CHANGES IN U937 CELL VIABILITY INDUCED BY STRESS
FACTORS
- POSSIBLE ROLE OF CALMODULIN
INTRODUCTION
Numerous studies have shown that various stress factors (e.g. chemically derived cytotoxic agents, physical factors) can influence normal cell function. According to some experimental results, one of the cell stressors may be exposure to magnetic fields (MFs). Because of the wide distribution of MF sources in the environment, magnetic fields have recently been intensively examined especially after not consistent reports considering their possible carcinogenic- or therapeutic role (1-4). The observed bio-effects remain controversial as the mechanism(s) underlying the effects of MF action on cells are still not known, in particular, non-thermal effects have not been fully recognized (5-8). The most often considered options are radical pair mechanism and related changes of reactive oxygen species (ROS) chemistry which can occur even for very weak MF (9-12), and modulation of intracellular ions mobilization (13-15).
Nevertheless, the biophysical models proposed so far, did not quantitatively describe and incontestable link all observed bio-effects with specific intracellular target(s) of MF. Interestingly, caused bio-effects vary depending on cell types and the parameters of MF (e.g. magnetic flux density, frequency, signal waveform, time of exposure) itself (5, 16). It is also experimentally verified, that significant bio-effects (e.g. changes in cell viabillity) have been observed for simultaneous action of MF and cytotoxic drugs (17-19). This scenario could be fulfilled when energy of used MF is not enough to cause direct lethal lesions, thus cytotoxic agent must be used to decrease the ‘natural cellular defense mechanisms’. To this day, mechanism(s) of action of various cytotoxic drugs were tested on different cell types in a time- and dose-dependent manner (20-22). Synergic effects induced by chemical- and physical factors, may lead to cell dysfunction and/or cell death.
A multitude of cellular processes are controlled and regulated by Ca2+ ions (23, 24), thus it is not surprising that a leading hypothesis concerning action of exogenous stressors on living cells is related to the disturbance of Ca2+ homeostasis and Ca2+- triggered signalling pathways (16, 25-27). Indeed, numerous studies performed on various cell model systems have revealed relation between cell response to stress factor(s) and changes in the intracellular Ca2+ calcium level. For instance, in (28) authors have found changes in cytosolic and endoplasmic calcium content in U937 cells due to PMC or topoisomerase II inhibitor etoposide (VP16) action, leading to apoptotic cell death. However, the cell response can differ depending on whether the calcium signal develops as a local or global change, cell types, internal condition of the cell system and combination of stressors. The diversity of cell responses (e.g. viabillity versus intracellular calcium level) for the same stressors (e.g. PMC, etoposide and/or DCMF) was well-documented in (29), where authors have compared several primary cultures and cell lines. The Ca2+- mediated changes in various cell types including role of calmodulin were elaborated in (30).
Since the modulation of Ca2+- dependent signalling pathways at different stages is suggested as one of many hypothetical mechanisms underlying cell response to various stress factors, it was verified here for simple experimental model.
Current studies were aimed to elucidate influence of low-frequency magnetic field stimulation on cell viabillity and its effect on expression of calmodulin (CaM) - the most important calcium binding protein, and Hsp70 protein which plays a role of cell stress indicator and belongs to Ca2+- dependent-CaM-binding proteins.
The presented studies were focused not only on bio-effects triggered by exposure to MF alone, but also in combination with chemical stressor (synergic effect). As an inducer of apoptosis, puromycin (PMC) has been chosen, as it plays a multiple role in cell via inhibition of translation in protein synthesis process or via acting as ER Ca2+ leak channel opener and an ER stressor (22, 26).
MATERIALS AND METHODS
Cell culture
U937 cells (human leukemia cell line; ATCC, Rockville, MD) were cultured in RPMI 1640 medium (Gibco-BRL, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Gibco-BRL, USA), 0.2 M L-glutamine (diluted 100 times) and 50 mg/ml gentamicin (diluted 1000 times) (Sigma-Aldrich, Germany) at 37°C in 5% CO2 incubator of 90% humidity. These conditions were found optimal for U937 cells growth, and cells were passaged every four days. Cell viabillity was monitored by trypan blue exclusion method and counted with a haemocytometer. The experiments were performed on viable cells (≥ 98% viabillity) in the logarithmic phase of growth. For experiments with ACDCMF or PEMF, cells were seeded into 6-well- and 96-well culture plates to reach a final density of 2.5 × 105 cells/ml, respectively. In order to place cells in ACDCMF exposure device (2.5 h/day in threefold repetition), cells were transferred from plates to BD Falcon polystyrene test tubes. For PEMF stimulation culture plates were set directly in the apparatus pocket. Cells were harvested by centrifugation and washed with fresh medium after each stimulation. Procedure was verified to obtain the best accuracy and reproducibility. Cell death was induced with 50 µg/ml or 100 µg/ml (final concentration) of puromycin (PMC) added simultaneously with MF (3 × 2.5 h), in 24 h interval between repetitions. Control group of cells, cells after PMC and/or MF treatment were collected for flow cytometry and Western blot analysis.
