Dentistry as a part of medicine
Dentistry is tightly integrated with the general medicine as the health of oral cavity may contribute to health conditions of various organs of the body. This fact stresses the important role of oral cavity as the first part of GIT in numerous functions including;
a) participation in articulation of sounds and generation of speech as well as participation in the perception of the taste and b) serving as passage-way of air to lungs, especially during strong physical exercises or habitually as well as in some pathological conditions that involves nose and mouth. The major role of oral cavity is, however, the food intake and its preparation for swallowing and the transport of ingested food into further segments of GIT. This includes biting and mixing of food particles with saliva to form food “bolus” ready for swallowing and passing through the pharynx and the esophagus to the stomach. Numerous organs are involved in this process such as masticate muscles, temporo-mandibular joint, teeth as well as auxiliary tissues of tongue, lips and chicks. Additionally, saliva which is a product of three pairs of salivary glands (parotid, sublingual and submandibular) as well as numerous small salivary glands dispersed within the mucosa of the oral cavity, enables the binding of the food particles into a single easy to swallow “bolus”. Saliva enzymes start the digestion of carbohydrates with alpha-amylase and triglycerides with lingual lipase secreted by salivary glands (1).
During recent decades it has become apparent that oral cavity is inhabited by million of various microorganisms forming an oral bacterial biofilm. This biofilm does not appear to cause any damage to the host, but oral bacteria remain in dynamic physiological symbiosis with the immunological system of the host. This system prevents in healthy individuals the excessive growth of microbes in oral cavity and the invasion of pathological bacteria. Additionally, the bacteria themselves competing for food and place to survival and vegetation, may restrict the growth of pathogenic microorganisms which otherwise could harm the organism. Oral cavity as a very heterogeneous environment consisting of the numerous organs, provide different conditions for different types of bacteria. Some germs permanently inhabit oral cavity, while others may be only transiently contaminating this cavity, building a very complex environment and under physiological conditions living in symbiosis with the host. Nevertheless, this balance can be disturbed by numerous internal and external factors (1, 2).
The disruption of this balance may lead to pathological states in the oral cavity, which can further influence the health of the whole organism by carrying infection to other portions of the GIT and other body organs through airways as well as blood stream. Oral bacteria can also become a source of systemic inflammatory response, thus, affecting the prognosis and outcomes of therapies for general medical diseases such as cardiovascular, especially coronary and brain artery diseases.
H. pylori is present in both the oral cavity and the stomach
H. pylori is a microaerophilic, Gram negative, spiral and mobile bacterium which is believed to be one of the major factors responsible for gastritis, gastro-duodenal ulcers as well as gastric cancer (3 - 5). There are different opinions concerning the presence of H. pylori in the oral cavity. The major question remains whether bacteria are only transiently contaminating oral environment during oral processing of food (6, 7) or whether they constitute an integral portion of residual flora of the oral cavity that remains in symbiotic relationship with its host (8). Nevertheless, it is now quite certain that H. pylori may be present in oral cavity either temporarily or permanently. The first person who isolated H. pylori from dental plaques, soon after its discovery by R. Warren and B. Marshall was Krajden in 1989 (9).
It seems to be logical and most likely, that in the case of the gastric infection with H. pylori, oral cavity serves as the gate of this germ transmission to the GIT (“person to person transmission”). However, it is not clear, what is the role of this germ in oral cavity in the transmission process. Is it not clear whether the bacterium is only transiently stored in the mouth when passing to the stomach or whether oral cavity is a real bacterial reservoir, where H. pylori can multiply, achieving high enough number for entering the stomach and its infecting.
It is well-established that the principal ecological niche for H. pylori is the gastric mucosa. The bacterium, when reaching the gastric lumen quickly passes through the thick mucus-HCO3- layer adhering to the surface of the gastric mucosa due to its mobility by using its flagellas and attaches via its adhesins to the glycol-lipid receptors on the apical membrane of surface epithelial cells. Once mucosal infection is established it can last many years or even whole life time. The question arises if in the oral cavity similar ecological niche exists, where H. pylori could by attached and grow. Such locum for the germ in oral cavity could be e.g. dental pockets where from the bacteria could be spread to the esophagus and the stomach, where its natural ecological niche exists.
