Helicobacter pylori (Hp) is one of the most common pathogenic microbes in humans. It causes inflammation of gastric mucous, gastro-duodenal ulcers; it is also a first class carcinogen, participating in formation of gastric cancers and MALT
lymphoma. Clinical symptoms of Hp infection are mostly manifested in adulthood, despite of that this bacteria is also found in the pediatricians’ scope of interest, since epidemiological studies in the developing countries show that infection most often occurs in childhood, although then it is asymptomatic.
Poland is a country with high frequency of Hp infection incidence in adults (1). So far studies on Hp epidemiology in children has been limited, especially in the youngest children.
The work was aimed at establishment of frequency of Hp infection incidence in the population of children below 4 years of age from the municipal Cracovian region.
Research was approved of the Ethical Committee for Clinical Research at the
Collegium Medicum of the Jagiellonian University No. KE/II/67/KI/97,
and was financed by the grant KBN No. 4 PO5E 052 18.
MATERIAL AND METHODS
From 1999 until 2001 the study was carried out on randomly selected healthy children aged from 6 months to 4 years, attending Healthy Child Centres in Cracovia for their immunization or physical assessment of their health. The exclusion criteria were one month period on antibiotics prior to testing.
Information on studied children and their families was obtained with the use
of a questionnaire and the Hp infection was diagnosed using
13C
urea breath test (UBT).
The questionnaire consisted of 24 questions, which pertained to a child, his parents and his siblings, as well as socioeconomic conditions of a family.
Questions in the part referring to a child were related to its sex, birth weight, present body mass and the height, period of breastfeeding by the mother, using a pacifier, attending créches or kindergartens, appetite, present or past symptoms from the digestive tract, antibiotic treatment, hospital stays. In the part refereeing to the family questions pertained to the parents’ education level and occupation, average income, symptoms and illnesses of the digestive tract of the family members. Among them there were questions on abdominal pain, gastro-duodenal ulcers, Hp infection.
In the part on the standards of living there were questions on density of the living space, sources of drinking water, presence of pets, cigarette smoking.
Urea breath test (13C-UBT)
Testing was performed on an empty stomach, in case of babies it was at least 4 hours from the last feeding, and older children were tested after a night break from feeding. The test was carried out in accordance to a uniform protocol by one person.
Each child received the same amount of 50mg
13C-urea,
regardless of the body weight, dissolved in 50ml of apple juice of pH 4. After
3 minutes, 50 ml of apple juice was given again to children in order to flush
the remains of urea from the mouth cavity. Air was collected before administering
of
13C-urea and in the 20
th
and 30
th minute afterwards into aluminum sacs
Quintron (Boston, USA). Then, the air was aspirated with a 10 ml syringe and
was injected into vacuum test tubes (Labco vacutainer).
Exhaled
13CO
2
was measured using the isotope mass spectrometer HeliView (Medichems, South
Korea) in the Isotope Laboratory, Chair of Physiology, Jagiellonian University
Medical College.
Results were given as quotient of exhaling
13CO
2
into
12CO
2 i.e.
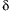
(DOB –
delta over baseline), and a positive result, evidencing infection,
was accepted when
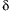
was greater than 3,5‰. In a case of positive result,
13C
UBT were performed in parents and other persons living in the same household
or taking care of the child.
Statistical methods
In statistical analysis of the material the following statistical data were
employed:
- t-Student Test - to compare a quantity variable – age.
 2
Test – to compare quality variables.
2
Test – to compare quality variables.- Exact Fisher Test - using which the impact of particular factors on Hp
infection was measured.
- Additionally quotient of chances and median unbiased confidence interval
for quotient of chances were calculated.
RESULTS
One hundred ninety eight children were tested aged between 5,61 months to 51,08 months (average 32 ± 11,75). Three children were excluded, because the air for breath test was not collected properly – after analysis of air in test tubes carbon dioxide was not detected. From further analysis 4 children were excluded because their parents did not fill in questionnaires and 6 children, because their d value was exactly 3,5‰, therefore these results were considered as equivocal.
Finally the analyzed group consisted of 185 children (79 girls and 106 boys), and Hp infection was found in 34 children, which was 18,38% of tested children.
Average age of infected children was 28,94 ± 14,01 months (ranging 5,61 – 50,36), and healthy children 33,85 ± 11,03 months (ranging 6,39 - 51,08). This difference was not statistically significant.
