The incorporation of labeled thymidine into DNA was determined by counting 0.5 ml DNA-containing supernatant in a liquid scintillation system. DNA synthesis was expressed as tritium disintegrations per minute per µg DNA (dpm/µg DNA).
Samples of mucosa, taken from the gum for determination of interleukin-1β concentration, were homogenized in ice-cold phosphate-buffered saline (PBS, 20 mM, pH 7.4). Homogenate was centrifuged at 1500 g for 10 min at 4°C. Content of interleukin-1β in the supernatant was measured using the BioSource Cytoscreen rat IL-1β kit (BioSource International, Camarillo, California, USA) based on ELISA. Concentration of interleukin-1β in gingival mucosa was expressed as ng per g of protein.
Statistical analysis
Results were expressed as mean ± S.E.M. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test using GraphPadPrism (GraphPad Software, San Diego, CA, USA). Differences were considered to be statistically significant when P was less than 0.05.
RESULTS
The gingival ulcer area
Topical application of acetic acid on the mucosa of the gum produced gingival ulcers in all rats subjected to this procedure. In rats with the intact pituitary gland treated with saline, one day after induction of gingival ulcers, the area of these ulcers was 16.5 ± 0.4 mm2 (Fig. 1). Gingival ulcers underwent spontaneous healing. In rats with the intact pituitary gland treated with saline, 3 days or 6 days after induction of gingival ulcers, the area of these ulcers was 11.2 ± 0.4 mm2 or 2.5 ± 0.1 mm2, respectively and the size of these ulcers was significantly smaller than that observed after 1 day after their induction (Fig. 1). In these rats, administration of ghrelin at all doses used led to a significant acceleration of spontaneous healing of gingival ulcers and this effect was observed at both times of observation, 3 and 6 days after induction of ulcers. Ghrelin given at a dose of 8 or 16 nmol/kg/dose exhibited maximal therapeutic effect. Hypophysectomy performed before induction of ulcers tended to increase the area of gingival ulcers, but neither after 1 nor 3 nor 6 days after induction of ulcer this effect was statistically significant (Fig. 1). On the other hand, hypophysectomy completely abolished the therapeutic effect of exogenous ghrelin in the healing of gingival ulcers (Fig. 1).
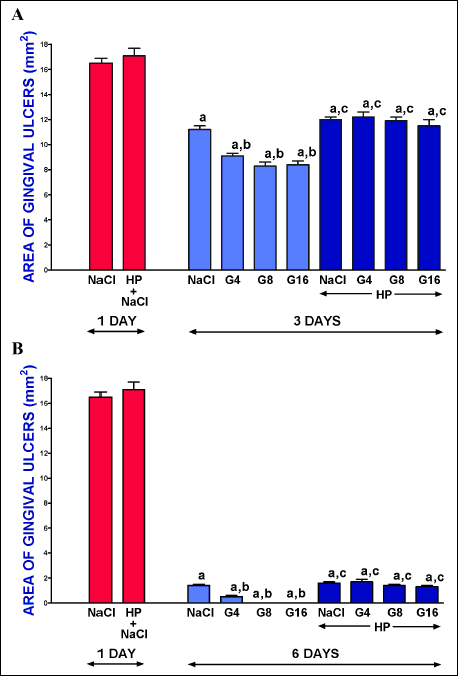 |
Fig. 1. Influence of intraperitoneal administration of saline (NaCl) or ghrelin given at a dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on the area of gingival ulcers in pituitary-intact or hypohysectomized (HP) rats. Observation was performed after 1 or 3 days (panel A), or after 1 or 6 days (panel B) after induction of ulcers. Mean ± standard error. N = 10 animals in each group. aP < 0.05 compared to a value observed 1 day after induction of gingival ulcers in pituitary-intact NaCl-treated rats; bP < 0.05 compared to a value observed 3 day (panel A) or 6 day (panel B) after induction of gingival ulcers in pituitary-intact NaCl-treated rats; cP < 0.05 compared to a value observed 1 day after induction of gingival ulcers in NaCl-treated hypophysectomized rats. |
Mucosal blood flow
Administration of ghrelin at a dose of 8 nmol/kg/dose failed to significantly affect mucosal blood flow in the gum in pituitary-intact rats without induction of gingival ulcers, and this lack of effect was observed after 3- and 6-day administration of ghrelin (Fig. 2). Three days after induction of gingival ulcers, mucosal blood flow in the gum was significantly reduced in all rats with ulcers. In pituitary-intact rats with gingival ulcers and treated with saline, mucosal blood flow was reduced by about 43% in comparison to a value observed in control pituitary-intact rats without induction of ulcers. In pituitary-intact rats with ulcers, administration of ghrelin improved mucosal blood flow and this effect was statistically significant after ghrelin given at a dose of 8 or 16 nmol/kg/dose (Fig. 2 panel A).
