The goal of the present study was to investigate the relationship between specific antibody profiles and host factors (age and sex), clinical presentation of TB infections, and cellular immune response measured as the TST response in children grouped into four diagnostic categories: primary and postprimary active disease, latent infection without radiographic chest abnormalities, and a TB-free control group.
Subjects
All patients came from the Department of Pediatric Pneumology of Warsaw Medical University in Warsaw, Poland. All subjects and their parents gave informed consent for participation in the study and the study protocol was approved by a local Ethical Committee. Serum samples from 224 children were examined. The subjects were Caucasian, HIV negative, and previously BCG vaccinated. They were divided into several groups depending on the results of clinical and radiological findings: 81 primary TB cases (41 cases of isolated lymphadenopathy, 33 cases of lymphadenopathy and mild lung parenchymal changes, 3 pleural TB, 2 kidney TB, 2 miliary TB including CNS involvement), 31 postprimary cases (cavitary and noncavitary), 30 cases of latent TB (diagnosis was made on the basis of TB contact and TST >15 mm, normal radiological picture, and lack of clinical symptoms and signs, all these children received isoniasid chemoprophylaxis), 82 control children with atopic rhinitis, asthma, nonspecific respiratory tract infections, and healthy children. No evidence of clinical, microbiological, or radiographic signs of pulmonary TB was seen in the control group. Demographic characteristics of the examined groups are presented in Table 1.
| Table 1. Characteristics of the children examined. |
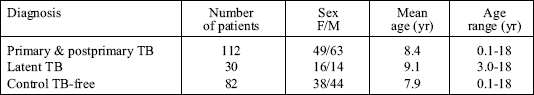 |
Diagnosis of TB was confirmed by sputum, bronchial lavage, or gastric lavage culture in 25 of the TB cases. The examination of acid fast bacilli was made using the Ziehl-Neelsen stain method, culture on Löwenstein-Jensen solid egg-based medium, or by a Bactec system. In the remaining 87 patients, TB diagnosis was reached through a panel decision on the base of suggestive clinical and radiological signs (in the absence of evidence for alternative infective or non-infective processes), epidemiological investigations, TST, bronchoscopy findings, and was reevaluated after specific antituberculosis treatment. Ninety patients did not receive antituberculosis treatment at the time of blood collection, and in the 22 remaining cases, the therapy was shorter than 1 month. Three patients received oral corticosteroids (1 case of pleural involvement, 2 cases of central nervous system involvement).
Analytical assays
Blood samples were collected, centrifuged, and serum was stored at -40°C until use. An array of commercial immunoenzymatic kits to detect IgG antibodies against A-60 (Immunozyme Mycobacterium; Assay Designs, Ann Arbor, MI, USA), 38 kDa plus 16 kDa (Pathozyme tb complex plus; Omega Diagnostics, Scotland), and IgG, IgA, and IgM antibodies to 38 kDa plus lipoarabinomannan (LAM) (MycoG, MycoA, and MycoM; Omega Diagnostics, Scotland) were applied. 38-kDa and 16-kDa are recombinant mycobacterial antigens specific for M. tuberculosis complex expressed in and purified from E. coli (11). A-60 is a huge molecule derived from tuberculin, composed of lipids, polysaccharides, and proteins found in all mycobacteria species. LAM is a component of the bacterial wall present in all mycobacteria (12). All tests are based on a solid double antibody sandwich ELISA. Sera diluted 1:50 or 1: 100 (according to the manufacturer's instruction) were added to microwells precoated with antigens. All samples were assayed in duplicates. In positive cases, an antigen-antibody complex reacted with peroxidase-labeled antihuman IgG (IgA or IgM) conjugate. Using H2O2/TMB as substrate, the enzymatic activity of peroxidase was measured at 450 nm with the use of an automated reading system ELX800 (Bio-Tek Instruments, Winooski, VT, USA). All the results, except for the IgM tests, were referred to the standard curve. Standards were provided for the generation of a semi-logarithmic reference curve. As the sera were diluted 1:50 or 1:100, the units extrapolated from the standard curve were multiplied by 50 (or 100) to obtain serounits for the result interpretation. IgM test results were expressed as the ratio of optical density (OD) of the examined sample to OD of the cut-off sample. Immuno-enzymatic assays were performed blindly by a laboratory technician.