The infection was found, respectively, in:
- a group under one year of life – in 4 children (44,4%)
- a group between one and three years of life - in 16 children (17,20%)
- a group of children above three years - in 14 children (16,86%) (
Fig.1).
 |
| Fig. 1.
Prevalence of Hp infection in different age groups. |
Because of small quantity the group of children under one year was not consider in statistical analysis. Differences in infection frequency among particular age groups were not statistically significant. Also Hp infection was not dependent on sex, birth weight, breastfeeding, use of pacifier, frequent antibiotics treatment and hospitalization of tested children. No correlation between Hp infection and their parents’ education level, cigarette smoking, density of living space or contacts with pets was found.
Only statistical significant finding was that Hp infection was more frequent
among children between the first and third year of life attending créches or
kindergartens (32,1%, p<0,02) (
Table 1).
| Table 1.
Hp infection in children attending creches or kindergarden. |
 |
| *p<0,02 , OR 3,93 (1,11-14,06) |
In the whole Hp infected group children had significantly more often good or medium appetite – 30 children (88%), and the bad one only 4 children (12%) - p<0,01.
There was no Hp infection impact on somatic development in infected children.
It was no association between Hp presence and any digestive tract disorders,
and half of infected children were completely asymptomatic. The occurrence of
Hp infection was found among 41 members in 13 infected families. In 21 families
nobody except a child was infected. This difference was statistically significant
(p<0,03). The statistically significant Hp infection in children was more frequent
if family members were also infected (OR=2,71, 95% median unbiased confidence
interval 1,1-6,47) (
Table 2). Risk of infection was noticeably higher
in a group of children above three years of age, but without statistical significance
(p<0,11).
| Table 2.
Hp infection in families |
 |
| p<0,03 , OR 2,71 (1,1-6,47) |
Infection of a mother, as a parameter analyzed separately, did not increase occurrence of Hp infection in children in a statistically significant manner.
DISCUSSION
Hp infection is causative agent of inflammatory process of a peptic ulcer disease (2 - 6). However, gastritis due to Hp etiology without duodenal ulcer often has a completely asymptomatic course (7-12). Therefore, there are no indications to treat the infection itself, without accompanying symptoms of ulcers (13). Since acquisition of Hp happens in childhood its early presence might be essential in the long process of the development to the stomach cancer. It is particularly important, because the presence of intestinal metaplasia in the stomach of Hp infected children has been described recently (14). The question arises, should pediatricians treat all Hp infected children?
Then epidemiological screening testing requires simple, cheap, highly sensitive
and specific methods. Moreover, in case of children such test should not be
invasive, either, not to arise reluctance in children and parents alike. That
is why UBT for children requires the use of non-radioactive carbon isotope
13C.
It seems that the test is a very useful method in conducting epidemiology studies
in children, however it is not perfect one (15 – 17). There are some essential
questions concerning timing of reading and cut- off value of
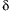
over the baseline. Our test was based on a protocol independently developed
by Rowland (18) and a group of Italian researchers (19). These authors showed
that the results at the 20
th and 30
th
minute were characterized by sensitivity reaching 89,9% and specificity 100%
in comparison to the golden standard, i.e. histological examination and rapid
urease test (19). The child’s age determined a meal for the test, therefore,
apple juice was chosen to be acceptable by different groups (15).
The cut-off value of
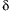
has been discussed for children. It is known that UBT outcome is a result of
two processes, one depends on the bacteria, and the other on its host. Hp bacteria
releases labeled
13CO
2,
from administered
13C-urea, which is eliminated
together with a much larger amount of
12CO
2,
coming from metabolic processes. Therefore, the degree of a
13CO
2
dilution by a host’s CO
2 determines the degree
of the increase of its elimination, defined as quotient
13CO
2
/CO
2. In an average adult person
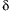
value over 2,4‰ is evidencing the infection, and expresses the increase of elimination
of
13CO
2 only
by 26 ppm, from 10882 ppm to 10908 ppm. Production of CO
2
changes depending on age (adults>children), sex (men>women), weight, height.
Therefore, the same amount of urea labelled with
13C
hydrolyzed in a child’s stomach will cause a proportionally higher increase
of
13CO
2 elimination
than in an adult person (20).
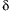
value in children, therefore, must be higher to eliminate a danger of too large
a number of falsely positive results. It seems that
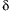
value >3,5‰, used in our test, is sufficient to avoid this error (21-23), however,
some authors stress the fact that the children’s age affects greatly the results
of UBT and postulate to increase the
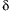
value to 5‰ in children under the age of two years (15).