Magnetic field exposure system
Cells were exposed to homogenous 6 mT static magnetic field (DCMF) combined with perpendicularly oriented sinusoidal component ACMF (35 Hz, 6.5rms mT) or to (45 ± 5) mT, 50 Hz pulsed electromagnetic field (PEMF). The MFs were produced by two separate exposure systems. The details of the applied experimental apparatus are given elsewhere (17, 18). Briefly, the AC coil was fed from a signal generator (Agilent 33120A, Hewlett-Packard, USA) and an amplifier (Magnoter D56, MARP Electronic, Poland). The DC coils arranged in series were fed from stabilized DC power supply (Unitra, Poland). The signals were first calibrated, allowing to collect calibration curves for static- and alternating components of the magnetic field. The homogeneity of the MF in the sample volume was tested and ensured with the accuracy of a few% by using a Gaussmeter (F.W. Bell 6010, Bell Technologies, USA). The MF- exposed samples and control samples were maintained in an incubator at conditions 5% CO2 and 37.0 ± 0.1°C. Control samples were set in area free of MFs produced by exposure devices. The background MF for static-and alternating component was as follows: < (0.05 ± 0.01) mT and < (0.03 ± 0.01)rms mT, respectively. Cell exposure to ACDCMF or PEMF was repeated three times (2.5 h per each day), in 24 h intervals between repetitions.
The choice of parameters for ACDCMF and PEMF was related to the following reasons: their proximity with parameters used in magnetotherapy and with the most abundant power line frequency; low-frequency signalling transduction in human body; and occurrence of non-thermal effects.
Western blot
Cytosolic extracts of cells were isolated using the following procedure: U937 cells 24 hours after last stimulation with PMC and/or MF were collected in ice-cold PBS by centrifugation (1200 rpm for 12 min. at 4°C) and washed in PBS twice. Cell pellet was lysed in 100 µl or 1 ml of cell lysis buffer (Invitrogen, USA) supplemented with 1 mM PMSF (Sigma-Aldrich, Germany) and protease inhibitor cocktail (Sigma-Aldrich, Germany) for 30 minutes, on ice. The samples were vortexed by 10 seconds at 10 minutes intervals. Next step, extracts were transferred to microcentrifuge tubes and centrifuged at 13,000 rpm for 10 minutes at 4°C. Supernatants were collected to further validation for proteins content using spectrophotometric measurement of absorbance at 280 nm (NanoDrop1000) and device for electrophoresis (Invitrogen, USA). Equal amount of lysate for each sample and molecular weight Novex® Sharp Protein Standard (Invitrogen, USA), were separated in 4 – 12% Bis-Tris GelNuPAGE® electrophoresis (40 min), using MES SDS running buffer (Invitrogen, USA). Separated proteins were dry blotted onto nitrocellulose iBlot®gel transfer stacks in iBlot Gel transfer device for 7 minutes. The nitrocellulose membrane was blocked with WesternDotTM blocking buffer (Invitrogen, USA) overnight at 4°C. Blocking procedure was followed with overnight at 4°C exposure to mouse antihuman monoclonal primary antibodies against calmodulin (CaM) (Invitrogen, USA) and against Hsp70 (RD Systems, USA), and beta-actin loading control antibody (Invitrogen, USA) as a control of immunobloting. The antibodies were diluted 1:500, 1:4000 and 1:1000 at final concentration, respectively. According to manufacturer’s procedure detection step was developed with Western blot detection kit (Invitrogen, USA), goat antimouse secondary polyclonal antibody (diluted 1:4000 at final concentration) with alkaline phosphatase and substrat Fast BCIP/NBT (Sigma-Aldrich, Germany). The obtained signal from protein detection was examined under VIS light using Transluminator equipped with PhotoCapt software (Vilber Lourmat, France). Quantitative protein levels were assessed by analysis of the grayscale level with the use of freely available software for image analysis (ImageJ 1.45s, USA). The relative band densities were normalized to appropriate β-actin bands used as reference protein.
Flow cytometry
Percentage of viable/non-viable cells was estimated with a BD FACSCalibur (San Jose, CA) flow cytometer equipped with CellQuest Pro software (BD, San Jose, CA), 24 h after last stimulation with cytotoxic agent and/or MF. Cells were stained with Annexin V-APC labeled (AnV-APC, BD Biosciences, USA) and with 7-amino-actinomycin D (7-AAD; BD Biosciences, USA) according to the manufacturer’s protocol. The details of the applied experimental procedure are given elsewhere (31, 32). AnV-APC – 7-AAD assay allowed to distinguish between viable cells and cells undergoing cell death (damaged cells). Annexin V-APC positive and double-positive (Annexin V-APC and 7-AAD) cells were analyzed as damaged ones. viable population was registered as Annexin V-APC and 7-AAD negative. Unstained cells and cells stained with AnV-APC or 7-AAD alone were used as controls to adjust the fluorescence compensation settings at first. Number of events collected for each sample was ≥ 104 to obtain reliable statistics.
Statistical analysis
Statistical evaluation of the experimental data was performed with U-Mann Whitney and Kruskal-Wallis tests considering P < 0.05 as the minimum level of significance (Statgraphics Centurion XVII). Data are presented as ratios, mean values, and standard deviation (S.D.) of three independent experiments, each performed at least in duplicate.
