Recently, a number of cytokines and growth factors (IL-1ß, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, VEGF, PDGF-AA, EGF), eicosanoids and nitric oxide (NO), some of them corresponding with clinical disease markers, have been demonstrated in EBC from cystic fibrosis, bronchial asthma, and COPD patients (3-7). In contrast to BALF, the technique of the exhaled breath condensate (EBC) collection is simple, quick, safe, non- invasive, and suitable for patients of any age and clinical status (8). However, except for oxidative stress (H2O2) and NO, no other mediators have been evaluated in EBC form sarcoidosis patients, although, as pointed above, airway monitoring is considered a key element of the recommended medical strategy (9, 10).
In the present study we attempted to answer the question of whether airway inflammatory markers in pulmonary sarcoidosis patients might be assessable in EBC and, if so, to what extend they might reflect the disease activity in the lungs, compared with the assessment based at the content of these markers in BALF.
The study was approved by a local Ethics Committee and informed consent was obtained from all study subjects. Nine patients (4 women and 5 men) aged 30-57 years with newly-detected pulmonary sarcoidosis were used for the study. The diagnosis was confirmed by typical clinical representation, histology, and HRCT findings. No patient was on steroid treatment at the time of the study or during the preceding 3 months.
EBC collection was performed according to a standard protocol (8). Subjects breathed tidally for 10-15 min, using a-nose-clip, into a special chamber of a condenser (EcoScreen, Jaeger, Germany). The collected condensate was stored in aliquotes at -70°C until further examination.
BALF collection was performed as part of routine diagnostics. The recovered BALF was filtered through sterile gauze and centrifuged (4°C, 400 x g for 15 min.). The supernatant was collected and frozen at -70°C. According to the study protocol, EBC was collected the day before or in the morning prior to bronchoscopy.
The measurement of cytokines in EBC and BAL was performed by a quantitative enzyme immunoassay technique (ELISA) using commercial kits (R&D) according to manufacturer recommendations. Optical density was measured at 450 nm using a spectrophotometric reader Elx800 (Bio-Tek Instruments, Winooski, VT, USA). The cytokine concentration was expressed in pg/ml (IL-6, TNF-alpha, PAI-1) or in ng/ml (IGF-1).
Data are presented as means ±SD. Statistical analysis was performed using Student’s t-test for the comparison of mean values and Pearson’s test for the analysis of correlations.
TNF-alpha, IL- 6, IGF-1 and PAI-1 concentrations were detectable in EBC of all sarcoidosis patients, except for IGF-1 that was not measurable in both EBC and BAL from one subject.
Interestingly, the levels of TNF-alpha, IGF-1, and PAI-1 in EBC and BAL did not differ statistically; the respective values were TNF-alpha 3.80 ±1.79 vs. 3.34 ±2.81 pg/ml, IGF-1 7.76 ±5.99 vs. 6.09 ±4.44 pg/ml, PAI-1 0.82 ±0.42 vs. 0.94 ±0.44 ng/ml. By contrast, the IL-6 concentration in EBC was significantly lower compared with that in BAL; 0.23 ±0.07 vs. 4.08 ±3.48 pg/ml, respectively (P< 0.001) (Fig. 1).
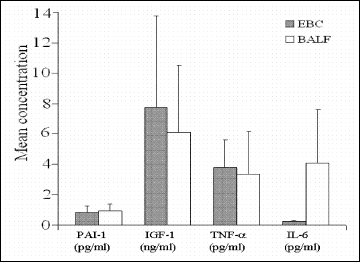 |
Fig. 1. Mean cytokine levels in the exhaled breath condensate (EBC) and broncholaveolar lavage fluid (BALF) in pulmonary sarcoidosis patients. |
There were positive correlations between the content of TNF-alpha (r=0.79, P<0.001), IGF-1 (r=0.94, P<0.001), and PAI-1 (r=0.81, P<0.001) (Fig. 2A, B, C) and a negative one for IL-6 (r=-0.47, P<0.05) (Fig. 2D) in EBC and BALF.
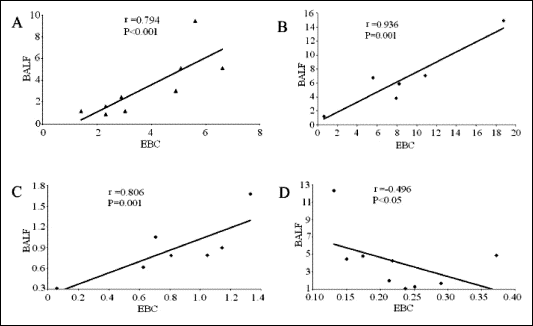 |
| Fig. 2. Correlations between the levels of TNF-alpha (A), IGF-1 (B), PAI-1 (C), and IL-6 (D) in exhaled breath condensate (EBC) and bronchoalveolar lavage fluid (BALF) from sarcoidosis patients. |
The immunopathology of pulmonary sarcoidosis is complex and involves a wide range of cell types and mediators (1, 2). Activated lymphocytes and macrophages are considered the main prominent cells regulating granulomatous inflammation in the lung and determining the further disease course to spontaneous regression or pulmonary fibrosis. Both cell types produce a considerable number of proinflammatory cytokines and growth factors. The mediators evaluated in the present study are influential in shaping several important phases in the sarcoidosis pathomechanism, such as driving alveolitis and granuloma formation (IL-6, TNF-alpha), persistence of interstitial inflammation (IGF-1), and progression into lung fibrosis (PAI-1).