One of the first investigations on the influence of oral H. pylori on the stomach condition was carried out by Miyabayashi et al. (10). This study was performed on 47 patients and confirmed the existence of the relationship between the gastritis caused by H. pylori infection and with oral colonization with this germ. Moreover, these authors also attempted to elucidate the resistance of oral H. pylori to typical (triple) anti- H. pylori therapy used to eradicate the germ from the stomach. They reported that patients with oral H. pylori were at significantly greater risk of gastric reinfection following successful therapy. Therefore, this study emphasized a clear link between the presence of H. pylori in oral cavity and its infection of gastric mucosa. Other authors also showed direct correlation between poor oral cavity health and H. pylori reinfection of stomach (11). H. pylori was found in the oral cavity of virtually each patient who presented with poor hygienic status. Song et al. (12) investigated further the particular characteristics of oral cavity microenvironment. They found that distal parts of the oral cavity, which are less oxygenated, contain higher numbers of bacteria. Our previous studies (13, 14) carried out in patients using dentures, indicated that all patients with the H. pylori present in the oral cavity showed also the gastric infection with this germ. According to our finding, if the H. pylori was present in the oral cavity of patients with dentures, the infection with this germ also occurred in the stomach. On the contrary, gastric H. pylori infection does not necessarily indicates the presence of bacteria in the oral cavity. It does not exclude the possibility that H. pylori for stomach infection passes through the oral cavity. Oral cavity seems to be the only gate of the H. pylori infection of the stomach, which occurs in 70% among Polish population, most probably by “person-to-person” transmission.
The study involved 40 men (25-70 yrs), who gave their informed consent to participate in the study that has been approved by Ethics Committee of Medical College of Jagiellonian University. The presence of urease active bacteria was demonstrated in the oral cavity by the use of 13C-urea breath testing (UBT) similarly to the technique described in details before (15). Briefly, after collecting two baseline breath samples, each patient was asked to confine in oral cavity 10 ml of water solution containing 40 mg of 13C-urea and phenol red as a volume recovery marker. While the solution was kept in the oral cavity for 5 min, patient was asked to inflate at one min interval plastic bags through his nose (nasal collection). That was followed by spitting of entrapped 13C-solution, washing out the mouth and finally proceeding 13C-UBT by collecting the breath samples at 6, 10 and 20 min time points from the start of testing. The spitted fluid was used to estimate the recovery factor. Gastric H. pylori status was determined using capsulated 13C-UBT as described previously (16).
Fig. 1 demonstrates the UBT in H. pylori positive (N=20) and negative patients (N=20). The presence of bacteria originating from the mouth cavity (saliva or supragingival plaques and determined by culture on agar-horse was independent of the presence or absence of gastric H. pylori infection determined by UBT. In some cases the bacteria was detected in the oral cavity even when in the same patients that were eradicated from the gastric H. pylori by typical triple therapy.
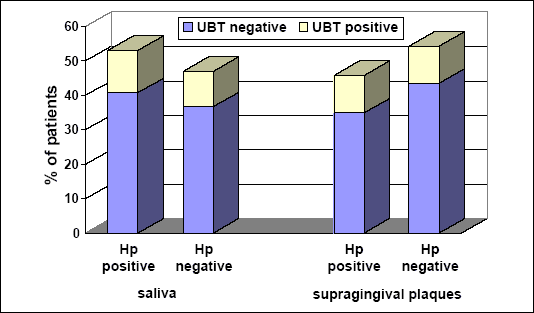 |
| Fig. 1. The positive and negative microbiology tests in saliva (on left) and supragingival plaques (on right). Gray color reflects percent of patients with negative UBT and white with positive UBT. |
RAPD – PCR
Most important question remained whether the bacteria detected by culture of mouth contents and that cultured from the gastric biopsy samples were identical or not. To answer this question, we employed Random Amplification of Polymorphic DNA (RAPD). Bacterial cultures were prepared from smears collected from oral cavities and from the gastric biopsies of selected patients. Total bacterial DNA was isolated from the samples of H. pylori cultures using DNAzol according to the manufacturer’s instruction. Isolated DNA was subject to RAPD reaction using specifically designed primers:
1) CCg CAg CCA A;
2) AAg AgC CCg T;
3) AAC gCg CAA C.