Six days after induction of gingival ulcers, mucosal blood flow in the gum in pituitary-intact rats reached a value similar to that observed in control pituitary-intact rats without induction of ulcers (Fig. 2 panel B). Administration of ghrelin in these rats led to significant increase in mucosal blood flow in the gum. In the case of ghrelin given at a dose of 4 nmol/kg/dose this effect was significant in comparison to a value observed in pituitary-intact rats treated with saline after induction of ulcers. In the case of ghrelin given at a dose of 8 or 16 nmol/kg/dose this effect was additionally significant in comparison to a value observed in control pituitary-intact rats without induction of ulcers (Fig. 2 panel B). Hypophysectomy alone or in combination with administration of ghrelin at doses used was without any effect on mucosal blood flow in the gum in rats with gingival ulcers and this lack of effect was observed after 3, as well as 6 days after induction of these ulcers (Fig. 2).
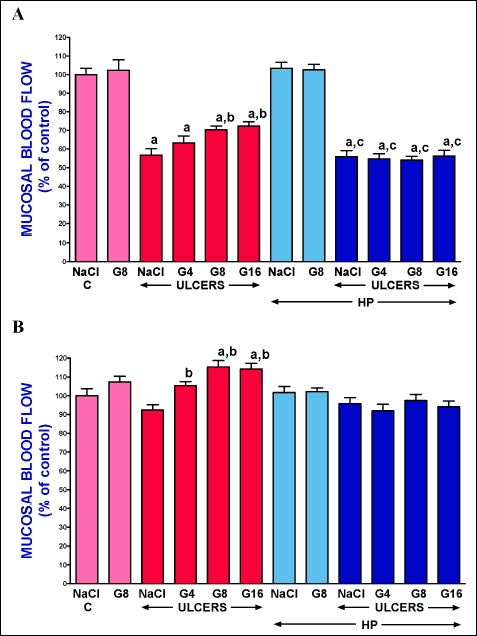 |
Fig. 2. Influence of intraperitoneal administration of saline (NaCl) or ghrelin given at a dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) and induction of gingival ulcers (ULCERS) on mucosal blood flow in the gum in pituitary-intact or hypohysectomized (HP) rats. Observation was performed after 3 days (panel A) or 6 days (panel B) after induction of ulcers or sham-operation. Mean ± standard error. N = 10 animals in each group. aP < 0.05 compared to a value observed in control pituitary-intact NaCl-treated rats without induction of ulcers (C); bP < 0.05 compared to a value observed in pituitary-intact rats treated with NaCl after induction of ulcers; cP < 0.05 compared to a value observed in hypophysectomized NaCl-treated rats without induction of ulcers. |
Mucosal DNA synthesis
Six days after induction of gingival ulcers, DNA synthesis in gingival mucosa was significantly increased in pituitary-intact, as well as hypophysectomized rats (Fig. 3). In pituitary-intact rats with gingival ulcers, administration of ghrelin led to an additional increase in mucosal DNA synthesis and this effect was significant after ghrelin given at a dose of 8 or 16 nmol/kg/dose. In the case of hypophysectomized rats with gingival ulcers, administration of ghrelin was without any effect on mucosal DNA synthesis in the gum (Fig. 3).
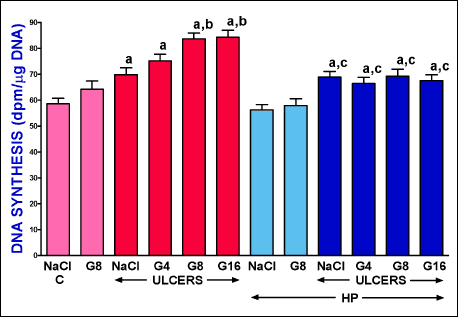 |
Fig. 3. Influence of intraperitoneal administration of saline (NaCl) or ghrelin given at a dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) and induction of gingival ulcers (ULCERS) on mucosal DNA synthesis in the gum in pituitary-intact or hypohysectomized (HP) rats. Observation was performed after 6 days after induction of ulcers or sham-operation. Mean ± standard error. N = 10 animals in each group. aP < 0.05 compared to a value observed in control pituitary-intact NaCl-treated rats without induction of ulcers (C); bP < 0.05 compared to a value observed in pituitary-intact rats treated with NaCl after induction of ulcers; cP < 0.05 compared to a value observed in hypophysectomized NaCl-treated rats without induction of ulcers. |
Mucosal and serum concentration of interleukin-1β
Induction of gingival ulcers significantly increased mucosal concentration of pro-inflammatory interleukin-1β in the gum and 6 days after induction of ulcers this effect was observed in pituitary-intact, as well as hypophysectomized rats (Fig. 4 panel A).
