The initiating factors up-regulating T lymphocytes and macrophages in the alveoli at the outset of the disease are as yet unknown (3). Similarly, there is still much to be discovered and understood about the pathological immune setting enabling the formation of alveolitis and granuloma and about the mechanisms responsible for spontaneous disease resolution or progression. There are some literature data strongly suggesting that the inflammatory angiogenesis might play a substantial role in the pathomechanism of sarcoidosis (4, 5, 6). Persistent stimulation of inflammatory cells and of the structural cells of the respiratory tract results in an unrestrained cytokine and mediator release that, in turn, fosters pathological neovascularization. We have previously reported that cells isolated from bronchoalveolar lavage (BAL), i.e., derived from the lower respiratory tract, are characterized by high proangiogenic activity (7). A subset of non-CD4+, non-CD8+ lymphocytes was shown to be the most important population in this process. The aim of the present study was to explore the possible relationship between the activity of the inflammatory process, as assessed by its cellular components and other parameters characterizing the disease progression, in (lung function tests) and outside the respiratory tract (splenomegaly). The study group consisted of the subjects who had participated in a previous investigation on BAL cell proangiogenic activity (7).
The study protocol was approved by the Ethics Committee of the National Institute of Tuberculosis and Lung Diseases in Warsaw, Poland.
Patient characteristics
The study population consisted of 40 patients, nonsmokers (15 females, 25 males) with sarcoidosis diagnosed by clinical and roentgenlogic evaluations and biopsy confirmed who underwent bronchoscopic bronchoalveolar lavage (BAL) for clinical reasons, either for diagnostic purposes or to assess the disease activity (Table 1). Sarcoidosis was classified as a stage I process in 18, stage II in 12, and stage III in 10 patients. None of the patients were receiving oral or inhaled steroids at the time of BAL or during the past 3 months.
BAL and BAL analysis
BAL was performed for diagnostic purposes with informed consent, according to the European Task Group standards (8). Shortly, fifty milliliters of sterile 0.9% saline solutions at room temperature was instilled through a flexible fiberoptic bronchoscope four times to a total volume of 200 ml with harvesting of the fluid under immediate gentle vacuum. The recovered BAL fluid was filtered through sterile gauze, spun (4°C, 400 g), washed twice in PBS, and the cells were counted. Cytospun preparations were prepared for the cell differential count. Cells were >90% viable, as assessed by trypan blue exclusion. The smears were stained with May-Grünwald-Giemsa staining and determined by counting a minimum of 600 cells. Lymphocyte phenotyping (CD4+, CD8+) was estimated by a monoclonal antibody staining procedure by using an LSAB+ kit (DAKO, Carpinteria, USA).
Lung function tests consisted of pulmonary static compliance and pulmonary diffusing capacity evaluated by the single breath technique. The spleen size was assessed by the ultrasonographic examination. Data were presented as mean (x) ±SE. The Pearson test was used to analyze correlations between the studied groups.
A routine assessment of sarcoidosis activity consists of the evaluation of the intensity of the inflammatory process by analyzing the BAL-derived biological material, of lung function to estimate the degree of its possible impairment, and finally of the disease extrapulmonary involvement.
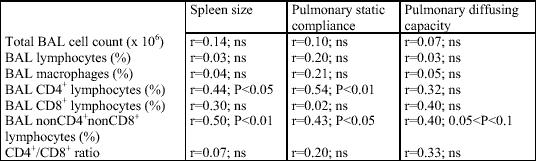
In the studied group of patients with active sarcoidosis, the relative counts of CD4+ and non-CD4+, non-CD8+ lymphocytes in the bronchoalveolar lavage fluid significantly correlated with the extrapulmonary disease activity marker, the spleen longitudinal dimension (r=-0.44, P<0.05 and r=0.50, P<0.01, respectively). Moreover, a close relationship between lung function parameters and BAL cell content was demonstrated. Lung static compliance positively correlated with the relative count of the CD4+ cells (r=0.54, P<0.01) and negatively with that of the non-CD4+, non-CD8+ lymphocytes (r=-0.43, P<0.05) and with the spleen longitudinal dimension (r=-0.44, P<0.05) (Fig. 1, Table 2). There was also an apparent trend toward a significant correlation between the diffusion lung capacity and the relative count of the non-CD4+, non-CD8+ lymphocytes (r=-0.40, 0.05 < P <0.1) (Fig. 2, Table 2).