RESULTS
Changes in U937 cells viabillity and modulation of CaM and Hsp70 levels upon exposure to ACDCMF or PEMF alone, or when combined with 50 µg/ml PMC are shown in Figs. 1 - 3. viabillity coefficient S, was assessed as a ratio of viable cells in the sample exposed to MF and/or PMC to viable cells in the relevant control sample (sham one (C) or only puromycin treated (PMC)). As shown in Fig. 1 magnetic fields alone did not exert significant changes in cell viabillity, regardless of type of the applied MF, which stays in accordance with our previous reports (17, 18). viabillity coefficient S was close to 1, for both types of MF exposure. Application of PMC has reduced percentage of viable cells in the treated sample about twofold in comparison to sham one.
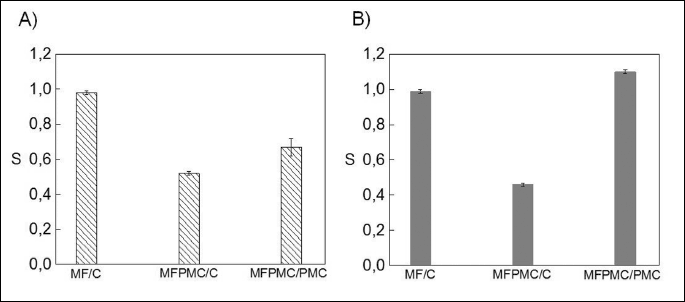
However, simultaneous action of MF and PMC significantly influenced the U937 cell viabillity in type of MF- dependent manner. Applied ACDCMF enhanced lethal changes caused by cytotoxic agent (S was equal to 0.67 ± 0.05), while for PEMF the effect was opposite (S = 1.1 ± 0.01) indicating on its likely protective role against PMC- induced cell death. The applied here lower dose of cytotoxic agent influenced the effectiveness of cell death process in non-linear manner compared to our previous results, while maintaining the same trend of changes in cell viabillity. These results indicate on variety of possible cell responses to applied parameters of MF itself and/or intensity of chemical stressor.
The data in Figs. 2 and 3 have shown, the differences in the amount of CaM and Hsp70 proteins between control-, PMC and/or MF stimulated samples. Observed changes in levels of expression of these proteins correspond with the effectiveness of cell death process, indicated by viabillity coefficient S (Fig. 1A, 1B). Examination of immunoblots developed with anti-CaM antibody have shown, that detected amount of CaM has been increased in PMC-treated samples about 35% compared to control ones. In case of samples simultaneously stimulated with PMC and MF, the level of CaM was further elevated (0.36 ± 0.02) (Fig. 2A) or reduced (0.19 ± 0.01) (Fig. 2B) in dependence on the type of applied MF. There was no significant differences in the expression level of calmodulin between control- and only MF-treated samples for both types of magnetic exposure.
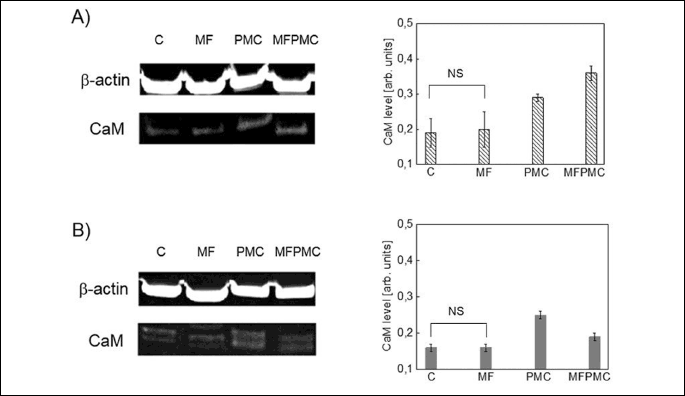
The same tendency of lesions in the detected amount of CaM was preserved for higher dose (100 µg/ml) of PMC and/or ACDCMF, or ten times higher volume (1 ml) of cell lysis buffer (not shown on the graph). In this case the detected amount of CaM was in the range of (0.32 ± 0.01 ¸ 0.38 ± 0.01) for PMC-treated samples, and has been elevated to (0.47 ± 0.01 ¸ 0.51 ± 0.01) for samples simultaneously stimulated with ACDCMF and PMC. The lower values (in the brackets) of CaM content are related to the samples lysed in 1 ml of cell lysis buffer instead of 100 µl. The two different volumes of the reagent were used to verify the reproducibility of the observations in accordance with the applied methodology. Because the tendency of changes was preserved in both cases, the observations are rather attributed to the intracellular mechanisms not to the technical details.
Western blot analysis of Hsp70 protein products in U937 cells in the absence and in the presence of ACDCMF and/or PMC (Fig. 3) has shown, that the Hsp70 level was strictly correlated with the amount of CaM (Fig. 2A) in the relevant samples. Examination of immunoblots developed with anti-Hsp70 antibody have shown, that amount of Hsp70 protein in control- and only MF-treated samples was preserved without significant changes. However, Hsp70 protein content has been increased, in both, PMC-treated sample and in sample simultaneously stimulated with magnetic field and PMC, compared to control one. The highest content of Hsp70 and CaM proteins has been related to the lowest U937 cell viabillity under action of applied ACDCMF and PMC stress factors.