Administration of ghrelin at all doses used led to significant reduction in the ulcer-induced increase in mucosal concentration of interleukin-1β. This effect was maximally pronounced after ghrelin given at a dose of 8 or 16 nmol//kg/dose. In the case of hypophysectomized rats, administration of ghrelin failed to affect the ulcer-induced increase in mucosal DNA synthesis in the gum (Fig. 4 panel A).
In control pituitary-intact rats without induction of gingival ulcers and treated with saline, serum concentration of pro-inflammatory interleukin-1β reached a value of 52.3 pg/ml (Fig. 4 panel B). Neither induction of ulcers nor hypophysectomy nor administration of ghrelin significantly affected serum concentration of interleukin-1β observed after 6 days after induction of gingival ulcers.
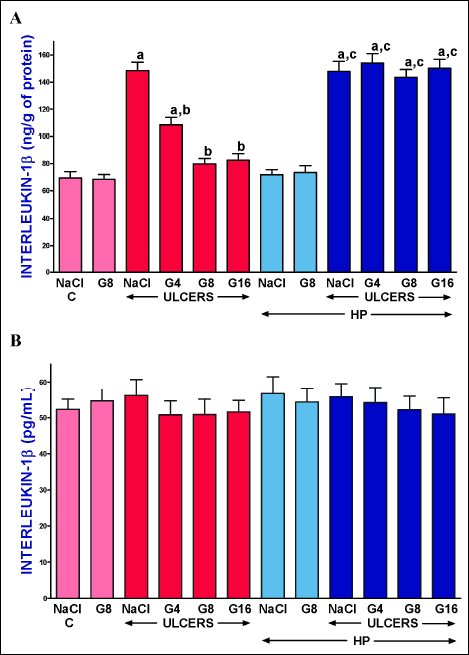 |
Fig. 4. Influence of intraperitoneal administration of saline (NaCl) or ghrelin given at a dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) and induction of gingival ulcers (ULCERS) on mucosal concentration of interleukin-1β in the gum (panel A) or on serum concentration of interleukin-1β (panel B) in pituitary-intact or hypohysectomized (HP) rats. Observation was performed after 6 days after induction of ulcers or sham-operation. Mean ± standard error. N = 10 animals in each group. aP < 0.05 compared to a value observed in control pituitary-intact NaCl-treated rats without induction of ulcers (C); bP < 0.05 compared to a value observed in pituitary-intact rats treated with NaCl after induction of ulcers; cP < 0.05 compared to a value observed in hypophysectomized NaCl-treated rats without induction of ulcers. |
Serum concentration of growth hormone
Serum concentration of growth hormone reached a value of 103.5 ± 6.1 ng/ml in control pituitary-intact rats without induction of gingival ulcers (Fig. 5). In pituitary-intact rats, administration of ghrelin at doses used significantly increased serum level of growth hormone. Maximal increase in serum concentration of growth hormone was observed after ghrelin given at a dose of 8 or 16 nmol/kg/dose and this effect was observed in pituitary-intact rats with ulcers, as well as in pituitary-intact rats without induction of ulcers. Induction of gingival ulcers was without any effect on serum growth hormone concentration determined 6 days after induction of these ulcers. Hypophysectomy decreased serum level of growth hormone below a detection limit and administration of ghrelin was without any effect on serum concentration of growth hormone in hypophysectomized rat (Fig. 5).