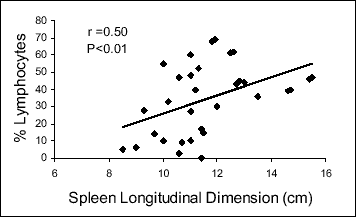 |
Fig. 1. Correlation between spleen size and relative counts of non-CD4+, non-CD8+ BAL lymphocytes in patients with active sarcoidosis. |
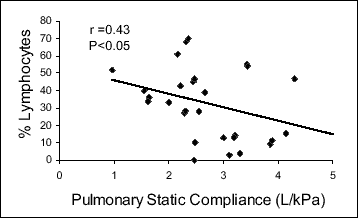 |
Fig. 2. Correlation between pulmonary static compliance and relative counts of non-CD4+, non-CD8+ BAL lymphocytes in patients with active sarcoidosis. |
| Table 1. Clinical characteristics of patients. |
 |
| Table 2. Correlation between the results of BAL cellular components, spleen size, and lung function; ns - statistically nonsignificant. |
 |
Sarcoidosis is considered a systemic disease, although, as a rule, only one organ is involved. The pathological process is most often limited to the lungs and mediastinal and hilar lymph nodes, but the liver, spleen, skin, other lymph nodes, and eyes might be drawn in as well (9, 10, 11). In the present study, extrapulmonary activity of sarcoidosis was assessed on the basis of spleen enlargement evaluated with an ultrasonographic examination. Spleen involvement in sarcoidosis is for the most part asymptomatic (12). However, postmortem examinations confirm the presence of solitary granuloma in the spleen of 38-77% of sarcoidosis patients, while a thin-needle biopsy does in 24-59% (13). Similarly, the results of ultrasonographic or radiological examinations show positive results in 28-60% patients, while a physical examination (palpation) does only in 3-15 % (13, 14). Therefore, the ultrasonographic examination applied in the present study is considered a reliable and approved method of spleen enlargement, and indirectly the disease activity, evaluation. Israel et al (15) and Mana et al (16) have confirmed that splenomegaly along with the race, erythema nodosum, mediastinal or hilar limphadenopathy, and disseminated parenchymal changes in lung X-ray are the strongest predictive factors for sarcoidosis activity and persistence. It is also commonly accepted that splenomegaly often goes in tandem with a higher risk of systemically diffuse disease and with poor effectiveness of corticosteroid treatment (15, 17). The spleen size strongly correlates with the increased serum angiotensin converting enzyme, but not with the results of lung X-ray examination (18). Besides, Hoogsteden et al (19) have reported that in 57% of sarcoidosis patients with the confirmed involvement of extrapulmonary disease, X-ray examinations turn normal, while the relative count of lymphocytes in BAL is increased, reflecting the activity of an inflammatory process. However, to the best our knowledge, there are no data in the literature analyzing at the same time the extrapulmonary involvement of sarcoidosis and the cellular composition of BAL.
The relationship between BAL morphology and lung function has been more thoroughly examined in the past. In early 1980s, a high lymphocyte count in BAL was considered a key sign of the unfavorable sarcoidosis outcome accompanied by gradual lung deterioration. However, subsequent studies do not confirm that hypothesis (20, 21). At present, high BAL lymphocytosis is perceived as a sign of the active alveolitis at the very first stage of the disease and is believed to be a good prognostic sign in pulmonary sarcoidosis (21). Foley et al (22) and Verstraeten et al (23) have demonstrated a close relationship between the high BAL lymphocytosis and the subsequent improvement of lung function in patients with active disease as compared with a group of non-active patients. The present study corroborated those findings by showing the relationship between the relative count of non-CD4+, non-CD8+ lymphocytes and CD4+ lymphocytes and lung static compliance in the group of active sarcoidosis patients (Table 1). The correlations observed, positive between the relative count of BAL non-CD4+, non-CD8+ lymphocytes and the spleen longitudinal dimension and negative with the lung static compliance, representing disease activity outside the pulmonary system and lung function impairment, respectively, seem to confirm that this particular lymphocyte population participates in the pathomechanisms of sarcoidosis. We have previously shown that the relative count of this lymphocyte population significantly correlates with a high proangiogenic activity of BAL cell homogenates (7). The present data suggest a more complicated function of the non-CD4+, non-CD8+ lymphocytes. Their notable correlation with the spleen size might imply, for example, a direct relationship with the development of a disseminated multiorgan form of the disease. A similar conclusion invokes a negative relationship between lung static compliance, a direct indicator of lung function impairment, and an indirect marker of progression of lung fibrosis, demonstrated in the present study. It is commonly accepted that angiogenesis actively participates in regenerative processes, including fibrotic remodeling of the persistently inflamed lungs (24, 25). The presented data are in absolute accordance with our previous studies devoted to the proangiogenic activity of the homogenated BAL cells. However, further examinations are required to evaluate the detailed phenotypic and functional characteristics of these cells.
