Studies show that plasma proteins present in the perivascular zone form scaffolding that supports newly formed blood vessels (4). Cellular aggregates seen in these areas show the ultrastructural features of endothelial cells but also some unusual features, such as the presence of cytoplasmic filaments. Using electron microscopy, we defined stages of new blood vessel formation. Morphological characteristics of endothelial cells, components of the basal membrane material and, other structures of cerebral parenchyma adjacent to new blood vessels suggest the contribution of stem cells to vasculogenesis in the areas of the cerebral cortex that are in direct contact with the posttraumatic lesion (5). Immunocytochemistry using the Flk-1 antibody, which interacts with receptors for the vascular endothelial growth factor (VEGF), a marker of immature endothelial cells, confirmed these suggestions and showed that the development and growth of new blood vessels in the brain is not limited to angiogenesis but may also involve other processes, with a likely contribution of immature endothelial cells. These ultrastructural findings show that surgical trauma of the cerebral cortex may induce new vessel formation at the site of surgical intervention and in the areas adjacent to damaged cerebral parenchyma. Thus, evaluation of the later phases of cerebral parenchyma response to surgical brain trauma is of interest. The objective of our study was to assess the ultrastructural characteristics of the early and late phases of damaged cerebral parenchyma in rats, 14, 30, 90 and 180 days following surgical trauma.
The study was approved by a local Ethics Committee. Male adult Wistar rats weighing 200-250g were anesthetized with ketamine hydrochloride - 20 mg/kg, i.m. Traumatic brain injury was induced in the frontotemporal region of the cerebral cortex after scull exposure as described earlier (6). After recovery from anesthesia, the rats remained under standard laboratory conditions for 14 to 180 days. Then, the animals were euthanized and the material for microscopic studies was dissected and processed for transmission electron microscopy. Each experimental group consisted of 3 operated on and 3 non-operated animals; the latter were used as controls.
The results showed that 14 days following surgical brain trauma in rats, fibroblast-like cells filled with ribosome-rich endoplasmic reticulum and containing elongated nuclei were present at the site of the trauma (Fig. 1A). Cytoplasmic processes of these cells were connected and formed a network around bundles of collagen fibers. Both these cells and striated collagen fibers were present in this area until 6 months following trauma.
 |
Fig. 1. Panel A - New capillary vessel (nv) with the direct contact with astrocyte (As) and fibroblast processes (F), 14 days after surgical operation, mag. x 8k; Panel B - Lesion border surrounded by basement membrane-like material (asterisks) with an adjacent astrocyte (As) containing glial filaments, 30 days after surgical procedure, mag. x 12k. |
In the zone immediately adjacent to the traumatic lesion, capillaries showing ultrastructural features of young vessels were seen after 14 days, surrounded by astrocyte processes. These vessels lacked a fully developed basal membrane and were surrounded only by basal membrane-like material. The vessel walls contained pericytes. These vessels were in direct contact with the astrocyte plasmatic processes (Fig. 1A). In addition to capillaries, larger precapillary vessels were seen. Both capillary and precapillary vessel formation also occurred in more remote areas until 3 months following trauma. Collagen fibers were found within the basal membrane of precapillary vessels in the remote areas.
A characteristic feature of the zone surrounding the traumatic lesion was the presence of basal membrane-like material defining lesion borders, with adjacent astrocyte plasmatic processes containing numerous glial filaments (Fig. 1B). Subsequently, the number of glial filaments in astrocytes increased, and the filaments filled morphologically differentiated processes of varying morphology. From 30 days following trauma, nerve fibers and synaptic endings were also seen in the zone adjacent to the traumatic lesion (Fig. 2A). At 6 months, the layer of basal membrane-like material and plasmatic processes of fibroblast-like cells formed a distinct border separating cerebral parenchyma from the posttraumatic lesion. Astrocytes and blood vessels were present adjacent to the layer of basal membrane-like material. In addition to glial filaments, irregular fibers of 7Ė9 nm in diameter were seen in astrocytes. A basal membrane that often formed a network with collagen fibers surrounded capillaries.
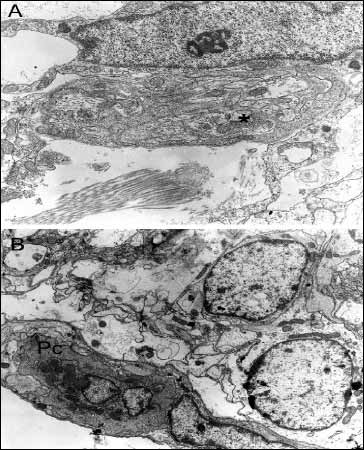 |
Fig. 2. Panel A - Synaptic ending (asterisk) seen in the zone adjacent to traumatic lesion, 30 days after surgical injury, mag. x 15k; Panel B - Borderline layer of a glial scar, with a phagocytic cell (Pc), 3 months after surgical injury, mag. x 15k. |
From 30 days following trauma, macrophages filled with phagolysosomes were seen in the traumatic lesion and adjacent areas. After 3 and 6 months, phagocytic cells were localized both in the borderline layer of the scar and in the remote areas (Fig. 2B). Microscopic pictures of the brain at 6 months following trauma showed large loss of cerebral parenchyma (Fig. 3A). In addition to macrophages, the glial scar contained astrocytes with rich lysosomal apparatus, suggesting these cells contributed to phagocytosis. Using electron microscopy, populations of perivascular macrophages were also seen.