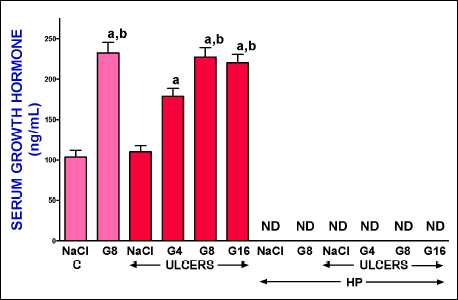 |
Fig. 5. Influence of intraperitoneal administration of saline (NaCl) or ghrelin given at a dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) and induction of gingival ulcers (ULCERS) on serum concentration of growth hormone in pituitary-intact or hypohysectomized (HP) rats. Observation was performed after 6 days after induction of ulcers or sham-operation. ND means non-detectable. Mean ± standard error. N = 10 animals in each group. aP < 0.05 compared to a value observed in control pituitary-intact NaCl-treated rats without induction of ulcers (C); bP < 0.05 compared to a value observed in pituitary-intact rats treated with NaCl after induction of ulcers. |
Serum concentration of insulin-like growth factor-1
In saline-treated pituitary-intact control rats without induction of ulcers, serum concentration of IGF-1 was 410.3 ± 17.8 ng/ml (Fig. 5). Induction of gingival ulcers was without significant effect on serum concentration of IGF-1 observed 6 days after induction of these ulcers. Administration of exogenous ghrelin significantly increased serum level of IGF-1 in pituitary-intact rats. Ghrelin given at a dose of 4 nmol/kg/dose led to about a 2-fold increase in serum level of IGF-1; whereas ghrelin given at a dose of 8 or 16 nmol/kg/dose caused about a 3-fold increase in this parameter in pituitary-intact rats (Fig. 6). Hypophysectomy reduced serum concentration of IGF-1 by about 90% and neither induction of gingival ulcers nor administration of ghrelin significantly affected this parameter in hypophysectomized rats (Fig. 6).
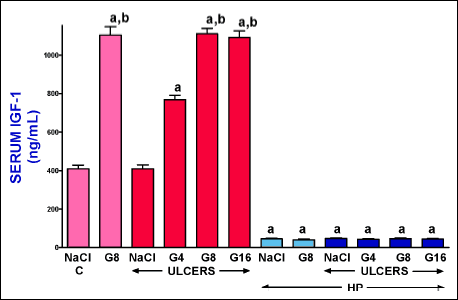 |
Fig. 6. Influence of intraperitoneal administration of saline (NaCl) or ghrelin given at a dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) and induction of gingival ulcers (ULCERS) on serum concentration of insulin-like growth factor-1 (IGF-1) in pituitary-intact or hypohysectomized (HP) rats. Observation was performed after 6 days after induction of ulcers or sham-operation. Mean ± standard error. N = 10 animals in each group. aP < 0.05 compared to a value observed in control pituitary-intact NaCl-treated rats without induction of ulcers (C); bP < 0.05 compared to a value observed in pituitary-intact rats treated with NaCl after induction of ulcers. |
DISCUSSION
Previous research showed that administration of ghrelin prevents reduction in cell proliferation in the mucosa membrane in the oral cavity and exhibits therapeutic effect in the healing of gingival and lingual ulcers (26). Our current observations performed on pituitary-intact rats are in agreement with these findings and extent our knowledge in this matter. We found that administration of exogenous ghrelin accelerates the healing of gingival ulcers. Ghrelin given at all doses used led to significant reduction in the area of gingival ulcers. Three-day treatment with ghrelin given at the most effective doses, 8 or 16 nmol/kg/dose decreased reduced the area of ulcers by about 26%. After next three days of treatment with these doses of ghrelin, gingival mucosa was fully healed. This effect of ghrelin administration was accompanied by an increase in DNA synthesis, improvement of blood flow and reduction in interleukin-1β concentration in mucosa of the gum. Integrity of the mucosa is maintained by dynamic balance between cell renewal by mitosis and cell loss as a result of cell exfoliation from surface layers of the mucosa membrane. Insufficient cell renewal or increased cell loss may lead to atrophy and/or ulceration. On the other hand, increased cell proliferation or reduced cell loss may result in mucosal hyperplasia. DNA synthesis, measured by incorporation of thymidine into DNA, is an index of cell proliferation (40). Our current observation showed that spontaneous healing of gingival ulcers is associated with a stimulation of DNA synthesis in gingival mucosa. In pituitary-intact rats, this effect was additionally enhanced by treatment with ghrelin.