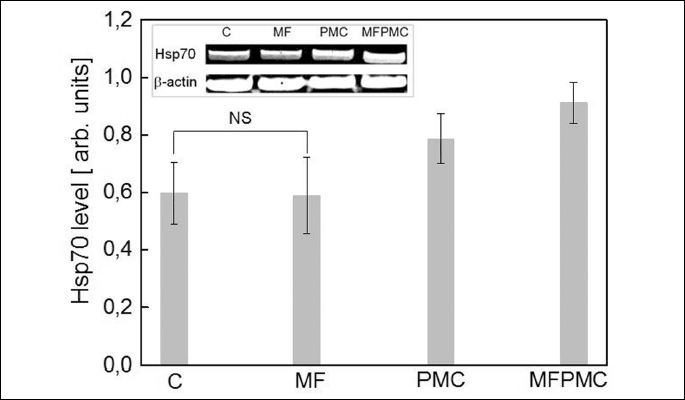
Therefore, calmodulin orchestrates action of various proteins involved in cell death- or cell survival signalling, it is likely, that observed modulations of the levels of CaM and Hsp70 proteins might influence cell functioning. The role of CaM-dependent signalling pathways in maintaining of cell homeostasis was confirmed in (24). The observed relation between intracellular levels of CaM and Hsp70 proteins and cell fate under applied inconvenient conditions, indicates the direction of further studies concerning MFs’ influence on leukemic cells survival.
DISCUSSION
Foregoing findings concerning action of MF alone on cell function are consistent with conclusions presented by some other groups (29, 33, 34). However, there are also reports presenting contrary results (2, 35, 36). Existing controversies reveal dependence between caused bio-effects, cell types and exposure conditions (8). The use of a known cytotoxic agent (PMC) reduced population of viable cells in dose-dependent manner, which stays in agreement with other studies (20, 28). Puromycin is the apoptotic inducer that plays a multiple role in cell via inhibition of translation in protein synthesis process or via acting as ER Ca2+ leak channel opener and an ER stressor (22, 26). This multitasking role of PMC makes it a good candidate for performed studies. Combined action of MF with PMC influenced cell viabillity differently for two types of MF exposure systems, and these effects cannot be explain through MF’s interaction with PMC itself. There is no evidence for changes in PMC or other apoptotic agents transport into the cell due to similar conditions of MF- exposure (magnetic flux density and time of MF’s application) (37-39, 41). Thus, the synergic action of MF and PMC might rather determine internal changes in the studied system, which finally could trigger signal cascades leading to cell survival or cell death. Indeed, it has been shown, that changes in cell functioning might be the result of the modulation of apoptosis-related genes expression, which regulate transduction of multiple signal pathways (39). In the study of Kaszuba-Zwoinska et al. (40), the variations in content of Bcl-2 family gene members and AIF gene under PEMF and/or PMC stimulation in MonoMac6 cells were presented. The authors have observed, that PEMF interaction elevated Bcl-2 mRNA in PMC treated cells, but diminished mRNA of pro-apoptotic Bax gene. Thus, used PEMF fulfilled protective role against of cytotoxic agent- induced cell death. The synergic action of magnetic fields and chemical stressors in cells are also supported by findings of other authors. In the study of Keczan et al. (42), the authors have focused on PEMF and/or tunicamycin- induced endoplasmic stress in three different cell lines. They have observed changes in the level of ER stress- and apoptosis markers in dependence on cell types and applied stressor(s). For human liver carcinoma (HepG2) cells, exposure to PEMF has reduced the elevated level of ER chaperons and apoptosis marker induced by tunicamycin, thus improving cell viabillity.
The similar protective effect for PEMF and PMC was observed in the studied here U937 cells. The discrepancy between results for ACDCMF or PEMF are most likely related to the parameters of used MF itself. It has been indicated before, that measurable bio-effects are dependent not only on magnetic flux density and/or frequency, but also on combinations of static- and alternating MF components, which might determine occurrence of resonant effects (8). Although, the physical mechanism of MF action has not yet been fully elucidated and is still unclear (6, 7, 10, 15, 13, 25). There is still lack of data concerning the linkage: physical factor (MF) - intracellular target of MF - measured bio-effects (cell response). The problem is all the more urgent, that one can notice rising exposure to magnetic fields and observed health problems in populations living close to magnetic fields sources (43, 44). In addition, MF are already used in medicine for treatment of some disorders, despite of lack of well-established theoretical model of their action on cells (45, 46).
However, as the calcium homeostasis is crucial for normal cell functioning, the processes of calcium mobilization and Ca2+- dependent signalling cascades are considered as a very likely ‘cellular targets’ of MF (15, 26, 30). The great role in calcium homeostasis is fulfilled by the heterogeneous group of Ca2+- binding proteins (CBP) capable of decoding very small changes in calcium intracellular concentration. For instance, calmodulin is an ubiquitous, versatile Ca2+- binding protein, which triggers multiple cellular processes including programmed cell death (47-49). To the group of well-characterized calmodulin target proteins belongs protein kinases, calcineurin (CaN), nitric oxide synthase (NOS), inositol 3-kinase, Ca2+-ATPase, IP3 receptors (IP3R), cytoskeleton proteins, receptors and ion channels (e.g. SOC, endoplasmic reticulum-translocon (TLC)), heat shock proteins (50-57). The concentration of CaM in resting cells is kept low, but could be down- or upregulated in accordance with modulation of intracellular Ca2+ level as a response to chemical carcinogens, ionizing and non-ionizing radiation or phase of the cell cycle (58-60).