In recent years the development of non-invasive diagnostic methods can be observed. Testing for presence of Hp antigen in stools using a mono- and polyclonal antibodies by the Elisa method is simpler and cheaper than UBT testing. High sensitivity and specificity of this test was shown (88-100% and 83-100%, respectively) (24). This method can also be used to control eradication, earlier than UBT, most likely a week following the completion of treatment (24).
Poland is a country of high Hp incidence among adults (1). However, there are few data pertaining to children. In study of Bielanski and co-workers was shown, that 28,65% of children at 3-5 years and 40% at age 18 was infected. Anti-Hp antibodies were detectable in only 45% patients at age 3-8. IgA-antibodies results were consistent neither with anti-Hp IgG Ab nor UBT (25,26). Frequency of infection incidence confirmed in this research is relatively high -18,38%, taking into consideration the fact, that it relates to young children, under the age of four. Increase of infection incidence frequency co-related with the age of tested children has not been confirmed at the tested age group.
In our study attending a créche by a child was a significant risk factor in Hp infection incidence in children. According to data from France and Sweden attending a créche or a kindergarten was not a factor increasing Hp infection in children (27, 28). French authors, however, showed the discrepancy between their data and reports on higher risk of infection among residents of social service homes for neurological patients, orphanages or submarine crews (27, 29). There are evidences that Hp infection is affected by density of a living space, what has been proven in developing countries (30, 31). This factor should be taken into account when parents consider sending a child to a créche or a kindergarten in view of a risk of spreading Hp infection among siblings and families (30, 32). The hypothesis on increased susceptibility to Hp infection in childhood and transmitting infection among children is based on such premises as hygienic habits insufficiently adopted at this age as well as close physical contact with infected adults or children (in childcare facilities) (30 - 32). In developed countries these factors may be of lesser importance due to a high sanitary and hygienic standards of such facilities and the fact that children attending them usually come from higher socioeconomic conditions, what is rather a protective factor against Hp infection (27, 30, 31). In Poland a créche is usually attended by children from various milieus.
In our study Hp infection did not cause any specific clinical symptoms, and a half of infected children had completely asymptomatic course. According to the literature a large percentage of children with abdominal pain are infected with Hp, but eradication of infection is not relieving of clinical symptoms (33). In ESPGHAN expert committee statement on Hp infection treatment in children, the indication for the triple therapy is only when there is confirmed an ulcer or chronic inflammation of the stomach (33, 34).
In available literature there are contradictory reports whether Hp infection significantly disturbs the growth of children (35 – 37). The authors, considering probably causes, stress the coexistence of infection with bad socioeconomic living standards as the most likely cause (36). However, they lean towards a thesis that the association is indirect, and the mechanism of this disorder requires further research. They also speculate that the decrease of a number of Hp infected persons is one of the reasons of developmental acceleration observed in developed countries (35). But the impact of infection on the children’s height in quoted literature was statistically significant only in older children, i.e. over eight years of age, and our children were younger. Research, recently published by Nwokolo and coworkers, showed that the level of ghrelin significantly drops in Hp infected persons and the eradication of this bacteria leads to the increase of the levels of ghrelin (38). Since ghrelin increases food intake and appetite there are suggestions that the increase of this hormone after Hp eradication may be responsible for excessive appetite followed by obesity, gastroesophageal reflux and a risk of esophagus cancer. A decreased ghrelin level in Hp infected individuals, especially in childhood, may contribute to retardation of their growth connected with lower BMI (Body Mass Index) (38).
Hp infection inside a family is one of the strongest epidemiological factor in spreading the disease, regardless of other socioeconomic and environmental factors. In this study it was confirmed that Hp prevalence in children was statistically significantly higher if family members were also Hp infected (OR=2,71). Risk of infection was noticeably higher in a group of children above three years of age.
It has been proven that Hp strains obtained from children and their parents have the identical DNA restrictive pattern, indicating that particular members of the family are infected by the same strain (39, 40). German authors stress the fact that the Hp infection incidence in the child’s mother, especially when she suffers from ulcers, has much greater impact on spreading of the infection. It seems understandable due to the closer contact, especially in the early childhood (41). This association was not shown in our study.
