NF-

B
is a cytoplasmic transcription factor, which is activated by a variety of inducers
including hormones, growth factors, viruses, chemicals, reactive oxygen species
(ROS), reactive nitrogen species (RNS), and cytokines. NF-

B
triggers genes associated with processes such as inflammation, inhibition of
apoptosis, growth and differentiation. In the unstimulated state, NF-

B
is bound in the cytosol to its inhibitory protein, I-

B
alpha.
The classical pathway of NF-

B
activation involves phosphorylation of I-

B
alpha
by IK kinase-1, release of I-aBa from NF-

B
subunits P65 and P50, which undergo nuclear translocation and modulation of
gene expression. Concomitantly, NF-

B
activates the I-

B
alpha
gene, resulting in synthesis and nuclear translocation of unphosphorylated I-

B
alpha
that binds to NF-

B
and translocates it back to the cytoplasm.
Cigarette smoke (CS) has been associated with a variety of human pathologies
including cardiovascular diseases and atherosclerosis, lung diseases, cancer,
and infertility (1). In the pulmonary system, CS is implicated in the development
and exacerbation of chronic bronchitis, asthma, and lung cancer (2). One of
the proposed mechanisms by which CS exerts its effect on the airways and lungs
is chronic activation of inflammatory processes by CS toxins (3, 4). Even mild
environmental exposure to CS ("passive smokers") causes pulmonary disease to
develop due to activation of the inflammatory system (5). This activation involves
continuous recruitment of neutrophils and macrophages (5). Superoxide and NO
present in CS react to form peroxynitrite anion (ONOO
-),
a RNS involved in protein nitration and nitrosylation, which has been associated
with a variety of pathological conditions (6). Our previous work (unpublished)
has shown that human lymphocytes exposed to mild, but not high, intensity CS
exhibited activation of NF-

B,
which is mediated by formation of peroxynitrite through a reaction between smoke
derived NO and endogenously produced superoxide. In the current study our aim
was to examine the activation of NF-

B
by SNAP, S-nitroso-
N-acetylpenicilamine (NO donor) and SIN-1, 3-morpholine-sydnoimine
(peroxynitrite donor) and evaluate the effect of CS-induced NF-

B
activation on lymphocyte apoptosis, as an indicator of chronic inflammation.
MATERIAL AND METHODS
The study was approved by an institutional Ethics Committee. A L8 rat myoblast cell culture system was used for the evaluation of NF-

B activation by SNAP and SIN-1, as previously described (7, 8). Human lymphocytes were used for the evaluation of cigarette smoke-induced
NF-

B activation and were cultured and grown, as previously described (9, 10).
Electromobility shift assay (EMSA).
The effect of SNAP treatment on NF-

B activation in L8 myoblasts was measured by the EMSA assay, as previously described (9). L8 cells were treated with 10 mM SNAP for 10, 20, 30, 60, and 120 min and with 20 mM SNAP for 10, 20, and 30 min. Equal amounts of nuclear extracts were added to each lane. Nuclear extracts were analyzed by EMSA using 32P-labeled kB consensus oligonucleotides sequence of NF-

B promoter. The effect of SIN-1 treatment on NF-

B activation was also evaluated by EMSA in the identical conditions as those described for SNAP.
FACS-Annexin V assay
NF-

B is activated by cigarette smoke and is also known to inhibit apoptosis in various cell lines, including human lymphocytes. Thus, we evaluated the effect of smoke-induced activation of NF-

B on apoptosis of human lymphocytes after exposure of lymphocytes to low intensity cigarette smoke for 8 h. Control lymphocytes were exposed to atmospheric air for the same period. Then, cells were collected, stained for Annexin V and proprium iodide, and then underwent counting and analysis by FACS assay.
RESULTS
NF-

B was activated
by SNAP in a time and concentration-dependent manner. Using both 10 mM and 20
mM SNAP concentrations, NF-