Thus, we have examined PMC and MFs (as the external stressors) on production of CaM and Hsp70 proteins in the model cell line. The Hsp70 protein is one of Ca2+-dependent-CaM-binding proteins crucial for cell survival. The modulation of the level of heat shock proteins can affect apoptosis process differently in various cell types. For instance, in the study of Leja-Szpak et al. (61) and related one (62), it was shown that kynuramines and melatonin stimulate production of heat shock proteins in pancreatic carcinoma cells (PANC-1), which resulted in the interruption of the intrinisic apoptotic pathway. In the study of Galea-Lauri et al. (63) for U937 cells, it has been verfied that heat shock proteins are implicated in the regulation of forms of cell injury that lead to programmed cell death. An excess of Hsp90 was associated with increased apoptosis when the cells were treated with a combination of TNF-alpha and cycloheximide, and what is more, new synthesis of Hsp72 did not protect against apoptosis. Chant et al. (64) have observed that susceptibility of acute myeloid leukaemia (AML) cells to apoptosis correlated with intracellular expression of Hsp70 and was associated with the presence of p53 and low levels of expression of Bcl-2.
Herein, the level of CaM and Hsp70 proteins was changed for particular combination of external stress factors, as well as cell viabillity. It indicates that CaM and CaM-dependent signalling molecules might posses a role in intracellular mechanism(s) responsible for cell response to used stimulation. Due to the fact that expression of CaM/Ca2+-dependent-CaM-binding proteins and cell survival are modulated with the changes in intracellular Ca2+ ions concentration, it is likely, that applied stress conditions have triggered calcium ions fluctuations in examined cells. Indeed, the increase in the Ca2+ leak through TLC under PMC treatment, can lead to cytosolic calcium ions elevation and to initiation of a number of cellular responses, for instance, upregulation of CaM (58, 59) and cell death (22, 28, 59, 65-67). Thus, our results seem to be consistent with findings indicating that changes in intracellular Ca2+ concentration can entail changes in CaM level, in consequence modulating Ca2+-CaM- dependent signalling pathways and cell functioning. For instance, according with the study of Dowd et al. (68), calmodulin gene expression was upregulated in WEHI7.2 lymphocytes undergoing glucocorticoid-mediated apoptosis. Authors have reported induction of CaM gene expression by dexamethasone, and other data supporting the putative role of CaM in apoptosis. The authors also claimed that CaM may play several key roles in cell death process, what should be intensively verified. In the study of Yu et al. (69), the mechanism of pancreatic beta cell loss in transgenic mice with elevated levels of calmodulin was presented. The authors speculate that overexpression of calmodulin sensitizes the beta cells to commit apoptosis via Ca2+- dependent activation of nitric oxide synthase, which suggests that the cell death process is Ca2+/CaM- dependent. Devireddy and Green (70) studies on IL-2-dependent T cells have revealed the role of a pro-apoptotic factor, RC3, whose ability to induce programmed cell death is related to its role in modulating intracellular CaM and/or Ca2+ levels. In the study of Pilla et al. (30), Ca2+ binding to CaM driven by increase in cytosolic Ca2+ concentration in response to chemical and/or physical insults (e.g. MF), activates the synthesis of the signalling molecule NO from L-arginine, which further triggers development of signalling cascades crucial for cell survival or death.
Also the obtained here relation between enhanced cell death in cells under stress conditions and the intracellular level of heat shock protein (Hsp70), stays in agreement with other data for leukemic cells. The observed in this study changes in both proteins (CaM and Hsp70) content and in U937 cell viabillity upon exposure to magnetic fields acting alone or when combined with cytotoxic agent, may be useful to indicate intracellular processes, which are likely activated by applied MFs.
However, in contrast to the general paradigm, assuming that rise of cytosolic Ca2+ level evoked by external stressor(s) is characteristic for cells committed to apoptosis, other studies have reported that elevation of calcium ions in cytosol can exert either a pro- or an anti-apoptotic effect, and also CaM may play a double role (71-73). Both types of effects can occur in dependence on cell types, conditions of cell system, dynamics and spatial range of calcium signal itself. For instance, Ca2+-calmodulin-dependent activity of calcineurin, was shown to promote apoptosis through enhancing binding of dephosphorylated pro-apoptotic molecule Bad with Bcl-xL protein (74). In turn, PKC isoforms can play double role (75).
In this context, the detailed explanation of our data needs further studies concerning various intensities of MF, influence of calcium channel blockers, and several downstream enzymes under the control of calmodulin. Also cooperative involvement of other mechanisms like stress proteins-mediated signalling pathways cannot be excluded. Thus far, we can assume, that observed differences in cell viabillity might be related to modulation of CaM and Hsp70 proteins concentrations as a cell response to varied stress conditions, but the detailed bio-mechanism requires more research.