Annual incidence of Hp infection in children depends on economic development of a country and ranges between 0,3-4% (42, 43). At fifth year of life the status of infection becomes stabilized, and the major risk factor is a low level of socio-economic standard (31). Children are much more susceptible to infection than adults, due to the insufficiently developed immunological system of the digestive tract.
Summing up, it can be confirmed that the frequency of Hp infection incidence in tested population of healthy children under the age of four in the municipal environment of Cracow is relatively higher (18,38%), when compared with that in Western European countries (Germany 11,3%, France 7,3%, Switzerland 6,5%). One can hope that the progressive improvement of socio-economic conditions also in our country shall cause the decrease of incidence to Hp infection in children. Special attention should be focused on health education popularizing hygienic habits, which has immense significance in limiting the spread of many infections, not only Hp. It is the more important since the decrease of Hp infection is expected to decrease the occurrence of gastric cancer. UBT is simple, highly reliable test, that can be considered as non-invasive “gold standard”, especially in children, where UBT detects Hp infection before immunological response is detectable.
REFERENCES
- Matysiak-Budnik T, Knapik Z, Megraud F, et al. Helicobacter pylori infection in Eastern Europe: seroprevalence in the polish population of Lower Silesia. Am J Gastroenterol 1996; 91: 2513-2515.
- Sipponen P, Marshall B. Gastritis and gastric cancer. Western Countries. Gastroenterol Clin North Am 2000; 29: 579-592.
- Moayyedi P, Feltbower R, Brown J, et al. Effect of population screening and treatment for Helicobacter pylori on dyspepsia and quality of life in the community: a randomised controlled trial. Lancet 2000; 355: 1665-1669.
- Danesh J, Whincup P, Walker M, et al. High prevalence of potentially virulent strains of Helicobacter pylori in the general male British population. Gut 2000; 47: 23-25.
- Ruskone-Fourmestraux A, Lavergne A, Aegerter P, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut 2001; 48: 297-303.
- Brenner H, Bode G, Boeing H. Helicobacter pylori infection among offspring of patients with stomach cancer. Gastroenterology 2000; 118: 31-35.
- Bode G, Rothenbacher D, Brenner H, et al. Helicobacter pylori and abdominal symptoms: a population-based study among preschool children in Southern Germany. Pediatrics 1998; 101: 634-637.
- Louis-Jacques O, Perman J. Gastroduodenal disorders in children. Curr Opin Gastroenterol 2000; 16: 522-526.
- Imrie C, Rowland M, Bourke B, Drumm B. Is Helicobacter pylori infection in childhood a risk factor for gastric cancer? Pediatrics 2001; 107: 373-380.
- Macarthur C. Helicobacter pylori, non-ulcer dyspepsia, and childhood recurrent abdominal pain. Pediatr Res. 2001;107: 140.
- Macarthur C. Is there an infectious etiology to abdominal pain in children? J Pediatr Gastroenterol Nutr 2000; 30: 112.
- Frank F, Stricker T, Stallmach T, et al. Helicobacter pylori infection in recurrent abdominal pain. J Pediatr Gastroenterol Nutr 2000; 31: 424-427.
- Huang F, Chang M, Hsu H, et al. Long term follow-up of duodenal ulcer in children before and after eradication of Helicobacter pylori. J Pediatr Gastroenterol Nutr 1999; 28: 76-80.
- Guarner J, Bartlett J, Whistler T, et al. Can pre-neoplastic lesions be detected in gastric biopsies of children with Helicobacter pylori infection? J Pediatr Gastroenterol Nutr 2003; 37: 309-314.
- Kindermann A, Demmelmair H, Koletzko B, et al. Influence of age on 13 C-urea breath test results in children. J Pediatr Gastroenterol Nutr 2000; 30: 85-91.
- Koletzko S, Feydt-Schmidt A. Infants differ from teenagers: use of non-invasive tests for detection of Helicobacter pylori infection in children. Eur J Gastroenterol Hepatol 2001; 13: 1047-1052.
- Amarri S, Celinska-Cedro D. Paediatrics in: The year in Helicobacter pylori
2001. Curr Opin Gastroenterol 2001; 17: S32-S37.
- Rowland M, Lambert I, Gormally S, et al. Carbon 13-labeled urea breath test for the diagnosis of Helicobacter pylori infection in children. J Pediatr 1997; 131: 815-820.
- The Bologna 13 C-Urea Breath Test User Group. The 13 C-urea breath test for the diagnosis of Helicobacter pylori infection in children. Ir J Med Sci 1997; 40: S70.
