The development of essential hypertension is accompanied by changes in the rheological properties of blood, particularly by increased red blood cell aggregation leading to further pathological complications (1, 2). Not long ago we demonstrated that rheological properties of red blood cells (RBC) were strongly affected by secretory function of endothelium (3). The effect of endothelium is achieved through the release of a variety of autocrine and paracrine substances, with endothelium-dependent prostacyclin (PGI2) and nitric oxide (NO), the best characterised and probably the most important among them (4, 5, 6). Since the secretory function of vascular endothelium appears to be disabled in hypertension (5, 7-9), hypertension-induced changes in RBC aggregation may result from accompanying dysfunction of endothelium.
In the treatment of hypertension angiotensin converting enzyme inhibitors (ACEI) are drugs of the first choice. Interestingly, ACEI seem to extend their pharmacological activity associated with suppression of angiotensin II beyond lowering of arterial blood pressure. It was found that after administration of ACEI, an increase might occur in the generation of bradykinin followed by augmented B2 receptor-mediated release of endothelial mediators (PGI2, NO) (10, 11). Such approach raises crucial questions: whether ACEI may modify RBC rheology during antihypertensive therapy and whether this phenomenon may depend on endothelial secretogogues. If so, lowering of blood pressure associated with improvement of rheological properties of RBC by ACEI may become a new principle of antihypertensive therapy.
The Institutional Review Committee approved the protocol for animal experiments.
Animal model of spontaneous hypertension
We decided on using commercially available inbred strain of spontaneously hypertensive male rats (SHR) aged 19-21 weeks originating from the Kyoto University, Japan as it is the most commonly studied animal model of arterial hypertension. In this model increased blood pressure was reported to correlate with impairment of endothelium dependent vasodilatation, just as in many humans with essential hypertension (8). The most appropriate control strain for SHR experimental model is age matched normotensive Wistar-Kyoto (WKY) rats to which SHR are genetically related.
Experiments in vivo
Experimental animals were divided into 14 experimental groups, each consisting of 8 hypertensive (SHR) and 8 control rats (WKY). Each group was injected (i.p.) daily, for 8 days, with one of the following: placebo or quinapril (100 µg/kg, a generous gift from Pfizer), or aspirin (1 or 50 mg/kg, Sigma), or indomethacin (20 mg/kg, Sigma), or NGnitro-L-arginine methyl ester (L-NAME - nitric oxide synthase inhibitor, Sigma) at a dose of 10 mg/kg, or bradykinin (Aldrich) at a dose of 30 µg/kg, or icatibant (B2 receptor antagonist, Hoechst Marion Roussel) at a dose of 100 µg/kg, or with combination of quinapril and aspirin at two doses, quinapril and indomethacin, quinapril and L-NAME, quinapril and icatibant, or bradykinin and icatibant.
On the 8-th day of treatment animals were anaesthetised with thiopental (50 mg/kg i.p., Biochemic GmbH), heparinized (800 U/kg i.v., Polfa) and 0.5 ml samples of blood were taken from the right carotid artery for measurement of RBC aggregability.
Experiments in vitro
Control WKY rats were anaesthetized with thiopental (50 mg/kg i.p.). Blood (8 ml) was collected from the right carotid artery into heparin (25 U/ml) solution and then erythrocytes were isolated using the method described by Jubelin and Giennan (12). Red blood cells were separated from white blood cells on a Ficoll packed column, washed three times in PBS solution (Imperia Lab. UK) and enriched with albumin (Sigma) and glucose (POCH S.A.) Cells were suspended in solution of PBS with 3% dextran for 30 min and again rinsed to remove dextran. Finally, cells were resuspended in PBS solution. Haematocrit was adjusted to the value of whole blood haematocrit. Microscopic examination of prepared suspension revealed neither white blood cells nor platelets. Samples of suspension of erythrocytes in PBS were incubated at 37°C for 30 min with placebo (saline) or with one of the following: quinapril (30 µg/ml), quinaprilat - active metabolite of quinapril (30 µg/ml, Pfizer), aspirin (3 mM), indomethacin (3 mM) or L-NAME (10 µM).