Moreover, cagA (298bp), vacA (447bp) (Fig. 2) and urease status (not shown) of H. pylori isolates was confirmed in PCR reaction with specific primers:
| cagA | sense: | ATA ATG CTA AAT TAG ACA ACT TGA GCG A; |
| antisense: | TTA GAA TAA TCA ACA AAC ATC ACG CCA T. | |
| vacA | sense: | GCT CAT TAC GGC TTC CAC; |
| antisense: | GCC TCG GAC CAG ATA GTT. | |
| urease A | sense: | GCC AAT GGT AAA TTA GTT; |
| antisense: | CTC CTT AAT TGT TTT TAC. |
RAPD reaction was performed to compare and discriminate strains of H. pylori in the collected samples. Analysis of H. pylori genomic DNA revealed no resemblance, in any analysed case and these results have been omitted for the sake of clarity. This implies no relation between H. pylori strains infecting oral cavity and stomachs.
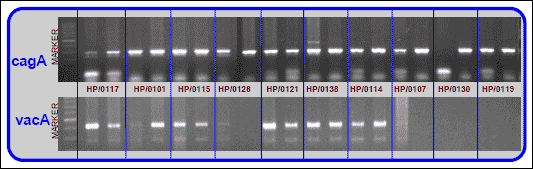 |
| Fig. 2. Cag A and vac A status in H. pylori cultures isolated from oral cavity and stomach of 10 patients. Each getween panel represents result of DNA analysis from the bacterial culture prepared from oral cavity (left band) and stomach (right band) of one patient. |
On the basis of our preliminary microbiological studies, we reached the conclusions that H. pylori is present in oral cavity of all of the examined patients with the dentures who had H. pylori infection in the stomach. In this context the question arises if oral cavity is the reservoir of H. pylori for the stomach or if the stomach is the source of the bacteria for the oral cavity. Theoretically, either way is feasible. H. pylori might get to the oral cavity in process of gastro-esophageal reflux or regurgitation. Even if there is no direct influence of gastric H. pylori on the oral cavity, the gastric H. pylori could still exert an indirect effect on the oral cavity through its various virulence and growth promoting factors released in the stomach to general circulation or via triggering the gastro-oral neural reflexes that function between various organs of the GIT. Based on the patients with diagnosed prosthetic stomatitis, we revealed the connection between gastric H. pylori infection and inflammation of oral mucosa in the area of contact with denture. We think that it is a consequence of the indirect influence of gastric H. pylori on the oral cavity mucosa. It is very likely that the H. pylori located in the stomach releases cytoxins such as CagA (encoded by cagA gene) or lipopolysacharides that reach the mucosa of oral cavity through bloodstream. The combination and overlay of simultaneous stimuli including direct irritation at the basis of the denture together with the toxic effects originating from blood H. pylori-related factors might damage the mucosa and result in various clinical manifestations of the symptoms.
On the other hand, Oshowo et al. (17) established in their study that although the reinfection of the stomach with the bacteria from oral cavity is possible, this occurs rather rarely and has minor impact on gastric re-infection. The majority of reports, however, indicate that the anti-H. pylori triple therapy does not influence the bacteria in the oral cavity, especially those present on dental plaques where they exist in the largest number. The failure of the triple therapy to affect oral H. pylori results from the fact that it is not possible to achieve the therapeutic anti-H. pylori concentrations of antibiotics in saliva and dental plaques (8, 12, 18-21). If this is true, the H. pylori eradication from the stomach in patients with bacteria present in the oral cavity, would be very difficult considering high rate of colonization of H. pylori in the saliva and dental plaques, that e.g. in Cracow region reaches the level of 84% and 100%, respectively.
Song (8) found that the presence of H. pylori in oral cavity is not associated with the gastric infection with this germ. However, according to out data based on our molecular studies using PCR for detection of H. pylori genome, we can conclude that the strain of H. pylori from the oral cavity is different from that from the stomach, which indicates that these two locations in GIT are infected independently and separately without any relationship between them. Nevertheless, we cannot exclude the potential influence of H. pylori in oral cavity on the stomach. Blaser and Berg (22) proposed that the H. pylori is highly diversible bacterium, capable of quick exchanging of its genetic (DNA) material from one bacterium to another and the strains originating in oral cavity and to those infecting the stomach. Such exchange of the genetic material from one strain to another, depends on the environmental conditions and this could help in best adjustment of germ to the surrounding conditions. The adjusting processing as well as genetic material exchange could take place in numerous different local niches in the oral cavity. The heterogeneous strains created may lead to developing resistance of bacteria to sudden changes in host environment, that is an important element of the symbiosis of H. pylori strains with the host. The dynamic balance between the environment and microbiological balance in the stomach may be disturbed when additional pathologic factors are introduced into these systems. These may include disorders of the immune system leading to decreased immune competence such as occurs with advancing age under physiological conditions. It is tempting to speculate if the factors leading to such disturbance of careful balance could be associated with the introduction into the system of new factor – e.g. oral H. pylori. Moreover, it is possible that life of H. pylori in the dental plaque, or denture-related inflammatory environment might actually “prepare it” for consecutive gastric infection by e.g. exchanging genetic material discussed above. This could result in the generation of strains, which would be capable of better survival and would push out the other less pathologic strains from the niche and environment. These processes would be very important in altering the balance facilitating oral, gastric and/or systemic infections. However, the responses of mucosa may be equally variable and often unpredictable (22).