- Ziegenhagen MW, Muller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med 2003; 253: 18-30.
- Perez RL, Rivera-Marrero CA, Roman J. Pulmonary granulomatous inflammation: From sarcoidosis to tuberculosis. Semin Respir Infect 2003; 18: 23-32.
- du Bois RM, Goh N, McGrath D, Cullinan P. Is there a role for microorganisms in the pathogenesis of sarcoidosis? J Intern Med 2003; 253: 4-17.
- Tolnay E. Vascular endothelial growth factor (VEGF) in granulomatous lung diseases. Eur J Clin Invest 1998; Suppl 1: A41.
- Takemura T, Hiraga Y, Oomichi M et al. Ultrastructural features of alveolitis in sarcoidosis. Am J Respir Crit Care Med 1995; 152: 360-366.
- Meyer KC, Kaminski MJ, Calhoun WJ, Auerbach R. Studies of bronchoalveolar lavage cells and fluids in pulmonary sarcoidosis. I. Enhanced capacity of bronchoalveolar lavage cells from patients with pulmonary sarcoidosis to induce angiogenesis in vivo. Am Rev Respir Dis 1989; 140: 1446-1449.
- Chorostowska-Wynimko J. NonCD4+ and nonCD8+ cells are the main source of angiogenic activity of BAL cells homogenates from sarcoidosis patients. Eur Respir J 1998; 12 (Suppl 28): 332 (Abstract P2175).
- Rennard SI, Aalbers R, Bleecker E et al. Bronchoalveolar lavage: performance, sampling procedure, processing and Assessment. Eur Respir J Suppl 1998; 26: 13S-15S.
- Kidd D, Beynon HL. The neurological complications of systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20: 85-94.
- Kok HS. The eye in lung disease. Hosp Med 2003; 64: 111-113.
- Schwartzbauer HR, Tami TA. Ear, nose, and throat manifestations of sarcoidosis. Otolaryngol Clin North Am 2003; 36: 673-684.
- Robertson F, Leander P, Ekberg O. Radiology of the spleen. Eur Radiol 2001; 11: 80-95.
- Fordice J, Katras T, Jackson RE et al. Massive splenomegaly in sarcoidosis. South Med J 1992; 85: 775-778.
- Folz SJ, Johnson CD, Swensen SJ. Abdominal manifestations of sarcoidosis in CT studies. J Comput Assist Tomogr 1995; 19: 573-579.
- Israel HL, Karlin P, Menduke H, DeLisser OG. Factors affecting outcome of sarcoidosis. Influence of race, extrathoracic involvement, and initial radiologic lung lesions. Ann NY Acad Sci 1986; 465: 609-618.
- Mana J, Salazar A, Manresa F. Clinical factors predicting persistence of activity in sarcoidosis: a multivariate analysis of 193 cases. Respiration 1994; 61: 219-225.
- Kataria YP, Whitcomb ME. Splenomegaly in sarcoidosis. Arch Intern Med 1980; 140: 35-37.
- Warshauer DM, Semelka RC, Ascher SM. Nodular sarcoidosis of the liver and spleen: appearance on MR images. J Magn Reson Imaging 1994; 4: 553-557.
- Hoogsteden HC, van Dongen JJ, Adriaansen HJ et al. Bronchoalveolar lavage in extrapulmonary sarcoidosis. Chest 1988; 94: 115-118.
- Rust M, Bergmann L, Kuhn T et al. Prognostic value of chest radiograph, serum-angiotensin-converting enzyme and T helper cell count in blood and in bronchoalveolar lavage of patients with pulmonary sarcoidosis. Respiration 1985; 48:231-236.
- Drent M, Jacobs JA, de Vries J, Lamers RJ, Liem IH, Wouters EF. Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur Respir J 1999; 13: 1338-1344.
- Foley NM, Coral AP, Tung K, Hudspith BN, James DG, Johnson NM. Bronchoalveolar lavage cell counts as a predictor of short term outcome in pulmonary sarcoidosis. Thorax 1989; 44: 732-738.
- Verstraeten A, Demedts M, Verwilghen J et al. Predictive value of bronchoalveolar lavage in pulmonary sarcoidosis. Chest 1990; 98: 560-567.
- Peao MN, Aguas AP, de Sa CM, Grande NR. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Rec 1994; 238: 57-67.
- Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis 1989; 140: 513-524.