B
activation and nuclear translocation was identified already after 10 min, followed
by a gradual decrease (
Fig. 1). Using SIN-1, NF-

B
activation and nuclear translocation was identified after 10 min, but no decrease
was noted through 120 min (
Fig. 2). In the FACS-Annexin V assay, lymphocytes
exposed to mild intensity of cigarette smoke for 8 h, which activated NF-

B,
exhibited a decrease in the fraction of apoptotic cells from 22% to 13% compared
with lymphocytes exposed to atmospheric air (
Fig. 3A). The mean decrease
in apoptotic cells in 3 identical experiments was from 27% to 19% (
Fig. 3B).
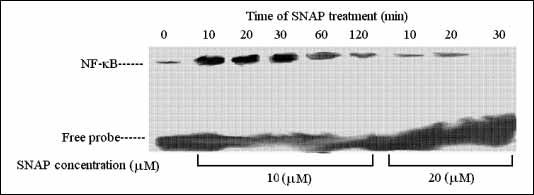 |
Fig. 1.
Time-dependent activation of NF- B
by two concentrations of SNAP using EMSA. SNAP concentrations in µM. B
by two concentrations of SNAP using EMSA. SNAP concentrations in µM. |
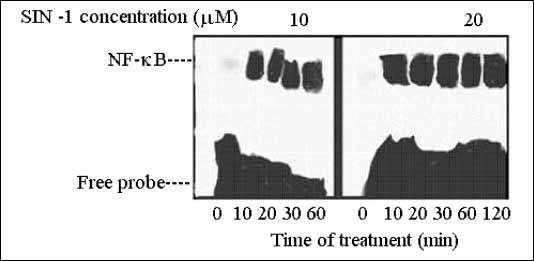 |
Fig. 2.
Time-dependent activation of NF- B
by two concentrations of SIN-1. SIN-1 concentrations in µM. B
by two concentrations of SIN-1. SIN-1 concentrations in µM. |
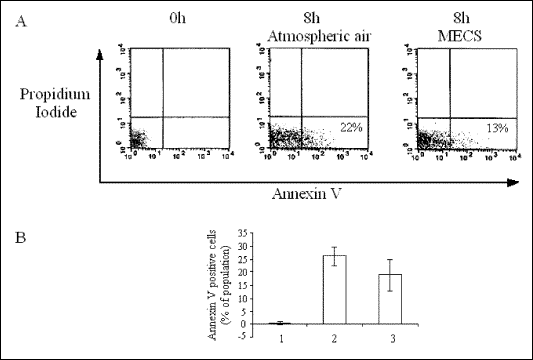 |
| Fig. 3.
A - Staining for Annexin V and propidium iodide as an index for lymphocyte
apoptosis following exposure to low intensity cigarette smoke. In each
square, cells in the lower right quadrant (Annexin V positive, Propidium
Iodide negative) represent apoptotic cells. Left square - cells immediately
after collection, right square - following 8 h of exposure to mild intensity
cigarette smoke, middle square - following 8 h of exposure to atmospheric
air. MECS - Mild Exposed Cigarette Smoke. B - Mean ±SD of apoptotic cells
from three different experiments. |
DISCUSSION
SNAP activates NF-

B
in the classical pathway in the rat myoblasts system, which may lead to transient
inflammatory response. As shown in
Fig 1, upon SNAP treatment, the NO
produced activates IKK, which phosphorylates I-

B
alpha,
leading to its ubiquitination and subsequent degradation. The released activated
NF-

B translocates
to the nucleus and activates a variety of genes, including the gene for I-

B
alpha
itself, leading to its
de novo synthesis, which causes feedback inhibition
and deactivation of NF-

