A basic question is: Why does mouth breathing not ease nocturnal respiration? What is the mechanism underlying observations that nasal obstruction causes episodes of obstructive apnea (or hypopnea) when the subject has a patent oral airway? In the present study we investigated the possible influence of breathing through the nose on genioglossus muscle reactivity to chemical stimulation in progressive hypoxia conditions.
The populations of 35 younger subjects [G-I, 20-30 years old] and 35 older subjects [G-II, 41-55 years old], who volunteered for the study, were investigated. All subjects were healthy, normotensive, non-snorers, but the older subjects showed greater resting values of systolic (SYS), diastolic (DIAS) blood pressures and body mass index (BMI) (Fig. 1).
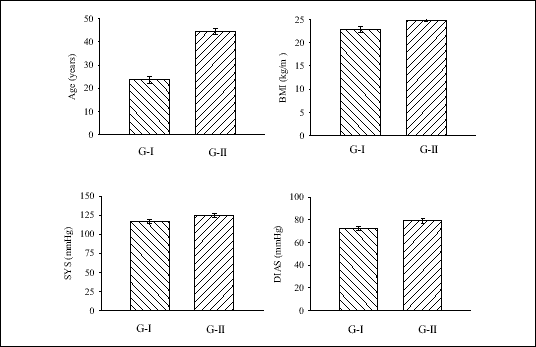 |
| Fig. 1. Age, systolic (SYS) and diastolic (DIAS) blood pressure, and body mass index (BMI) in both investigated groups: G-I (younger subjects) and G-II (older subjects). |
GG-EMG activity and hypoxic response
An electromyogram from the genioglossus muscle (GG-EMG) was recorded from gold cup surface electrodes. The electrodes were attached to the skin using double-sided adhesive disks and collodion. The GG electrodes were positioned in the midline 1 cm apart and halfway between the chin and the hyoid bone (7). The raw EMG signal was amplified 100 to 1000 times with 30 and 1000 Hz low- and high-frequency filters and integrated with a RC circuit. The integrator band was 70 to 1500 Hz.
In order to activate the arterial chemoreceptors, the progressive normo- and isocapnic hypoxia test was used. Progressive hypoxia was induced by a rebreathing method (8), using an MES-Lungtest-System (MES, Cracow, Poland). A mask covering the nose and mouth, nose or mouth, separately, was connected to the instrument setup. End-tidal CO2 was continuously monitored, as was arterial O2 saturation (SaO2) with a finger oximeter.
Protocol
All measurements were done in awake subjects in the sitting position. Subjects were allowed to breathe quietly through the apparatus until the end-tidal CO2 stabilized. Measurements were obtained during a 1-min baseline period and during progressive normocapnic hypoxia. The rebreathing test was performed three times by each subject when he breathed separately through the nose alone (N), through the mouth alone (M), and during the combined nose and mouth breathing (N&M), in a voluntary order. During the test, end-tidal CO2 remained within 0.2% of the baseline value. During baseline time and hypoxia test, breath-by-breath values of SaO2, changes in inspiratory drive and in GG-EMG-activity were determined and analyzed for the groups G-I and G-II. The inspiratory drive was estimated from the continuous determinations of minute ventilation (MV) and tidal volume/inspiratory time (VT/TI) ratio. Slopes of the regression curves MV/SaO2 (l/min/%), VT/TI/SaO2 (l/min/%), and GG-EMG-activity/SaO2 (%of baseline/%) were compared in both groups for the three different experimental conditions: N, M, and N&M breathings. Values are expressed as means ±SE. Student's t-test was used for the statistical analysis.
The values of both MV and VT/TI did not differ in the two investigated groups during the baseline control time (1 min before the rebreathing test). All subjects showed a linear increase in MV, VT/TI and in GG-EMG-activity in response to progressive isocapnic hypoxia. The increases in MV and VT/TI did not differ during N, M, N&M breathing in each group, but in the older group (G-II) these increases were significantly reduced in comparison with the younger group (G-I) ((P<0.01) (Fig. 2).