Appropriate blood flow plays an important role in the protection and healing of mucous membrane in the gut (14, 41). Adequate blood supply allows a delivery of oxygen, bicarbonate, nutritious substances, hormones and growth factors, and removes carbon dioxide, hydrogen ions and other toxic agents. Local improvement of mucosal blood flow reduces damage and accelerates the healing of the mucosa membrane in the digestive tract (41-43). In our present study, we found that induction of gingival ulcers by acetic acid leads to initial reduction in mucosal blood flow in the gum, followed by subsequent increase in this parameter during spontaneous healing of these ulcers. In pituitary-intact rats, administration of ghrelin significantly improved mucosal blood flow in the gum leading to faster healing of gingival ulcers. These observations indicate that improvement of mucosal blood flow is involved in therapeutic effect of ghrelin in the treatment of acetic acid-induced gingival ulcers in rats with the intact pituitary gland.
Our current study also showed that treatment with ghrelin decreases the ulcer-induced increase in mucosal concentration of interleukin-1β in pituitary-intact rats. This cytokine is a well-known mediator of inflammation and plays a crucial role in the induction of local and systemic acute phase response and in the release of other members of the pro-inflammatory cytokine cascade (44, 45). Administration of interleukin-1 receptor antagonist prevents the rise in serum concentration of pro-inflammatory interleukin-6 and TNF-α, and decreases severity of systemic inflammation (45-48). These data taken together with our current results indicate that treatment with exogenous ghrelin reduces the local inflammatory process in the course of gingival ulcers in pituitary-intact rats. This finding shows the next mechanism of beneficial effect of ghrelin in gingival mucosa. On the other hand, our current study showed that neither induction of gingival ulcers nor administration of ghrelin affected serum level of interleukin-1β. This observation indicates that induction of gingival ulcers causes the development of local but not systemic inflammation.
Our current study also showed that administration of exogenous ghrelin leads to increase in serum level of growth hormone and insulin-like growth factor-1. Both these hormones exhibit anabolic effects and are involved in cell survival and proliferation (31, 32), as well as in the protection and healing of different organs in the digestive system (49-54). Moreover, the presence of growth hormone receptor and IGF-1 receptors was found in the oral cavity (33-37). These data may suggest that therapeutic effect of ghrelin in the healing of oral mucosa damage is related to the ghrelin-induced release of endogenous growth factor and IGF-1. On the other hand, there are observations showing that human oral epithelial cells express mRNA for ghrelin and ghrelin receptor, and ghrelin protein is present in gingival epithelium and fibroblasts located in the lamina propria in the gum (29). Moreover, ghrelin is present in high concentrations in saliva (27-29), it causes that the mucous membrane of the oral cavity is permanently exposed to huge amount of ghrelin. This group of findings suggests that therapeutic effects of ghrelin in the gum may depend on direct action of this peptide on oral mucosa. For this reason, to check the role of endogenous growth hormone and IGF-1 in therapeutic effects of exogenous ghrelin in the healing of gingival ulcers, we performed the study using hypophysectomized rats.
In this part of study, we performed hypophysectomy using the transauricular technique according to a method described by Falconi and Ross (38). We chose this method because of our experience in using it. Moreover, this method reduced the possibility of developing inflammation of the central nervous system. The disadvantage of this method is the lack of visual inspection whether the pituitary was completely removed. On the other hand, our current results showed that hypophysectomy performed via the transauricular approach decreased serum level of growth hormone below a detection limit. This observation clearly demonstrates that the method used leads to effective removal of the pituitary gland.
Our current study also showed that elimination of circulating growth hormone was associated with a decrease in serum concentration of IGF-1 by about 90%. On the other hand, unexpectedly these effects of hypophysectomy did not affect the healing rate of gingival ulcers in rats without administration of ghrelin. Also blood flow and DNA synthesis in gingival mucosa was not altered in these rats in comparison to values observed in pituitary-intact rats treated with saline. Above findings indicates that basal levels of growth hormone and IGF-1 do not play an important role in maintaining integrity of gingival mucosa. This thesis is supported by observation that ghrelin is present in high concentration in saliva (27-29). Furthermore, the study performed by Oath et al. found in humans showed that the concentration ghrelin in gingival reticular fluid is approximately 500-fold greater than in saliva (29). Probably, for this reason, the direct action of exogenous ghrelin on gingival mucosa cannot be revealed due to a local high concentration of endogenous ghrelin in basal condition.