Measurement of red blood cell aggregability
Aggregability was measured with the use of a fully automatic erythrocyte aggregometer type MA-1 (Myrenne gmbh, Germany) equipped with infrared photometric head for readings of density of RBC suspension. In such type of instrument as this one, test samples (whole blood or isolated erythrocytes in suspension) are applied at a volume of 20 µl into the measuring chamber where erythrocytes are subjected for 20 seconds to high shear rate (600/sec) in aim to disperse all their aggregates. Aggregation of red blood cells is induced by lowering of shear rate from high during hydrodynamic aggregate dispersion to zero (standstill). Mean extent of aggregation (MEA) is measured automatically for 10 seconds and displayed in arbitrary units of MEA as a four-digit-number.
Measurement of arterial blood pressure
Arterial blood pressure was measured indirectly on rat tail artery with the use of a commercial rat sphygmograph.
Statistical analysis
All results were expressed as arithmetical means ± SD of n numbers of experiments and analyzed by Student`s T test for paired means to determine the significance of the response; "p" values of less than 0.05 were considered as statistically significant.
RBC aggregability, experiments ex vivo
In accordance with our expectations aggregabilty of RBC in hypertensive rats was significantly higher than in control WKY animals. In arbitrary units of MEA, aggregabilty of WKY and SHR was 6.0±1 and 9.2±2 (n=6, p<0,05), respectively (Fig. 1). Angiotensin converting enzyme inhibitor, quinapril, at a dose of 100 µg/kg administered i.p. for 8 days improved RBC aggregabilty in normotensive rats (improvement from 6.0±1 to 4.0±1, n=6, p<0.05) but surprisingly not in SHR animals (7.6±3 vs. 9.2±2 in control, the difference not significant for n=6) (Fig. 1). Beneficial effect of quinapril on RBC aggregation observed in normotensive animals did not occur when this drug was injected in combination with aspirin (1 or 50 mg/kg) or with indomethacin (20 mg/kg) or with L-NAME (10 mg/kg) (Fig. 1). In such conditions administration of quinapril in combination with the above drugs induced even worsening (p<0.05) of RBC aggregability (MEA units were increased up to 8.2±2 and 15.3±2 in combination with aspirin at two doses respectively, up to 10.8±3 with indomethacin, and 9.3±4 with L-NAME) (Fig. 1). However, much the same damaging effects on RBC aggregability was observed when aspirin (MEA=8.1±4 for a dose of 1 mg/kg and 14.0±4 for 50 mg/kg), indomethacin (MEA=12.2±2) or L-NAME (MEA=11.1±3) were each administered into normotensive animals without quinapril (Fig. 2).
 |
| Fig.
1. The effect of quinapril (Q) at a dose of 100 µg/kg administered
i.p. in vivo for 8 days alone or in combination with aspirin at
a dose of 1 mg/kg (ASA-1) or 50 mg/kg (ASA-50) or with indomethacin at
a dose of 20 mg/kg (IND) or with NGnitro-L-arginine methyl ester (L-NAME)
at a dose of 30 µg/kg on the aggregability of red blood cells in normotensive
(A) or spontaneously hypertensive rats (B). WKY - control aggregability of normotensive animals, SHR - control aggregability of hypertensive animals. All results expressed as arithmetical means ± SD for n=8; * - p < 0.05, ** - p < 0.01, n.s. - not significant as compared to corresponding controls. |
In contrast with normotensive rats, aggregability of RBC in spontaneously hypertensive rats was not affected either by quinapril or by indomethacin and by L-NAME, given separately or in combination (Fig. 1 and 2). The only compound that significantly worsened RBC aggregability in SHR was aspirin (up to 21.0±6 at a dose of 50 mg/kg) (Fig. 2). This effect of aspirin was not dose-dependent (Fig. 2) and it seemed to be rather less pronounced when aspirin was administered in combination with quinapril (Fig. 1) (MEA= 21.0±6 for aspirin at a dose of 50 mg/kg versus aspirin+quinapril MEA=17.2±4) than when it was given separately (Fig. 2).
 |
| Fig. 2.