Conclusions
The exposure to MFs combined with PMC affects U937 cell viabillity and level of both CaM and Hsp70 proteins. Observed changes are dependent on parameters of applied MF, and are not consequence of direct interaction between the field and the apoptosis inducer (PMC). From our data, it could be hypothesized that occurred modulation of cell viabillity might be result of Ca2+- dependent signalling perturbation, as calmodulin acts as the primary intracellular calcium sensor. Calmodulin regulates numerous proteins in response to changes in the intracellular calcium content (e.g. Hsp70), thus triggering multitude of cell signalling cascades which eventually might lead to cell survival or to cell death. Nonetheless, the detailed mechanism underlies the interaction between used MFs and model cell line is still not recognized and needs further studies. Understanding the intracellular bio-effects evoked by MFs act in synergy with chemical agents is crucial to develop new strategies for noninvasive cancer therapy or for protection against rising exposure to these stress factors.
Abbreviations: 7-AAD, 7-amino-actinomycin D; ACMF, alternating magnetic field; AML, acute myeloid leukaemia; AnV-APC, annexin V allophycocyanin labeled; BCIP, 5-bromo-4-chloro-3-indolyl phosphate; [Ca2+]cyt, cytosolic Ca2+ concentration; CaM, calmodulin; CaN, calcineurin; CBP, calcium binding proteins; DCMF, static magnetic field; ER, endoplasmic reticulum; f, frequency; HepG2, human liver carcinoma; IL-2, interleukin-2; IP3, inositol 1,4,5-trisphosphate; IP3R, inositol 1,4,5-trisphosphate receptors; NBT, nitro blue tetrazolium chloride; NOS, nitric oxide synthase; PANC-1, pancreatic carcinoma cells; PEMF, pulsed electromagnetic field; PKC, protein kinase C; PMC, puromycin; rms, root mean square; S, cell viabillity coefficient; SOC, store-gated channels; TLC, translocon; U937, human leukemia cell line; VP16, topoisomerase II inhibitor etoposide; β-actin, actin isoform.
Acknowledgments: The authors thank Dr. Paulina Chorobik for her generous help with NanoDrop spectrophotometer measurements and her valuable discussions. This work was supported by grants no. K/ZDS/006424 and K/ZDS/001542.
Conflict of interests: None declared.
REFERENCES
- Bowman JD, Thomas DC, London SJ, Peters JM. Hypothesis: The risk of childhood leukemia is related to combinations of power-frequency and static magnetic fields. Bioelectromagnetics 1995; 16: 48-59.
- Huang L, Dong L, Chen Y, Qi H, Xiao D. Effects of sinusoidal magnetic field observed on cell proliferation, ion concentration, and osmolarity in two human cancer cell lines. Electromagn Biol Med 2006; 25: 113-126.
- Zmyslony M, Rajkowska E, Mamrot P, Politanski P, Jajte J. The effect of weak 50 Hz magnetic fields on the number of free oxygen radicals in rat lymphocytes in vitro. Bioelectromagnetics 2004; 25: 607-612.
- Lagroye I, Percherancier Y, Juutilainen J, Poulletier de Gannes F, Veyret B. ELF magnetic fields: animal studies, mechanisms of action. Prog Biophys Mol Biol 2011; 107: 369-373.
- Albuquerque WW, Costa RM, Fernandes T de S, Porto AL. Evidences of the static magnetic field influence on cellular systems. Prog Biophys Mol Biol 2016; 121: 16-28.
- Eveson RW, Timmel CR, Blocklehurst B, Hore PJ, McLauchlan KA. The effects of weak magnetic fields on radical recombination reactions in micelles. Int J Radiat Biol 2000; 76: 1509-1522.
- Gartzke J, Lange K. Cellular target of weak magnetic fields: ionic conduction along actin filaments of microvilli. Am J Cell Physiol 2002; 283: C1333-C1346.
- Stavroulakis P. Biological Effects of Electromagnetic Fields. Berlin, Heidelberg, Springer-Verlag 2003.
- Mannerling AC, Simko M, Mild KH, Mattsson MO. Effects of 50-Hz magnetic field exposure on superoxide radical anion formation and HSP70 induction in human K562 cells. Radiat Environ Biophys 2010; 49: 731-741.
- Mattsson M, Simko M. Grouping of experimental conditions as an approach to evaluate effects of extremely low-frequency magnetic fields on oxidative response in in vitro studies. Front Publ Health 2014, 2: 1-11.
- Tang R, Xu Y, Ma F, et al. Extremely low frequency magnetic fields regulate differentiation of regulatory T cells: potential role for ROS-mediated inhibition on AKT Bioelectromagnetics 2016; 37: 89-98.
- Ghodbane S, Lahbib A, Sakly M, Abdelmelek H. Bioeffects of static magnetic fields: oxidative stress, genotoxic effects, and cancer studies. Biomed Res Int 2013; 2013: 602987. doi: 10.1155/2013/602987
- Panagopoulos DJ, Karabarbounis A, Margaritis LH. Mechanism for action of electromagnetic fields on cells. Biochem Biophys Res Commun 2002; 298: 95-102.
- Ross CL, Siriwardane M, Almeida-Porada G, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res 2015; 15: 96-108.
- Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med 2013; 17: 958-965.
- Teodori L, Goehde W, Valente MG, et al. Static magnetic fields affect calcium fluxes and inhibit stress-induced apoptosis in human glioblastoma cells. Cytometry 2002; 49: 143-149.