We have to admit, however, that the detection of H. pylori in the oral cavity does not necessarily provides an evidence that the oral cavity is the reservoir of bacteria for the further parts of GIT. Certain number of viable bacteria is required for successful infection of gastric mucosa and the bacteria may be present in the oral cavity in the number too low to infect gastric mucosa after passing into the stomach with saliva or swallowed food. It was shown for the first time by B. Marshall, who drank the pure culture of H. pylori, that caused an immediate acute hemorrhagic and erosive gastritis confirmed by gastroscopy but no ulcer developed. That was the proof that the oral cavity may serve as the gate for the transmission of the H. pylori to the stomach (23).
The risk of the stomach infection with H. pylori originating from the oral cavity is significantly increased when aphthous ulcerations are present in the mouth. It has been established that the amount of H. pylori in the oral cavity is much higher when aphthous ulcerations occur (24 - 26). Therefore, when H. pylori is attempted to be eradicated when aphthous ulcerations are present, the risk of the reinfection of the stomach and the possibility of the transmission of the bacteria between humans (“mouth to mouth” transmission) greatly increase.
The group of the patients, which should be considered as a group of high risk of the H. pylori gastric reinfection from the oral cavity, includes individuals with extensive dentures. These subjects have been already established as the group with increased detection of fungi in the mouth, which usually manifests as the pathology described as prosthetic stomatopathy (stomatitis prothetica mycotica). Fungi can serve as the vector for bacteria H. pylori (see below), which allows these bacteria to survive in the oral cavity and after getting to the hostile acidic environment of the stomach, to infect its mucosa. The correlation between H. pylori and yeast-like fungi has not been established yet (27).
In our own studies with toothless patients using complete dentures and exhibiting proliferative form of mucosal inflammation type papillaris or with fibrosis of the prosthetic area, are in 100% H. pylori positive in the stomach. We found that there is no gastric H. pylori infection without H. pylori presence in the oral cavity in these selected subjects. Moreover, we can further speculate that there is no proliferative prosthetic stomatopathy without gastric H. pylori infection, which might indicate that the influence of some H. pylori cytotoxins and noxious substances such as LPS can encourage the proliferative changes even in the distant parts of the organism. We think that such distant changes caused by gastric H. pylori infection require the involvement of additional, as yet unrecognized factors required to develop mucosal proliferation. In the case of described patient it may be also the trauma of the oral mucosa caused by the denture. This motion can prove the fact that in the case of patients with gastric H. pylori infection who do not use dentures, we didn’t observe any proliferative changes within mucous membranes.
- Majewski S. Propedeutyka protetyki stomatologicznej dla studentow medycyny. Instytut Stomatologii WL CMUJ, Krakow 2006.
- Majewski S. Podstawy protetyki w praktyce lekarskiej i technice dentystycznej. SZS-W, Krakow, 2000.
- Konturek P, Kania J, Konturek J, Nikiforuk A, Konturek S, Hahn R. H.
pylori infection, atrophic gastritis, cytokines, gastrin, COX-2, PPAR
 and impaired apoptosis in gastric carcinogenesis. Med Sci Monit 2003; 9(7):
SR53-66.
and impaired apoptosis in gastric carcinogenesis. Med Sci Monit 2003; 9(7):
SR53-66. - Konturek PC, Konturek SJ. Role of Helicobacter pylori infection in gastro-duodenal secretion and in pathogenesis of peptic ulcer and gastritis. J Physiol Pharmacol 1994; 45(3): 333-350.
- Konturek SJ. Etiopatogeneza choroby wrzodowej. Wydawnictwo MP. Krakow, 1996.
- Checchi L, Felice P, Acciardi C, et al. Absence of Helicobacter pylori in dental plaque assessed by stool test. Am J Gastroenterol 2000; 95(10): 3005-3006.