The effect of aspirin at a dose of 1 mg/kg (ASA-1) and 50 mg/kg (ASA-50),
indomethacin at a dose of 20 mg/kg (IND) or NGnitro-L-arginine
methyl ester (L-NAME) at a dose of 30 µg/kg, each administered i.p. in
vivo for 8 days on the aggregability of red blood cells in normotensive
(A) or spontaneously hypertensive rats (B). WKY - control aggregability of normotensive animals, SHR - control aggregability of hypertensive animals. All results expressed as arithmetical means ± SD for n=8; * - p < 0.05, ** - p < 0.01, n.s. - not significant as compared to corresponding controls. |
Quinapril-induced improvement of RBC aggregabilty in normotensive rats (but not in SHR) was completely abolished by simultaneous administration of B2 receptor anatagonist, icatibant, which per se had no effect on aggregability (Fig. 3). Beneficial effect of quinapril on RBC aggregability in normotensive animals was also mimicked by bradykinin (MEA=3.9±2 vs. 4.0±1 for quinapril) injected for 8 days and this phenomenon was successfully reversed by icatibant (Fig.4).
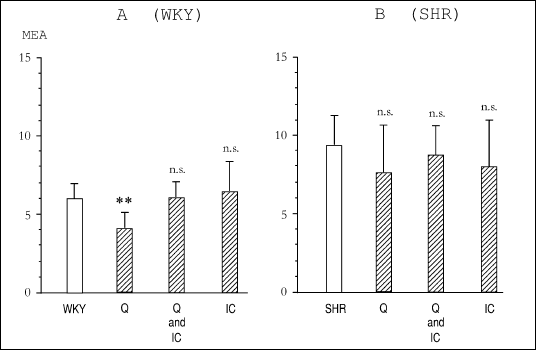 |
| Fig. 3.
The effect of quinapril (Q) at a dose of 100 µg/kg or icatibant (IC) at
a dose of 100 µg/kg, each administered i.p. in vivo for 8 days
separately or in combination on the aggregability of red blood cells in
normotensive (A) or spontaneously hypertensive rats (B). WKY - control aggregability of normotenisive animals, SHR - control aggregability of hypertensive animals. All results expressed as arithmetical means ± SD for n=8; * - p < 0.05, ** - p < 0.01, n.s. - not significant as compared to corresponding controls. |
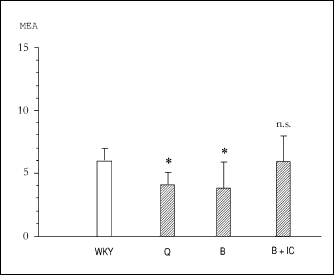 |
Fig. 4. The effect of quinapril
(Q) at a dose of 100 µg/kg or bradykinin (B) at a dose of 30 µg/kg, each
administered i.p. in vivo for 8 days on the aggregability of red
blood cells in normotensive rats. In B+IC experimental group bradykinin
was injected in combination with icatibant (100 µg/kg). WKY - control aggregability of normotenisive animals. All results expressed as arithmetical means ± SD for n=8; * - p < 0.05, ** - p < 0.01, n.s. - not significant as compared to corresponding control. |
Experiments in vitro
Aggregability of isolated red blood cells from normotensive animals was not affected by previous incubation (30 min at 37°C) with quinapril, indomethacin, L-NAME (Fig. 5), and quinaprilat (active metabolite of quinapril; results not shown). Only aspirin at a concentration of 3 mM significantly increased RBC aggregability (up to 10.1±2, n=6, p<0.001) as compared to placebo (Fig. 5).