TST was performed in all individuals of this study using two tuberculin units of PPD of M. tuberculosis, strain RT-23, supplied by the Copenhagen World Health Organization reference laboratory (Copenhagen, Denmark) Testing and reading was done according to international guidelines (13). Both intradermal injections of 0.1 ml solutions were administered by a trained nurse into the volar surface of the left forearm. The diameters of indurations were measured 72 h after inoculations; induration of
Data analysis
Results are expressed as means ąSD. Results of antibody titers were compared by the U Mann-Whitney test. Statistical significance was accepted at a level of P<0.05. Univariate and multivariate analysis of variance with the Tukey post-hoc test were used to estimate the independent association of antibody response with the patients' demographic and clinical characteristics. The Pearson test was used for the analysis of correlations between the age of patients and antibody production. A commercial Graph Pad Prism version 4 was used for all statistical data elaboration.
The mean antibody levels in the groups examined are presented in Table 2. In the group of children with pulmonary TB, the IgG levels were higher than those in the control group, but the difference was significant only for the antibodies against 38kDa and 16kDa. These differences in the IgG levels, although in general smaller, persisted also in children with lymph nodes involvement. No differences were observed between the IgM levels in the groups examined.
| Table 2. Antibody levels in the groups of children studied. |
 |
| Values are means ąSD. |
To evaluate the dynamics of antibody production in relation to age, the analysis was performed in children stratified into the following age-groups: 0-2, 3-5, 6-9, and 10-18 years old. The mean antibody levels were compared with those in the control group (Table 3). The IgG and IgA levels were significantly higher only in the two oldest groups. No differences between the TB and control groups were observed in children below 6 years of age. The IgM level did not differ between any of the groups examined.
| Table 3. Antibody levels in different age groups of TB children compared with non-TB controls. |
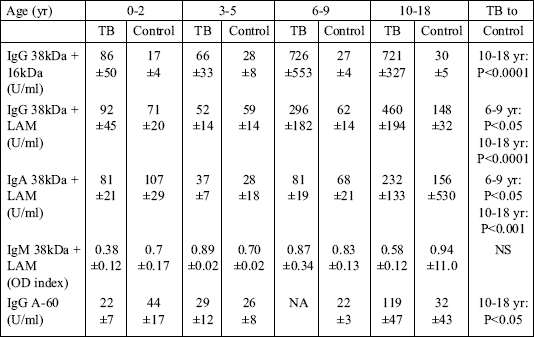 |
| Values are means ąSD. NA - data not available. |
Humoral responses to all the antigens examined positively correlated with the age of children (data not shown). The correlation coefficients were the following: for IgG anti 38-kDa r=0.57, P<0.0001; for IgG anti 38+16-kDa r=0.39, P<0.001; for IgG anti 38kDa+LAM r=0.34 P<0.05; for IgA r=0.29, P<0.05; for IgM r=0.47, P<0.0001; and for IgG anti A-60 r=0.44, P<0,05.
A univariate analysis of variance was used to assess the association between the humoral immune response, on the one side, and sex, phase of the disease, and the presence of a cavity, on the other side. For this purpose the children were divided into the following groups: pulmonary TB (pulm TB), lymph node TB (lymph TB), cavitary TB (yes) and no cavitary TB (no). The results of the analysis are graphically illustrated in Fig. 1, Fig. 2, Fig. 3, Fig. 4, and Fig. 5 below.