- Klein P, Malaty H, Czinn S, et al. Normalizing results of 13 C-urea breath testing for CO2 production rates in children. J Pediatr Gastroenterol Nutr 1999; 29: 297-301.
- Koletzko S, Feydt-Schmidt A. Infants differ from teenagers: use of non-invasive tests for detection of Helicobacter pylori infection in children. Eur J Gastroenterol Hepatol 2001; 13: 1047-1052.
- Cadranel S, Corvaglia L, Bontems P, et al. Detection of Helicobacter pylori Infection in children with standarized and simplified 13 C-urea breath test. J Pediatr Gastroenterol Nutr 1998; 27: 275-280.
- Bazzoli F, Cecchini L, Corvaglia L, et al. Validation of the 13 C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a multicenter study. Am J Gastroenterol 2000; 95: 646-650.
- Rothenbacher D, Bode G, Brenner H. Diagnosis of Helicobacter pylori infection with a novel stool antigen-based assay in children. Pediatr Infect Dis J 2000; 19: 364-366.
- Bielanski W, Kacka K, Karczewska E , et al. Capsulated mini-dose 13 C-urea breath test for studies on the prevalence of Helicobacter pylori infection in children. J Physiol Pharmacol 1997; 48 suppl1:17.
- Bielanski W, Plonka M, Dobrzanska M, et al. Studies on the prevalence of Helicobacter pylori infection in polish children and in adult population using modified capsulated urea breath test. Gut 1997; 41 suppl 3: A 163
- Wizla-Derambure N, Michaud L, Ategbo L, et al. Familial and community environmental risk factors for Helicobacter pylori infection in children and adolescents. J Pediatr Gastroenterol Nutr 2001; 33: 58-63.
- Tindberg Y, Blennow M, Granstrom M. Clinical symptoms and social factors in a cohort of children spontaneusly clearing Helicobacter pylori infection. Acta Paediatr 1999; 88: 631-635.
- Hammermeister I, Janus G, Schamarowski F, et al. Elevated risk of Helicobacter pylori infection in submarine crews. Eur J Clin Microbiol Infect Dis 1992; 11: 9-14.
- Goodman K, Correa P, Tengana Aux H, et al. Helicobacter pylori infection in Colombian Andes: a population based study of transmission pathways. Am J Epidemiol 1996; 144: 290-299.
- Everhart J. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am 2000; 29: 559-578.
- Goodman K, Correa P. Transmission of Helicobacter pylori among siblings. Lancet 2000; 355: 358-362.
- Drumm B, Koletzko S, Oderda G. Helicobacter pylori infection in children: a consensus statement. JPGN 2000; 30: 207-213.
- Ernst P, Gold B. Helicobacter pylori in childhood: new insights into the immunopathogenesis of gastric disease and implications for managing infection in children. J Pediatr Gastroenterol Nutr 1999; 28: 462-473.
- Perri F, Pastore M, Leandro G, et al. Helicobacter pylori and growth delay in older children. Arch Dis Child 1997; 77: 46-49.
- Pehlivanoglu E, Ertem D, Harmanci H. Does Helicobacter pylori infection affect growth? 34th Annual Meeting of ESPGHAN 2001, Abstracts Book, P146.
- Kehrt R, Becker M, Brosicke H, et al. Prevalence of Helicobacter pylori infection in Nicaraguan children with persistent diarrhea, diagnosed by the 13 C-urea breath test. J Pediatr Gastroenterol Nutr 1997; 25: 84-88.
- Nwokolo CU, Freshwater DA, Hare PO, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut 2003; 52: 637-640.
- Dzierzanowska D, Gzyl A, Rozynek E, et al. PCR for identification and
typing of Helicobacter pylori isolated from children. J Physiol Pharmacol
1996; 47: 101-114.
- Miehlke S, Genta R, Graham D, et al. Molecular relationships of Helicobacter pylori strains in a family with gastroduodenal disease. Am J Gastroenterol 1999; 94: 364-368.
- Rothenbacher D, Bode G, Berg G, et al. Helicobacter pylori among preschool children and their parents: evidence of parent-child transmission. J Infect Dis 1999; 179: 398-402.
- Xia H, Talley N. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implication. Am J Gastroenterol 1997; 92: 1780-1787.
- Everhart J. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am 2000; 29: 559-578.