 |
Fig. 5. The effect of quinapril
(Q), aspirin (ASA), indomethacin (IND) or L-NAME on aggregability of erythrocytes
from normotensive rats in vitro. All results expressed as arithmetical means ± SD for n=6; * - p < 0.05, ** - p < 0.01, n.s. - not significant as compared to corresponding control. |
Arterial blood pressure
In normotensive rats (WKY) mean arterial blood pressure (BP) stayed at the normal level from 115 to 125 mmHg (n=50). In spontaneous hypertension group BP was increased up to 195-225 mmHg (n=50). The treatment with quinapril for 8 days did not affect BP in normotenisive animals but in SHR mean arterial blood pressure was normalized and equaled 110-150 mmHg (n=15). Normalization of BP by quinapril in SHR group was not influenced by aspirin, indomethacin, L-NAME, bradykinin or icatibant. Bradykinin, however, in about 5 min time from its each i.p. injection, had induced a fall in BP down to 110-135 (n=6) which then lasted for about 25 min. This phenomenon was slightly more pronounced in WKY than in SHR and it did not matter whether bradykinin was administered alone or in combination with quinapril. Aspirin at two doses (1 and 50 mg/kg), indomethacin and L-NAME when administered for 8 days did not change BP per se, both in WKY (115-125 mmHg) and in SHR (185-200 mmHg).
Essential hypertension, one of the most important risk factors in cardiovascular diseases, is linked both with dysfunction of vascular endothelium and increased aggreability of erythrocytes (1, 2, 8, 13). However, patophysiological mechanism of the correlation between the function of vascular endothelium and RBC rheology in hypertension remains unclear. Not long time ago we found that rheological properties of RBC in vivo were regulated endogenously by balanced generation of two endothelial secretogogues: prostacyclin and nitric oxide (3, 14). Although this phenomenon applies to normally functioning endothelium, it is reasonable to speculate that in hypertension the interaction between red blood cells and vascular endothelium may be impaired and antihypertensive therapy may reverse it.
Regarding the first of the above speculations, here we confirm that in hypertensive rats RBC aggregability is augmented. But then we demonstrate that in experimental hypertension the antihypertensive agent, quinapril (ACEI), beside being very effective in normalizing of arterial blood pressure, does not significantly inhibit RBC aggregability. On the other hand, quinapril very efficiently improves RBC aggregability in normotensive rats having no effect on mean arterial blood pressure. Is there any rationale for such results? Keeping in mind that impaired secretory function of endothelium is a general feature of hypertension and that RBC rheology remains under predominant control of endothelium-derived secretogogues, mainly NO and prostacyclin (14-17), we are putting forward an explanatory hypothesis that antihypertensive therapy has nothing to do with improvement of RBC rheology unless and until secretory function of endothelium has been impaired. Moreover, it does not matter whether endothelial dysfunction results from or accompanies hypertension or it is induced, for example, by pharmacological inhibition of endothelial function. Indeed, in our experiments, contrary to the observations in hypertensive rats, quinapril significantly improves RBC aggregability of control animals in which vascular endothelium is supposed to work efficiently but this effect is completely abolished by simultaneous administration of cyclooxygenase and/or nitric oxide synthase inhibitors. In favour of the indispensable role of endothelial secretion in endogenous regulation of RBC aggregabilty we were able to deliver two additional proofs. First was that quinapril did not influence rheological properties of erythrocytes in vitro when they were isolated from blood vessels and secondly, pharmacological inhibition of endothelial secretion of prostacyclin and NO augmented RBC aggregability in normotensive rats in vivo almost to the same extent that occurred in experimental hypertension. It must also be added that in comparison with normotensive rats the secretory function of endothelium in SHR was reduced approximately 75-86%, as assessed by us with the use of a standard organ-bath myography from the attenuation of endothelium-dependent vasodilatations of rat aorta rings to acetylcholine (4 experiments, results not shown). Accordingly, it seems evident that the lack of the effect of quinapril on RBC aggergability in hypertension may result directly from dysfunction of vascular endothelium. Under normal conditions when the release of endothelial mediators is sustained quinapril works very efficiently. Obviously then, a different question is what is the mechanism by which this antihypertensive drug is able to improve blood rheology in normotensive animals.