- Kaszuba-Zwoinska J, Wojcik K, Bereta M, et al. Pulsating electromagnetic field stimulation prevents cell death of puromycin treated U937 cell line. J Physiol Pharmacol 2010; 61: 201-205.
- Wojcik-Piotrowicz K, Kaszuba-Zwoinska J, Rokita E, et al. Influence of static and alternating magnetic fields on U937 cell viabillity. Folia Med Cracov 2014; 4: 21-33.
- Zmyslony M, Palus J, Jajte J, Dziubaltowska E, Rajkowska E. DNA damage in rat lymphocytes treated in vitro with iron cations and exposed to 7 mT magnetic fields (static or 50 Hz). Mutat Res 2000; 453: 89-96.
- Sanwal V, Pandya M, Bhaskaran, M. et al. Puromycin aminonucleoside induces glomerular epithelial cell apoptosis. Exp Mol Pathol 2001; 70: 54-64.
- Skierski J. Cytotoxic assays of chemical substances. [in Polish]. Postepy Biol Komorki 2008; 35: 147-163.
- Hammadi M, Oulidi A, Gackiere F, et al. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J 2013; 27: 1600-1609.
- Bootman MD, Collins TJ, Peppiatt CM, et al. Calcium signaling-an overview. Cell Dev Biol 2001; 12: 3-10.
- Islam Md Shahidul: Calcium Signaling. Springer Netherlands, 2012.
- Liboff AR, Cherng S, Jenrow KA, Bull A. Calmodulin-dependent cyclic nucleotide phosphodiesterase activity is altered by 20 µT magnetostatic fields. Bioelectromagnetics 2003; 24: 32-38.
- Wojcik-Piotrowicz K, Kaszuba-Zwoinska J, Rokita E, Thor P. Cell viabillity modulation through changes of Ca2+-dependent signalling pathways. Prog Biophys Mol Biol 2016; 121: 45-53.
- Golbach LA, Philippi JG, Cuppen JJ, Savelkoul HF, Verburg-van Kemenade, BM. Calcium signalling in human neutrophil cell lines is not affected by low-frequency electromagnetic fields. Bioelectromagnetics 2015; 36: 430-443.
- Cerella C, Mearelli C, Coppola S, et al. Sequential phases of Ca2+ alterations in pre-apoptotic cells. Apoptosis 2007; 12: 2207-2219.
- Tenuzzo B, Chionna A, Panzarini E, et al. Biological effects of 6 mT static magnetic fields: a comparative study in different cell types. Bioelectromagnetics 2006; 27: 560-577.
- Pilla AA. Electromagnetic fields instantaneously modulate nitric oxide signaling in challenged biological systems. Biochem Biophys Res Commun 2012; 426: 330-333.
- Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 1992; 13: 204-208.
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods 1995; 184: 39-51.
- Capri M, Mesirca P, Remondini D, et al. 50 Hz sinusoidal magnetic fields do not affect human lymphocyte activation and proliferation in vitro. Phys Biol 2004; 1: 211-219.
- Ikeda K, Shinmura Y, Mizoe H, et al. No effects of extremely low frequency magnetic fields found on cytotoxic activities and cytokine production of human peripheral blood mononuclear cells in vitro. Bioelectromagnetics 2003; 24: 21-31.
- Hisamitsu T, Narita K, Kasahara T, Seto A, Yu Y, Asano K. Induction of apoptosis in human leukemic cells by magnetic fields. Jpn J Physiol 1997; 47: 307-310.
- Tofani S, Barone D, Cintorino M, et al. Static and ELF magnetic fields induce tumor growth inhibition and apoptosis. Bioelectromagnetics 2001; 22: 419-428.
- Fanelli C, Coppola S, Barone R, et al. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J 1999; 13: 95-102.
- Liu Y, Qi H, Sun RG, Chen WF. An investigation into the combined effect of static magnetic fields and different anticancer drugs on K562 cell membranes. Tumori 2011; 97: 386-392.
- Tenuzzo B, Vergallo C, Dini L. Effect of 6 mT static magnetic field on the bcl-2, bax, p53 and hsp70 expression in freshly isolated and in vitro aged human lymphocytes. Tissue Cell 2009; 41: 169-179.
- Kaszuba-Zwoinska J, Chorobik P, Kuszczak K, Zaraska W, Thor JP. Pulsed electromagnetic field affects intrinsic and endoplasmatic reticulum apoptosis induction pathways in MonoMac6 cell line culture. J Physiol Pharmacol 2012; 63: 537-545.
- Marcantonio P, Del Re B, Franceschini A, et al. Synergic effect of retinoic acid and extremely low frequency magnetic field exposure on human neuroblastoma cell line BE(2)C. Bioelectromagnetics 2010; 31: 425-433.
- Keczan E, Keri G, Banhegyi G, Stiller I. Effect of pulsed electromagnetic fields on endoplasmic reticulum stress. J Physiol Pharmacol 2016; 67: 769-775.
- Schuz, J, Dasenbrock C, Ravazzani P, et al. Extremely low-frequency magnetic fields and the risk of childhood leukemia: a risk assessment by the ARIMMORA consortium. Bioelectromagnetics 2016; 37: 183-189.
- Terzi M, Ozberk B, Deniz OG, Kaplan S. The role of electromagnetic fields in neurological disorders. J Chem Neuroanat 2016; 75: 77-84.