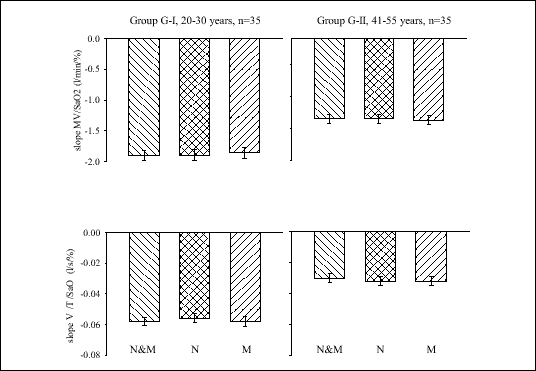 |
| Fig. 2. Linear regression slopes of MV and VT/TI on SaO2 during nose and mouth (N&M), nose alone (N), and mouth alone (M) breathing in G-I (left) and G-II (right) groups. |
Fig. 3 shows the linear relationships between the arterial oxygen saturation and GG-EMG-activity during the three ways of breathing employed. In both younger (G-I; Fig. 3A) and older (G-II; Fig. 3B) subject groups, the most prominent increases in GG-activity in response to hypoxia occurred during the nose and the combined nose and mouth breathing paradigm, whereas during the mouth breathing the increases were smaller (P<0.01). There also were significant differences between the young and old groups in that the responses were reduced in the older group across all the experimental conditions (P<0.01).
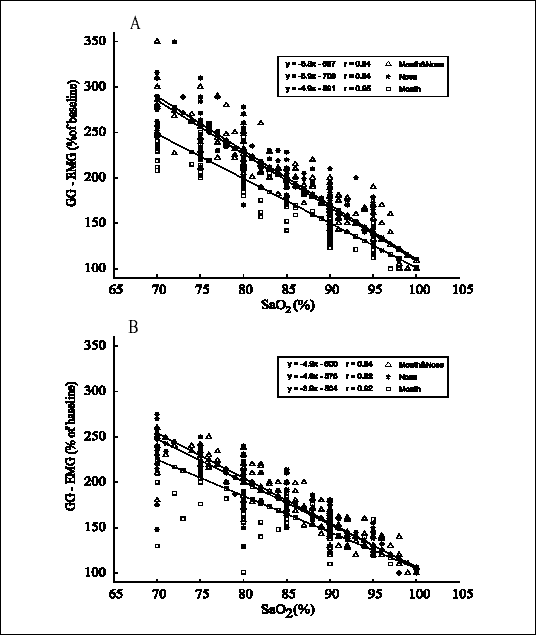 |
| Fig. 3. Increases in GG-EMG-activity during progressive isocapnic hypoxia during the nose, mouth, and nose+mouth ways of breathing in the groups of younger (Panel A) and older (Panel B) subjects. Lines are linear regression slopes of EMG activity on arterial oxygen saturation. |
Olsen et al (3) have proposed a possible explanation for the differences observed between oral and nasal airway resistances. Their results suggested that the oral airway resistance is greater than that of the nasal airway when the patient is asleep. “During sleep, the nasal airway seems to be the preferred route of respiration. Oral breathing appears to be a high-resistance pathway during sleep” (3). When a total nasal obstruction occurs, the inspiratory effort is greater for the breathing to occur across the more resistive oral pathway. It follows that the oral airway seems to be an ineffective channel during sleep.
Our results suggest an additional explanation. Linear increases in GG-EMG- activity in response to progressive isocapnic hypoxia in human subjects have been previously described (9, 10). In the present study, the most eminent increase in GG-EMG-activity in response to a rebreathing test was observed during breathing through the nose, with mouth closed, or through the mouth and nose simultaneously. This phenomenon was found in both younger and older subjects, although the reflex genioglossal response was smaller in the old, similarly to our previous study. Thus, nasal mucosa seems to play an important role in the regulation of upper airway flow, probably by activation of the dilator muscle of the upper airway. Mathew et al (10) showed that the response of upper airway muscles to negative pharyngeal pressure is abolished when nasopharyngeal topical anesthesia is administered. Several other studies indicated that the nasal airway is an active component of the respiratory system. Hypercapnia decreases nasal airway resistance proportionally to the increase in minute ventilation (11). Nasal cycle (alternating congestion and decongestion of the nasal airway) is another important aspect of nasal physiology (12, 13). Nasal reflexes have also been clearly demonstrated (14). Nasal obstruction changes the sensing of airflow and pressure, which may disrupt the neural and muscular control mechanisms for the maintenance of respiration during sleep (2). The nasal airway seems to be the preferred route of respiration not only in the awake state but also, and even more so, during sleep.