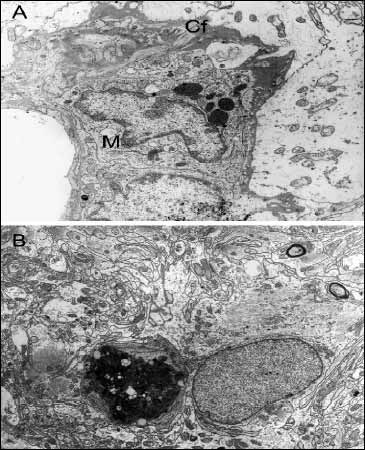 |
Fig. 3. Panel A - Part of a blood capillary vessel, with a macrophage (M) in the perivascular space, 6 months after surgical injury. Collagen fibrils (Cf) are seen in the basement membrane, mag. x 20k; Panel B - Neuronal cell death at some distance from the lesion border, 6 months after injury, mag. x 6k. |
Neuronal cell death occurred in cerebral parenchyma at some distance from the lesion border from 14 to 180 days following trauma (Fig. 3B). These areas showed an increased number of microglial cells and loss of cerebral parenchyma, which was likely associated with neuronal cell death.
Damage to the central nervous system initiates a series of cellular and molecular processes leading to the formation of a glial scar. Cells involved in these processes include not only astrocytes but also microglial cells, oligodendrocyte precursors, meningeal cells, and stem cells (7-9).
The initial phenomenon following cerebral parenchymal damage is cellular death within neurovascular units and other components of the neuropile (10, 11). During initial phases, we noticed capillary vessel formation supported by plasma protein scaffolding. Macrophages migrated to the lesion site, followed by phagocytic cells originating from the surrounding parenchyma (5, 12). Four days following surgical trauma, we could see fibroblast-like cells that were involved in the repair processes and collagen protein synthesis. Close interaction between these cells and astrocytes suggests their contribution to the formation of the glial scar (13, 14). The latter is eventually composed mainly of tightly connected astrocyte processes that form a network surrounding nearby fibroblasts.
Our study also showed that the process initiated in the cerebral parenchyma by surgical trauma does not terminate with the formation of a glial scar. Initial mechanisms of repair and reconstruction become disturbed after approximately 3 months following trauma of the cerebral parenchyma. At that time the number of macrophages is increased, with an associated marked microglial response. Astrocytes forming the glial scar show features of edema. The solid glial scar surrounded by basal membrane-like material becomes loosened. Macroscopic pictures of the brain 6 months after trauma show large loss of cerebral parenchyma suggesting that the glial scar is not a stable structure, and its remodeling involves various cells contributing to formation of the glial scar during different phases of this process.
Acknowledgments: This study was supported by grant No. 2P05A 028 28 from the Ministry of Education and Science, Poland.
- Berry M, Maxwell WL, Logan A et al. Deposition of scar tissue in the central nervous system. Acta Neurochir 1983; 32 (Suppl): 31-53.
- Cervos NJ, Lafuente JV. Traumatic brain injures: structural changes. J Neurol Sci 1991; 103 (Suppl): S3-S14.
- Fawcett JW, Asher RA. The glia scar and central nervous system repair. Brain Res Bull 1999; 49: 377-391.
- Frontczak-Baniewicz M, Gordon-Krajcer W, Walski M. The immature endothelial cell in new vessel formation following surgical brain injury in rat brain. Neuro Endocrinol Lett 2006; (in press).
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312-318.
- Frontczak-Baniewicz M, Walski M. New vessel formation after surgical brain injury in ratís cerebral cortex. Formation of the blood vessels proximally to the surgical injury. Acta Neurobiol Exp 2003; 63: 65-75.
- Abnet K, Fawcett JW, Dunnett SB. Interactions between meningeal cells and astrocytes in vivo and in vitro. Dev Brain Res 1991; 53: 187-196.
- David S, Bouchard C, Tsatas O, Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron 1990; 5: 463-469.
- Streit WJ. The role of microglia in brain injury. Neurotoxicology 1996; 17: 671-678.
- Preston E, Webster J, Small D. Characteristics of sustained blood-brain barrier opening and tissue injury in a model for focal trauma in the rat. J Neurotrauma 2001; 18: 83-92.
- Walski M, Gajkowska B. The changes in the ultrastructure of cerebrovascular junction after traumatic injury of the cerebral cortex. Neuro Endocrinol Lett 2001; 22: 19-26.
- Perry VH, Gordon S. Macrophages and the nervous system. Int Rev Cytol 1991; 125: 203-244.
- Bignami A, Dahl D. The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific protein (GFA) in mammalian and submammalian vertebrates. Neuropathol Appl Neurobiol 1976; 2: 99-110.
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci 1997; 20: 570-577.