In contrast to results observed in pituitary-intact rats, administration of ghrelin was without any effect on the release of endogenous growth hormone and IGF-1 in hypophysectomized rats. Also, in these rats, ghrelin also did not affect the healing rate of gingival ulcers, mucosal blood flow, DNA synthesis or gingival concentration of pro-inflammatory interleukin-1β. These findings taken together with observation performed on pituitary-intact rats indicate that therapeutic effect of ghrelin administration in gingival ulcers is based on the release of endogenous growth hormone and IGF-1.
Finally, we conclude that administration of exogenous ghrelin exhibits the therapeutic effect in the healing of gingival mucosal damage and this effects is mainly related to the ghrelin-induced release of endogenous growth hormone and IGF-1. Moreover this observation suggests that ghrelin could be potentially useful in the treatment of radiation- or chemotherapy-induced oral mucositis. However, this potential application in clinics requires additional experimental research.
Acknowledgements: Dr. Jakub Cieszkowski and Dr. Zygmunt Warzecha have contributed equally to this work.
Conflict of interests: None declared.
REFERENCES
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature 1999; 402: 656-660.
- Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 2001; 86: 4753-4758.
- Warzecha Z, Dembinski A. Protective and therapeutic effects of ghrelin in the gut. Curr Med Chem 2012; 19: 118-125.
- Muller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Mol Metab 2015; 4: 437-460.
- Davenport AP, Bonner TI, Foord SM, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev 2005; 57: 541-546.
- Gnanapavan S, Kola B, Bustin SA, Morris DG, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002; 87: 2988-2991.
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000; 407: 908-913.
- Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001; 50: 2540-2547.
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992-5995.
- Nesic DM, Stevanovic DM, Ille T, Petricevic S, Masirevic-Draskovic G, Starcevic VP. Centrally applied ghrelin affects feeding dynamics in male rats. J Physiol Pharmacol 2008; 59: 489-500.
- Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology 2003; 144: 353-359.
- Brzozowski T, Konturek PC, Konturek SJ, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept 2004: 120: 39-51.
- Iseri SO, Sener G, Yuksel M, et al. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol 2005; 187: 399-406.
- Ceranowicz P, Warzecha Z, Dembinski A, et al. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J Physiol Pharmacol 2009; 60: 87-98.
- Warzecha Z, Ceranowicz P, Dembinski M, et al. Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J Physiol Pharmacol 2014; 65: 95-106.
- Warzecha Z, Ceranowicz D, Dembinski A, et al. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med Sci Monit 2012; 18: BR181-BR187.
- Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006; 130: 1707-1720.
- Konturek PC, Brzozowski T, Engel M, et al. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J Physiol Pharmacol 2009; 60: 41-47.
- Maduzia D, Matuszyk A, Ceranowicz D, et al. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J Physiol Pharmacol 2015; 66: 875-885.
- Matuszyk A, Ceranowicz P, Warzecha Z, et al. Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int J Mol Sci 2016; 17: E1455. doi: 10.3390/ijms17091455.
- Dembinski A, Warzecha Z, Ceranowicz P, et al. Ghrelin attenuates the development of acute pancreatitis in rats. J Physiol Pharmacol 2003; 54: 561-573.
- Dembinski A, Warzecha Z, Ceranowicz P, et al. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF Res 2006; 16: 348-356.
- Warzecha Z, Ceranowicz P, Dembinski A, et al. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J Physiol Pharmacol 2010; 61: 419-427.
- Bukowczan J, Warzecha Z, Ceranowicz P, Kusnierz-Cabala B, Tomaszewska R, Dembinski A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr Pharm Des 2015; 21: 2284-2290.
- Kasimay O, Iseri SO, Barlas A, et al. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res 2006; 36: 11-19.
- Warzecha Z, Kownacki P, Ceranowicz P, Dembinski M, Cieszkowski J, Dembinski A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J Physiol Pharmacol 2013; 64: 657-668.
- Dynesen AW, Bardow A, Astrup A, Petersson B, Holst JJ, Nauntofte B. Meal-induced compositional changes in blood and saliva in persons with bulimia nervosa. Am J Clin Nutr 2008; 87: 12-22.
- Aydin S, Halifeoglu I, Ozercan IH, et al. A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects. Peptides 2005; 26: 647-652.
- Ohta K, Laborde NJ, Kajiya M, et al. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J Dental Res 2011; 90: 1286-1292.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Dual age-dependent effect of ghrelin administration on serum level of insulin-like growth factor-1 and gastric growth in young rats. Eur J Pharmacol 2006; 529: 145-150.