A number of animal studies have demonstrated that upper airway dilator muscles can be activated through reflex mechanisms, with afferent pathways originating in the upper airway itself (15, 16). Similar observations have been made in normal adult humans during wakefulness (17). In the genioglossus muscle, there also are both excitatory and inhibitory reflexes linked to jaw opening and closing, with the afferents responding to information arising in joint receptors in the temporomandibular joint (18). As the jaw falls open (during a sleep-induced loss of postural drive to the muscles), it rotates, so that the tongue moves posteriorly, narrowing the oropharynx. Furthermore, passive and active jaw openings inhibit genioglossus activity (19), a reflex that plays an important role in mastication that is activated by temporomandibular joint afferents. It seems reasonable to speculate that this reflex can explain smaller responses of the genioglossus muscle to hypoxia during mouth breathing, found in the present study. This reflex may likely contribute to obstructive apneas, since jaw opening during sleep is common in patients with obstructive sleep apnea. Additionally, it may contribute through a reduction of reflex activation of the genioglossus muscle, as compared with breathing through the nose. However, the role of this mechanism in the maintenance of upper airway muscle tone during sleep is yet unclear.
Acknowledgments: This study was supported by KBN grant Nr 4P05B07010.
- Willard BM. Jr. Obstructive sleep apnea: diagnosis by history, physical examination and special studies. In Snoring and Obstructive Sleep Apnea, DNF Fairbanks, S Fujita, T Ikematsu, FB Simmons (eds). New York, Raven Press 1987, pp. 19-39.
- Olsen KD. The nose and its impact on snoring and obstructive sleep apnea. In Snoring and Obstructive Sleep Apnea, DNF Fairbanks, S Fujita, T Ikematsu, FB Simmons (eds). New York, Raven Press 1987, pp. 200-226.
- Olsen KD, Suh KW, Staats BA. Surgically correctable causes of sleep apnea syndrome. Otolaryngol Head Neck Surg 1981; 89: 726-731.
- Taasan V, Wynne JW, Cassisi N, Block AJ. The effect of nasal packing on sleep disordered breathing and nocturnal oxygen desaturation. Laryngoscope 1981; 91: 1163-1172.
- Lavie P, Fischel N, Zomer J, Eliaschar I. The effects of partial and complete mechanical occlusion of the nasal passages on sleep structure and breathing in in sleep. Acta Otolaryngol 1983; 95: 161-166.
- McNicholas WT, Tarlo S, Cole P et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis 1982; 126: 625-628.
- Jeffries BJ, Brouillette RT, Hunt CE. Electromyographic study of some accessory muscles of respiration in children with obstructive sleep apnea. Am Rev Respir Dis 1984; 129: 696-702.
- Read DJC, Leigh J. Blood-brain tissue PCO2 relationship and ventilation during rebreathing. J Appl Physiol 1967; 23: 53-70.
- Tafil-Klawe M, Klawe JJ, Grote L, Schneider H, Peter JH, Śmietanowski M. Changes in upper airway resistance during progressive normocapnic hypoxia in patients with obstructive sleep apnea syndrome. Sleep Res 1995; 4(S1): 208-213.
- Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol 1982; 52: 438-444.
- McCaffrey TV, Kern EB. Response of nasal airway resistance to hypercapnia and hypoxia in man. Ann Otol Rhinol Laryngol 1979; 88: 247-252.
- Hasegawa M, Kern EB. The nasal cycle. Mayo Clin Proc 1977; 52: 28-34.
- Tafil-Klawe M, Hildebrandt G. Do changes of microvascular blood flow of nasal mucosa play a role in occurrence of the laterality rhythm of nasal breathing? In Chronobiology & Chronomedicine. C Gutenbrunner, G Hildebrandt, R Moog (eds). Peter Lang Frankfurt am Main, Berlin, New York, Paris, Wien 1991, pp. 320-324.
- Angell James JE, Daly M de B. Nasal reflexes. Proc R Soc Med 1969; 62: 1287-1293.
- Van Lunteren E, Van de Graaff WB, Parker DM et al. Nasal and laryngeal reflex responses to negative upper airway pressure. J Appl Physiol 1984; 6: 746-752.
- McNamara SG, Issa FG, Szeto E, Sullivan CE. Influence of negative pressure applied to the upper airway on the breathing pattern in unanaesthetized dogs. Respir Physiol 1986; 65: 315-329.
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol (London) 1991; 436: 15-29.
- Cistulli P, Sullivan CE. Pathophysiology of sleep apnea. In Sleep and Breathing. NA Saunders, CE Sullivan (eds). New York, Marcel Dekker 1994, pp. 405-448.
- Sauerland EK, Mizuno N. A protective mechanism for the tongue: suppression of genioglossus activity induced by stimulation of the trigeminal proprioceptive afferents. Experimentia 1970; 26: 1226-1227.