Quinapril is an antihypertensive agent which belongs to the family of tissue form of angiotensin converting enzyme inhibitors (ACEI) exerting their pharmacological activity via suppression of the generation of angiotensin II beyond lowering arterial blood pressure. Our group hypothesized that the administration of quinapril is likely to induce in rats in vivo a significant increase of the generation of bradykinin followed by augmented B2 receptor-mediated release of endothelial mediators, including prostacyclin and nitric oxide (10, 18). The potency of ACEI to change endothelial function through accumulation of endogenous bradykinin was only a few orders of magnitude lower than that of exogenous bradykinin (10). In the present paper we demonstrate that quinapril-induced improvement of RBC aggregability in normotensive rats was completely abolished by simultaneous administration of B2 receptor anatagonist, icatibant, which per se had no effect on RBC aggregability. Since the beneficial effect of quinapril on RBC aggregability in normotensive animals is also mimicked by bradykinin and, moreover, this phenomenon is successfully reversed by icatibant, in line with our previous findings on the effects of quinapril in peripheral and pulmonary circulation (10) we assume that quinapril-induced improvement of rheological properties of RBC in vivo seems very likely to depend upon bradykinin-mediated activation of the secretion of vascular endothelium.
As prostacyclin and nitric oxide play a protective role for RBC rheology (14, 15), the inhibition of its endothelial generation by cyclooxygenase and nitric oxide synthase inhibitors was supposed to increase aggregability of RBC. Indeed, as has been already said, indomethacin, aspirin and L-NAME when injected into control rats significantly augmented aggregability. In addition, indomethacin and L-NAME did not affect aggregability of erythrocytes in vitro, indicating that the most meaningful portion of endothelial mediators involved in the regulation of RBC rheological properties originated from vascular endothelium. However, aspirin increased aggregability in vivo even when used at a dose of 1 mg/kg which was expected to be too low to inhibit cyclooxygenase. Moreover, contrary to indomethacin, aspirin increased RBC aggregability also in isolated erythrocytes in vitro in the absence of endothelium and other blood cells as well as in hypertensive animals in which due to endothelial dysfunction worsening of RBC aggregability reached nearly maximum, so any further effect of endothelial inhibition on aggregability could not be demonstrated. Some time ago we revealed (3) this unique cyclooxygenase-independent property of aspirin to influence blood rheology under physiological conditions in normal rats, along with a few clinical studies (2, 19). Nevertheless the mechanism of this phenomenon still remains unclear. The most plausible explanation we can at the moment propose is that aspirin worsens rheological properties of red cells via direct unspecific effects on their membranes. As a matter of fact, such statement is well-grounded since asprin was found to induce acetylation of integral proteins of the red cells membrane leading to increased rigidity of the membranes (20). Aspirin, like e.g. methyl acetylphosphate, may also acetylate haemoglobin which in its acetylated form is known to modify proteins and/or lipids of the red cells membrane changing their rheological properties (21, 22).
In conclusion, under physiological conditions angiotensin converting enzyme inhibitor - quinapril - efficiently inhibits RBC aggregability and this effect is modulated by secretory function of vascular endothelium. However, in hypertension quinapril, in spite of its ability to lower arterial blood pressure, is unable to display beneficial effects on RBC aggregability due to the hypertension-induced (-accompanied) dysfunction of endothelial secretion. Aspirin reveals unique erythrocyte damaging properties, presumably independent of inhibition of cyclooxygenase but related to a direct membrane protein acetylation. Finally, we feel authorized to state that RBC aggregability is an important indicator of the secretory function of endothelium thanks to which it is becoming possible to measure the effectiveness of the drugs that have been claimed to reverse endothelial dysfunction during a long-term therapy. Apart from pleiotropic activity of ACEI which should be verified again on RBC aggregability after administration of these drugs for much longer time than in the present work, here we refer mainly to pleiotropic action of statins which is frequently associated with their ability to inhibit neointimal inflammation in atherosclerosis (23), and to up-regulate nitric oxide endothelial production (24, 25), and even to prevent thrombin-induced dysfunction of cultured endothelial cells (26).
- Lominadze D, Joshua IG, Schuschke DA. Increased erythrocyte aggregation in spontaneously hypertensive rats. Am J Hypertens 1998; 11: 784.
- Korbut RA, Adamek-Guzik T. The effect of aspirin on rheological properties of erythrocytes in essential hypertension. Przeg. Lek. 2002; 59: 71-75.