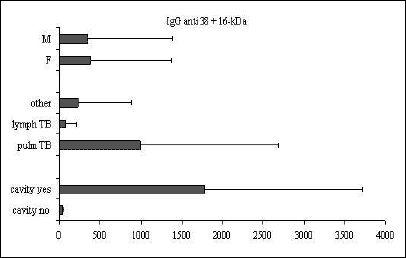 |
Fig. 1. Mean ąSD values of IgG anti-38-kDa+16-kDa in relation to sex, clinical presentation of TB, and the presence of cavity. |
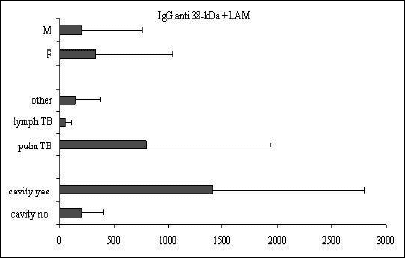 |
Fig. 2. Mean ąSD values of IgG anti-38-kDa+LAM in relation to sex, clinical presentation of TB, and the presence of cavity. |
 |
Fig. 3. Mean ąSD values of IgA anti-38-kDa+LAM in relation to sex, clinical presentation of TB, and the presence of cavity. |
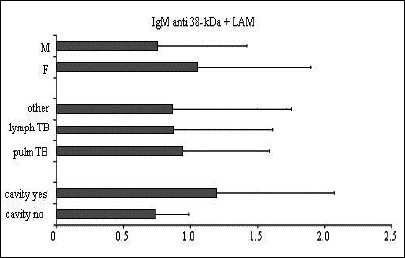 |
Fig. 4. Mean ąSD values of IgM anti-38-kDa+LAM in relation to sex, clinical presentation of TB, and the presence of cavity. |
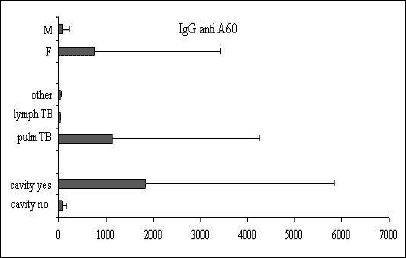 |
Fig. 5. Mean ąSD values of IgG anti-A-60 in relation to sex, clinical presentation of TB, and the presence of cavity. |
Significant differences between the IgG antibody levels in pulmonary vs. lymph node TB were observed for all the tests examined (anti 38+16-kDa, P<0.05; anti 38-kDa+LAM, P<0.01; and IgG anti A-60, P<0.05). For IgA and IgM no differences were observed between children in different phases of the disease. The presence of cavities was associated with a humoral response to all the IgG antigens examined (P<0.001) and to IgA (P<0.05). The mean antibody levels were higher in girls than in boys, but the difference did not achieve statistical significance.
Host immunity is considered to be the most important factor determining the risk of TB development (7). Infants with an immature immune system are at the highest risk of developing active disease following contact with a tuberculosis patient (2, 14). The risk decreases with age. Studies show that in 40% to 50% of infants with untreated TB infections the disease develops within 1 to 2 years (14). The risk decreases to 15% among older children (14). In 25% to 35% of children, TB is extrapulmonary (14). The clinical picture of the disease in children significantly differs from that in adults and may be strongly influenced by age, genetic (racial) factors, nutritional status, etc (1, 2, 5). All these factors are liable to influence immunoreactivity to mycobacterial antigens.
In the present paper, the analysis of humoral immune response in relation to demographic factors and to clinical forms of TB was performed in the group of children and adolescents. Serum antibody levels to Mycobacterium tuberculosis antigens were analyzed in children grouped into four diagnostic categories: active primary and postprimary disease, latent infection without radiographic chest abnormalities, and TB-free. The severity of lung involvement was assessed radiologically. In the examined population, humoral immune response was stronger in older children and in those with more extensive disease. However, the increase in the mean antibody levels resulted from the high antibody titer in only a few cases. In the majority of children, the humoral response was very weak. High antibody titers were usually associated with more extensive pulmonary or with adult-type (post-primary) TB. The antibody levels in isolated lymphadenopathy and in very young children were very low. This has also been observed by others (8-10). According to those previous authors, very low antibody levels resulted from the prevalence of early and limited forms of TB in children (8). Because of the lack of clear criteria for TB diagnosis in children, overdiagnosis also has to be considered. The difference in antibody production between the groups examined was seen for all the tests used, except for the IgM based assay. IgM levels did not differ between TB children, latent TB children, and control subjects. This phenomenon can be explained by the different mechanisms of the regulation of IgM synthesis. The IgM response is considered as a primary humoral response developed just after contact with antigens. IgM production is independent of the help provided by T lymphocytes. Antimycobacterial IgM is probably strongly influenced by environmental factors, such as BCG vaccination or contact with environmental mycobacteria (15). Beyazova et al (16) showed that the IgM levels anti A-60 are very low in nonvaccinated children, but the progressively increase during the first months after BCG administration. This observation has not been confirmed in India. The discrepancy can be explained by a potential influence of micro-flora in India on the humoral response to mycobacterial antigens (9). Delacourt et al (10) observed a significant difference in IgG and IgM anti A-60 between vaccinated and nonvaccinated children in France. Children with latent TB were included in that study. Differences between the latent TB and control groups were noticed only for the IgA class of antibodies (10). In our study, significant differences between the latent TB and control groups were not seen. This is consistent with the idea of strong cellular immune response in latent TB, outweighing weak antibody production.