- Ho KK, O'Sullivan AJ, Hoffman DM. Metabolic actions of growth hormone in man. Endocr J 1996; 43 (Suppl): S57-S63.
- Zapf J, Froesch ER. Insulin-like growth factors/somatomedins: structure, secretion, biological actions and physiological role. Horm Res 1986; 24: 121-130.
- Joseph BK, Savage NW, Young WG, Waters MJ. Prenatal expression of growth hormone receptor/binding protein and insulin-like growth factor-1 (IGF-1) in the enamel organ. Role for growth hormone and IGF-I in cellular differentiation during early tooth formation? Anat Embryol (Berl) 1994; 189: 489-494.
- Zhang CZ, Young WG, Waters MJ. Immunocytochemical localization of growth hormone receptor in rat maxillary teeth. Arch Oral Biol 1992; 37: 77-84.
- Mustafa W, Mustafa A, Elbakri N, Link H, Adem A. Reduced levels of insulin-like growth factor-1 receptor (IGF-1R) suppress cellular signaling in experimental autoimmune sialadenitis (EAS). J Recept Signal Transduct Res 2001; 21: 47-54.
- Magnucki G, Schenk U, Ahrens S, et al. Expression of the IGF-1, IGFBP-3 and IGF-1 receptors in dental pulp stem cells and impacted third molars. J Oral Sci 2013; 55: 319-327.
- Biggs BT, Tang T, Krimm RF. Insulin-like growth factors are expressed in the taste system, but do not maintain adult taste buds. PLoS One 2016; 11: e0148315. doi: 10.1371/journal.pone.0148315.
- Falconi G, Rossi GL. Transauricular hypophysectomy in rats and mice. Endocrinology 1964; 74: 301-303.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Role of sensory nerves in gastroprotective effect of anandamide in rats. J Physiol Pharmacol 2011; 62: 207-217.
- Dembinski A, Warzecha Z, Konturek SJ, Cai RZ, Schally AV. The effects of antagonists of receptors for gastrin, cholecystokinin and bombesin on growth of gastroduodenal mucosa and pancreas. J Physiol Pharmacol 1991; 42: 195-209.
- Sorbye H, Svanes K. The role of blood flow in gastric mucosal defence, damage and healing. Dig Dis 1994; 12: 305-317.
- Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol 2010; 24: 873-882.
- Leung FW, Su KC, Pique JM, Thiefin G, Passaro E, Guth PH. Superior mesenteric artery is more important than inferior mesenteric artery in maintaining colonic mucosal perfusion and integrity in rats. Dig Dis Sci 1992; 37: 1329-1335.
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009; 27: 519-550.
- Dinarello CA. A clinical perspective of IL-1 as the gatekeeper of inflammation. Eur J Immunol 2011; 41: 1203-1217.
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11: 633-652.
- Norman J, Franz M, Messina J, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery 1995; 117: 648-655.
- De Koning HD, Schalkwijk J, van der Ven-Jongekrijg J, Stoffels M, van der Meer JW, Simon A. Sustained efficacy of the monoclonal anti-interleukin-1β antibody canakinumab in a 9-month trial in Schnitzler's syndrome. Ann Rheum Dis 2013; 72: 1634-1638.
- Coerper S, Wolf S, von Kiparski S, et al. Insulin-like growth factor I accelerates gastric ulcer healing by stimulating cell proliferation and by inhibiting gastric acid secretion. Scand J Gastroenterol 2001; 36: 921-927.
- Wang X, Wang B, Wu J, Wang G. Beneficial effects of growth hormone on bacterial translocation during the course of acute necrotizing pancreatitis in rats. Pancreas 2001; 23: 148-156.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J Physiol Pharmacol 2003; 54: 575-590.
- Christensen H, Flyvbjerg A, Orskov H, Laurberg S. Effect of growth hormone on the inflammatory activity of experimental colitis in rats. Scand J Gastroenterol 1993: 28: 503-511.
- Chen T, Zheng F, Tao J, et al. Insulin-like growth factor-1 contributes to mucosal repair by β-arrestin2-mediated extracellular signal-related kinase signaling in experimental colitis. Am J Pathol 2015; 185: 2441-2453.
- Ceranowicz P, Warzecha Z, Dembinski A. Peptidyl hormones of endocrine cells origin in the gut - their discovery and physiological relevance. J Physiol Pharmacol 2015; 66: 11-27
A c c e p t e d : August 22, 2017