B
(
Fig 4).
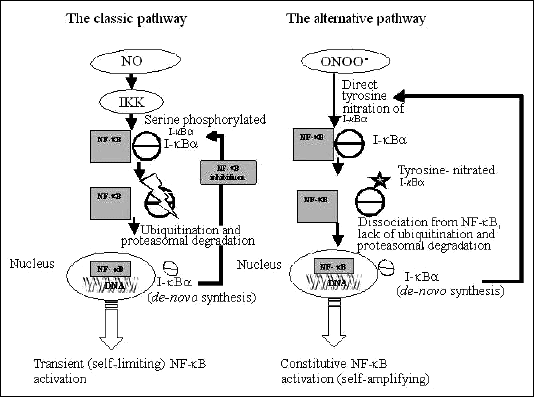 |
Fig. 4.
Proposed mechanism for the two distinct patterns of NF- B
activation by SNAP and SIN-1. B
activation by SNAP and SIN-1. |
On the other hand, NF-

B
activation by SIN-1 is mediated by an alternative pathway, leading to a prolonged
inflammatory reaction. In previous works it was shown to involve I-

B
alpha
nitration rather than phosphorylation. Upon activation of NF-

B
by SIN-1, the prolonged exposure of ONOO
- causes
nitration of I-

B
alpha,
rather than phosphorylation. This prevents its ubiquitination and further degradation,
and ultimately leads to non-transient, prolonged activation of NF-

B
(8) (
Fig. 4). Mild exposure to cigarette smoke induces NF-

B
activation, which can attenuate apoptosis in human lymphocytes and leads to
increased inflammatory response.
In summary, chronic inflammatory response evoked by peroxynitrite-induced activation of NF-

B may lead to tissue-specific deleterious biological consequences. In human lymphocytes, this may result in interference with apoptosis regulation and increased risk for chronic inflammation and malignant diseases. In rat myocytes, it may lead to increased protein breakdown and muscle atrophy. Therefore, we assume that peroxynitrite may be one of key mediators in physiological and pathological inflammatory processes.
Acknowledgments: This work was supported by the Krol foundation of Lakewood, NJ, and a grant from the Vice President of Research, Technion, Israel.
REFERENCES
- Mallampalli A, Guntupalli KK. Smoking and systemic disease. Med Clin North Am 2004; 88: 1431-1451.
- Eyre H, Kahn R, Robertson RM. Preventing cancer, cardiovascular disease, and diabetes - a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004; 109:3244-3255
- van der Vliet A, Cross CE. Oxidants, nitrosants, and the lung. Am J Med 2000; 109: 398-421.
- Radi R, Pelluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med 2001; 30: 463-488.
- Waldren CA, Vannais DB, Knowlton MS, Domenico KK, Smith CJ, Doolittle DJ. The role of glutathione in the toxicity of smoke condensates from cigarettes that burn or heat tobacco. Free Radic Biol Med 2003; 30: 1400-1406.
- Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med 2001; 30: 463-488.
- Bar-Shai M, Reznick AZ. Peroxynitrite induces an alternative NF-
 B activation pathway in L8 rat myoblasts. Antioxid Redox Signal 2006; 8: 639-652.
B activation pathway in L8 rat myoblasts. Antioxid Redox Signal 2006; 8: 639-652.
- Bar-Shai M, Reznick AZ. Reactive nitrogen species induce NF-
 B-mediated protein degradation in skeletal muscle cells. Free Radic Biol Med 2006; (in press).
B-mediated protein degradation in skeletal muscle cells. Free Radic Biol Med 2006; (in press).
- Sen CK, Khanna S, Reznick AZ, Roy S, Packer L. Glutathione regulation of tumor necrosis factor-alpha-induced NF-kappa B activation in skeletal muscle-derived L6 cells. Biochem Biophys Res Commun 1997; 237: 645-649.
- Hasnis E, Reznick AZ, Pollack S, Klein Y, Nagler RM. Synergistic effect of
cigarette smoke and saliva on lymphocytes- the mediatory role of volatile
aldehydes and redox active iron and the possible implications for oral cancer.
Int J Biochem Cell Bio 2004; 36: 826-839.