In our study, TST did not correlate with the humoral response. Similar observations in children with TB have been made by other authors (10, 16). This may be related to weaker antagonism between Th1 and Th2 components in children (17). It has been shown that cord-derived human dendritic cells have a profound defect in the production of IL-12, playing a central role in the induction of Th1 cells (17). Studies in mice indicate that the immune response at birth is often biased toward the Th2 response (17). BCG was proved to be a stimulant for both Th1 (INFg) and Th2 (IL-5, IL-13) type cytokines (17). The dogma has been that the Th1/Th2 responses are antagonistic (18). However, BCG primes both arms of the immune response, which may be the cause of suboptimal co-stimulation of dendritic cells and subsequent synthesis of both types of cytokines (17). The hypothesis that in children the dichotomy of immune response is not as complete as in adults may explain the lack of relations between TST and antibody production.
The analysis of demographic and clinical factors associated with the humoral response showed that in young children antibody production to mycobacterial antigens is very weak or even nonexisting in children below 5 years of age. Only in patients older than 6 and in adolescents, IgG and IgA antibody levels increased. IgM levels did not differ between any of the age-groups examined. Other authors have made similar observations (8, 10). For all the tests examined, including IgM, a strong positive correlation between age and humoral immune response was observed. A tendency for higher susceptibility for TB in girls before puberty, observed in the present study, has also been noted by other authors (8). After puberty the incidence of TB is much higher in men then in women, which can be linked to the protective effect of female sex hormones.
IgG levels against all the antigens examined were significantly higher in postprimary than in primary TB, which also is in line with the observations made by others (8-10). In contrast, no difference was observed for IgA and IgM. Delacourt et al (10) reported similar findings. The presence of cavities was associated with significantly higher IgG and IgA levels. Cavities are rare in children in Poland and they almost never occur in children aged below 10. Cavitation following primary infection occurs frequently during adolescence. The adult-type disease results from primary infection, endogenous reactivation, or exogenous reinfection. No association of the IgM level with any of the factors examined was found in the present study. Delacourt et al (10) had similar observations in France. However, authors in India noticed higher IgM levels in primary compared with postprimary TB in children (9). They explain this phenomenon by linking IgM to an early immune response to antigens.
Very few studies have discussed the problem of humoral immune responses in childhood TB. The use of ELISA for immunodiagnosis of TB has shown that antibody production to mycobacterial antigens is low in children (8-10, 19, 20). The most established tenet in mycobacterial disease is that strong T-cell responses are associated with disease localization and protection, and the antibody response is associated with disease dissemination (7). Among children with recent TB infections, active multiplication of mycobacteria occurs with or without the presence of radiographic abnormalities or clinical symptoms (2-4). For example, gastric aspirate cultures yield M. tuberculosis from a small proportion of recently infected children with normal chest radiographs (4). Patients with more extensive pulmonary tuberculosis show a more marked humoral response to infection as manifested by changes in serum levels of antibodies (9, 10). The intensity and duration of infection in addition to the genetic background may modify the antibody response. The immune response in the adult-type TB has been extensively studied. The Th1-type immune response is believed to be necessary for protection against mycobacterial pathogens, such as Mycobacterium tuberculosis (7). These facultative intracellular pathogens reside in mononuclear cells, which allow them to escape the immune response of the host (7). Humoral immunity is only strong in the presence of large numbers of organisms. When cellular immunity predominates in the setting of few organisms, the antibody response is negligible (7). Although immunity to tuberculosis is regulated by cell-mediated mechanisms, the knowledge of the humoral immune response at various stages of infection and disease could help elucidate the complex interaction between the host and the pathogen. A comprehensive immune profiling of antigen-specific responses in relation to the clinical phase of the disease is critical not only to understand the disease pathogenesis, but also for using this knowledge for diagnostic purposes. The basic differences in pathophysiology of TB in adults and children must be appreciated before new tests and new vaccines are applied in pediatrics.