- Korbut RA, Adamek-Guzik T, Madej J, Korbut R. Endothelial secretogogues and deformability of erythrocytes. J Physiol Pharmacol 2002; 53: 655-665.
- Moncada S, Higgs A. The L-arginine-NO pathway. N Engl J Med 1993; 329: 2002-2012.
- Gryglewski RJ, Botting RM, Vane JR. Mediators produced by the endothelial cell. Hypertension 1988; 12: 530-548.
- Ignarro LJ: Nitric oxide as a unique signaling molecule In the vascular system: a historical overview. J Physiol Pharmacol 2002; 53: 503-514.
- Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart 2001, 85: 342-350.
- Lind L, Granstam SO, Millgard J. Endothelium-dependent vasodilation in hypertension: a review. Blood Pressure 2000; 9: 4-15.
- Channon KM, Guzik TJ: Mechanism of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol 2002; 53: 515-524.
- Gryglewski RJ, Chłopicki S, Uracz W, Marcinkiewicz E. Significance of endothelial prostacyclin and nitric oxide in peripheral and pulmonary circulation. Med Sci Monit 2001; 7: 1-16.
- Lauth M, Cattaruzza M, Hecker M. ACE inhibitor and AT1 antagonist blockade of deformation-induced gene expression in the rabbit jugular vein through B2 receptor activation. Arterial Thromb Vasc Biol 2001; 21: 61-66.
- Jubelin BC, Gierman JL. Erythrocytes may synthesize their own nitric oxide. Am J Hypertens 1996; 9: 1214-1219.
- Cicco G, Pirelli A. Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clin Hemorh Microcirc 1999; 21: 169-177.
- Starzyk D, Korbut R, Gryglewski RJ. Effects of nitric oxide and prostacyclin on deformability and aggregability of red blood cells of rats ex vivo and in vitro. J Physiol Pharmacol 1999; 50: 629-637.
- Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol 2003; 284: H1577-H1584.
- Tsuda K, Kimura K, Nishio I, Masuyama Y. Nitric oxide improves membrane fluidity of erythrocytes in essential hypertension: An electron paramagnetic resonance investigation. Biochem Biophys Res Comm 2000; 275: 946-954.
- Sprague RS, Bowles EA, Olearczyk JJ, Stephenson AH, Lonigro AJ: The role of G protein ß subunits in the release of ATP from human erythrocytes. J Physiol Pharmacol 2002; 53: 667-674.
- Gryglewski RJ, Uracz W, Święs J, Chłopicki S, Marcinkiewicz E, Łomnicka M, Madej J. Comparison of endothelial pleiotropic actions of angiotensin converting enzyme inhibitors and statins. Ann NY Acad Sci 2001; 947: 229-245.
- Saniabadi AR, Fischer TC, McLaren M, Belch JF, Forbes CD. Effect of dipyridamole alone and in combination with aspirin on whole platelet aggregation, PGI2 generation, and red cell deformability ex vivo in man. Cardiovasc Res 1991; 25: 177-183.
- Watała C, Gwozdzinski K. Effect of aspirin on conformation and dynamics of membrane proteins in platelets and erythrocytes. Biochem Pharmacol 1993; 45: 1343-1349.
- Xu AS, Labotka RJ, London RE. Acetylation of human hemoglobin by methyl acetylphosphate. Evidence of broad regio-selectivity revealed by NMR studies. J Biol Chem 1999; 274: 26629-26632.
- Xu AS, Macdonald JM, Labotka RJ, London RE. NMR study of the sites of human hemoglogin acetylated by aspirin. Biochim Biophys Acta 1999; 1432: 333-349.
- Bustos C, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol 1998; 32: 2057-2064.
- Kaesemeyer WH, et al. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol 1999; 33: 234-241.
- Mital S, et al. Simvastatin upregulates coronary vascular endothelial nitric oxide production in conscious dogs. Am J Physiol 2000; 279: H2649-H2657.
- van Nieuw-Amerongen GP, et al. Simvastatin improves disturbed endothelial barrier function. Circulation 2000; 102: 2803-2809.