- Perera T, George MS, Grammer G, Janicak FG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul 2016; 9: 336-346.
- Kasperski J, Rosak P, Roj R, et al. The influence of low-frequency variable magnetic fields in reducing pain experience after dental implant treatment. Acta Bioeng Biomech 2015; 17: 97-105.
- Berchtold MW, Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim Biophys Acta 2014; 1843: 398-435.
- Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol 2000; 10: 322-328.
- Persechini A, Stemmer PM. Calmodulin is a limiting factor in the cell. Trends Cardiovasc Med 2002; 12: 32-37.
- Giurisato E, Gamberucci A, Ulivieri C, et al. The KSR2-calcineurin complex regulates STIM1-ORAI1 dynamics and store-operated calcium entry (SOCE). Mol Biol Cell 2014; 25: 1769-1781.
- Lee A, Wong ST, Gallagher D, et al. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 1999; 399: 155-159.
- Missiaen L, Parys JB, Weidema AF, et al. The bell-shaped Ca2+ dependence of the inositol 1,4,5-triphosphate-induced Ca2+ release is modulated by Ca2+/calmodulin. J Biol Chem 1999; 247: 13748-13751.
- Toutenhood SL, Strehler EE. The calmodulin multigene family as a unique case of genetic redundancy: multiple levels of regulation to provide spatial and temporal control of calmodulin pools? Cell Calcium 2000; 28: 83-96.
- Zhulke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 1999; 399: 159-162.
- Lang S, Erdmann F, Jung M, Wagner R, Cavalie A, Zimmermann R. Sec61 complexes form ubiquitous ER Ca2+ leak channels. Channels 2011; 5: 228-235.
- Huang M, Wei JN, Peng WX, et al. The association of CaM and Hsp70 regulates S-phase arrest and apoptosis in a spatially and temporally dependent manner in human cells. Cell Stress Chaperones 2009; 14: 343-353.
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol 2003; 5: 1051-1061.
- Rasmusen CD, Means AR. Effects of changes in calmodulin levels on cell proliferation. Environ Health Perspect 1990; 84: 31-34.
- McGinnis KM, Sharaiat-Madar Z, Gnegy ME. Cytosolic calmodulin is increased in SK-N-SH human neuroblastoma cells due to release of calcium from intracellular stores. J Neurochem 1998; 70: 139-146.
- Baran I. Calcium and cell cycle progression: possible effects of external perturbations on cell proliferation. Biophys J 1996; 70: 1198-1213.
- Leja-Szpak A, Pierzchalski P, Goralska M, et al. Kynuramines induce overexpression of heat shock proteins in pancreatic cancer cells via-5-hydroxytryptamine and MT1/MT2 receptors. J Physiol Pharmacol 2015; 66: 711-718.
- Leja-Szpak A, Jaworek J, Pierzchalski P, Reiter RJ. Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J Pineal Res 2010; 49: 248-255.
- Galea-Lauri J, Richardson AJ, Latchman DS, Katz DR. Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-alpha and cycloheximide: a possible role in immunopathology. J Immunol 1996; 157: 4109-4118.
- Chant ID, Rose PE, Morris AG. Susceptibility of AML cells to in vitro apoptosis correlates with heat shock protein 70 (hsp70) expression. Br J Haematol 1996; 93: 898-902.
- Bian X, Hughes FM, Huang Y, Cidlowski JA, Putney JW. Roles of cytoplasmic Ca2+ and intracellular Ca2+ stores in induction and suppression of apoptosis in S49 cells. Am J Physiol 1997; 272: C1241-C1249.
- Pinton P, Ferrari D, Di Virgilio F, Pozzan T, Rizzuto R. Molecular machinery and signalling events in apoptosis. Drug Dev Res 2001; 52: 558-570.
- McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys 1989; 269: 365-370.
- Dowd DR, MacDonald PN, Komm BS, Haussler MR, Miesfeld R. Evidence for early induction of calmodulin gene expression in lymphocytes undergoing glucocorticoid-mediated apoptosis. J Biol Chem 1991; 266: 18423-18426.
- Yu W, Niwa T, Miura Y, et al. Calmodulin overexpression causes Ca2+-dependent apoptosis of pancreatic β cells, which can be prevented by inhibition of nitric oxide synthase. Lab Invest 2002; 82: 1229-1239.
- Devireddy LR, Green MR. Transcriptional program of apoptosis induction following interleukin 2 deprivation: identification of RC3, a calcium/calmodulin binding protein, as a novel proapoptotic factor. Mol Cell Biol 2003; 23: 4532-4541.
- Chionna A, Dwikat M, Panzarini E, et al. Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur J Histochem 2003; 47: 299-308.
- Wood DE, Thomas A, Devi LA, et al. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 1998; 17: 1069-1078.
- Schmalzigaug R, Ye Q, Berchtold MW. Calmodulin protects cells from death under normal growth conditions and mitogenic starvation but plays a mediating role in cell death upon B-cell receptor stimulation. Immunology 2001; 103: 332-342.
- Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999; 284: 339-343.
- Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase C alpha in Bcl-2 phosphorylation and suppression of apoptosis. J Biol Chem 1998; 273: 25436-25442.
A c c e p t e d : July 25, 2017