- Savoldi E, Marinone MG, Negrini R, Facchinetti D, Lanzini A, Sapelli PL. Absence of Helicobacter pylori in dental plaque determined by immunoperoxidase. Helicobacter 1998; 3(4): 283-287.
- Song Q, Haller B, Ulrich D, Wichelhaus A, Adler G, Bode G. Quantitation of Helicobacter pylori in dental plaque samples by competitive polymerase chain reaction. J Clin Pathol 2000; 53(3): 218-222.
- Krajden S, Fuksa M, Anderson J, et al. Examination of human stomach biopsies, saliva, and dental plaque for Campylobacter pylori. J Clin Microbiol 1989; 27(6): 1397-1298.
- Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter 2000; 5(1): 30-37.
- Avcu N, Avcu F, Beyan C, et al. The relationship between gastric-oral Helicobacter pylori and oral hygiene in patients with vitamin B12-deficiency anemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 92(2): 166-169.
- Song Q, Lange T, Spahr A, Adler G, Bode G. Characteristic distribution pattern of Helicobacter pylori in dental plaque and saliva detected with nested PCR. J Med Microbiol 2000; 49(4): 349-353.
- Loster BW. Wspolzaleznosc infekcyjnych stanow chorobowych jamy ustnej i gornych odcinków przewodu pokarmowego u pacjentów uzytkujacych protezy zebowe. Wydawnictwo Uniwersytetu Jagiellonskiego, Krakow 2004.
- Czesnikiewicz-Guzik M, Karczewska E, Bielanski W, et al. Association of the presence the Helicobacter pylori in the oral cavity and in the stomach. J Physiol Pharmacol 2004; 55 Suppl 2: 105-115.
- Bielanski W, Konturek SJ, Dobrzanska MJ, Pytko-Polonczyk J, Sito E, Marshall B.J. Microdose 14C-urea breath test in detection of Helicobacter pylori. J Physiol Pharmacol 1996; 47(1): 91-100.
- Bielanski W, Konturek SJ. New approach to 13C-urea breath test; capsule-based modification with low-dose of 13C-urea in the diagnosis of Helicobacter pylori infection. J Physiol Pharmacol 1996; 47: 545-553..
- Oshowo A, Tunio M, Gillam D, et al. Oral colonization is unlikely to play an important role in Helicobacter pylori infection. Br J Surg 1998; 85(6): 850-852.
- Czesnikiewicz-Guzik M, Bielanski W, Guzik TJ, Loster B, Konturek SJ. Helicobacter pylori in the oral cavity and its implications for gastric infection, periodontal health, immunology and dyspepsia. J Physiol Pharmacol 2005; 56 Suppl 6: 77-89.
- Kignel S, de Almeida Pina F, Andre EA, Alves Mayer MP, Birman EG. Occurrence of Helicobacter pylori in dental plaque and saliva of dyspeptic patients. Oral Dis 2005; 11(1): 17-21.
- Kilmartin CM. Dental implications of Helicobacter pylori. J Can Dent Assoc 2002; 68(8): 489-493.
- Pytko-Polonczyk J, Konturek SJ, Karczewska E, Bielanski W, Kaczmarczyk-Stachowska A. Oral cavity as permanent reservoir of Helicobacter pylori and potential source of reinfection. J Physiol Pharmacol 1996; 47(1): 121-129.
- Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest 2001; 107(7): 767-773.
- Konturek P, Konturek S, Pierzchalski P, et al. Cancerogenesis in Helicobacter pylori infected stomach – role of growth factors, apoptosis and cyclooxygenases. Med Sci Monit 2001; 7(5): 1092-1107.
- Birek C, Grandhi R, McNeill K, Singer D, Ficarra G, Bowden G. Detection of Helicobacter pylori in oral aphthous ulcers. J Oral Pathol Med 1999; 28(5): 197-203.
- Riggio MP, Lennon A, Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med 2000; 29(10): 507-513.
- Thomas E, Jiang C, Chi DS, Li C, Ferguson DA, Jr. The role of the oral cavity in Helicobacter pylori infection. Am J Gastroenterol 1997; 92(12): 2148-2154.
- Siavoshi F, Salmanian AH, Akbari F, Malekzadeh R, Massarrat S. Detection of Helicobacter pylori-specific genes in the oral yeast. Helicobacter 2005; 10(4): 318-322.