- Majewska-Zalewska H. Gruzlica pluc u dzieci. Medipress, 1999; 2: 3-13.
- Eamranod P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int J Tuberc Lung Dis 2001; 5: 594-603.
- Starke JR. Childhood tuberculosis: ending the neglect. Int J Tuberc Lung Dis 2002; 6: 373-374.
- Klotz SA, Penn RL. Acid-fast staining of urine and gastric contents is an excellent indicator of mycobacterial disease. Am Rev Respir Dis 1987; 136: 1197-1198.
- KusJ. Gruzlica - sytuacja epidemiologiczna oraz wspolczesne poglady na leczenie i zapobieganie. Terapia i Leki 2002; XXX/LII/5-6: 26-29.
- Zwolska Z. Tuberculosis in the world and in Poland at the 20th century. Bull Pol Acad Sci Biol Sci 1996; 44: 261-271.
- Havlir D, Wallis RS, Boom H et al. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun 1991; 59: 665-670.
- Imaz MS, Domini M.A, Zerbini E et al. Evaluation of the diagnostic value of measuring IgG, IgM and IgA antibodies to the recombinant 16-kDa antigen of Mycobacterium tuberculosis in childhood tuberculosis. Int J Tuberc Lung Dis 2001; 5: 1036-1043.
- Gupta S, Bhatia R, Datta KK. Serological diagnosis of childhood tuberculosis by estimation of mycobacterial antigen 60 specific immunoglobulins in the serum. Tuber Lung Dis 1997; 78: 21-28.
- Delacourt C, Gobin J, Gaillard J-L et al. Value of ELISA using antigen 60 for the diagnosis of tuberculosis in children. Chest 1993; 104: 393-398.
- Young D, Kent L, Rees A, Lamb J, Ivanyi, J. Immunological activity of a 38-kilodalton purified from Mycobacterium tuberculosis. Infect Immun 1986; 54: 177-183.
- Alifano M, Sofia M, Mormile M et al. IgA immune response against the mycobacterial antigen A60 in patients with active pulmonary tuberculosis. Respiration 1996; 63: 292-297.
- Snider DE. The tuberculin skin test. Am Rev Respir Dis 1982; 125: 102-104.
- Brailey ME. Tuberculosis in white and negro children. II. The epidemiologic aspects of the Harriet Lane study. Cambridge, MA, Harvard University Press, 1958.
- Amicosante M., Paone G, Ameglio F et al. Antibody repertoire against the A60 antigen complex during the course of pulmonary tuberculosis. Eur Respir J 1993; 6: 816-822.
- Beyazova U, Rota S, Cevheroglu C et al. Humoral immune response in infants after BCG vaccination. Tubercle Lung Dis 1995; 76: 248-253.
- Ota M, Vekemans J, Schlegel-Haueter S et al. Influence of Mycobacterium bovis Bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol 2002; 168: 919-925.
- Hernandez-Pando R, Orozco H, Sampieri A et al. Correlation between the kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 1996; 89: 26-33.
- Starke JR, Correa AG. Management of mycobacterial infection and disease in children. Pediatr Infect Dis J 1995; 14: 455-70.
- Swaminathan S, Umadevi P, Shantha S, Radhakrishnan A, Datta O. Serodiagnosis of tuberculosis in children using two ELISA kits. Indian J Pediatr 1999; 66: 837-884.