The main aim of this preliminary study was to evaluate whether inflammatory markers might be measurable in the exhaled breath condensate from pulmonary sarcoidosis patients. Our results demonstrate, for the first time, that it is possible to assess the IL-6, TNF-alpha, IGF-1, and PAI-1 concentrations in EBC using a standard Elisa technique. The TNF-alpha, IGF-1, and PAI-1 levels in EBC were comparable with those found in BALF. It should be emphasized that both materials, EBC and BALF, were collected in close time proximity. Moreover, highly significant correlations were observed between the levels of TNF-alpha, IGF-1, and PAI-1 in both materials. It seems reasonable to conclude that the EBC reflects the production of these cytokines in the lungs as effectively as BALF.
However, the results concerning the IL-6 concentration were different. Its level in EBC was considerably lower than that in BALF, while the correlation between IL-6 in both materials was negative. The most likely explanation for this apparent inconsistency, as compared with other cytokines, might be a characteristic for IL-6 tendency to form molecular forms more complex than a simple monomer. While the molecular weight (Mw) of IL-6 monomer is only 25–30 kDa, the average size of more intricate structures it has a propensity to form is 100-150 kDa or 400-500 kDa (11). It has been shown that molecules detected in EBC, originating from the fluid lining the pulmonary tracts, might be no more than 100 kDa (12). All the other cytokines evaluated in the present study are either small molecule, as IGF-1 with Mw 7.5 kDa, or stay preferentially in a monomeric form, as TNF-alpha (Mw 17 kDa) or PAI-1 (Mw 45 kDa). That important distinction in the IL-6 performance rationalizes both its measurable, although low, levels in EBC, as compared with BALF, but also a negative correlation between its content in both materials. The higher IL-6 concentration in the airways the more it tends to form large-scale molecules that are not able to relocate into the exhaled air. Further experiments are ongoing to confirm this hypothesis.
This preliminary study confirmed that EBC might provide an easily accessible material for the assessment and monitoring of inflammatory mediators in pulmonary sarcoidosis. EBC reflects their production in the lungs as effectively as BALF, providing that the molecular characteristics of proteins being evaluated allow their easy transfer into the exhaled air. Therefore, detailed studies are required before accepting the EBC sampling as an equivalent to BALF. Since the collection of EBC is faster, less expensive, safer, and beyond any comparison much more comfortable for the patient, further efforts to assess the EBC usefulness in diagnostics and monitoring of pulmonary sarcoidosis are necessary.
- Moller DR. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoid Vasc Diffuse Lung Dis 1999; 16: 24-31.
- Sharma OP, Alam S. Diagnosis, pathogenesis and treatment of sarcoidosis. Curr Opin Pulm Med 1995; 392-400.
- Garey KW, Neuhauser MM, Robbins RA et al. Markers on inflammation in exhaled breath condensate of young healthy smokers. Chest 2004; 125: 22-26.
- Robroeks CM, Jobsis Q, Damoiseaux JG et al. Cytokines in exhaled breath condensate of children with asthma and cystic fibrosis. Ann Allergy Asthma Immunol 2006; 96: 349-355.
- Carpagnano GE, Foschino BMP, Cagnazzo M et al. Use of exhaled breath condensate in the study of airway inflammation after hypertonic saline solution challenge. Chest 2005; 128: 3159-3166.
- Leung TF, Wong GW, Ko FW et al. Analysis of growth factors and inflammatory cytokines in exhaled breath condensate from asthmatic children. Int Arch Allergy Immunol 2005; 137: 66-72.
- Gessner C, Scheibe R, Wotzel M et al. Exhaled breath condensate cytokine in chronic obstructive pulmonary disease. Respir Med 2005; 99: 1229-1240.
- Horvath I, Hunt J, Barnes PJ et al. Exhaled breath condensate: methodological recommendations and unresolved question. Eur Respir J 2005; 26: 523-548
- Wilsher ML, Fergusson W, Milne W, Wells AU. Exhaled nitric oxide in sarcoidosis. Thorax 2005; 60: 967-970.
- Kwiatkowska S, Luczynska M, Grzelewska-Rzymowska I, Nowak D, Zieba M. Comparison of oxidative stress markers in exhaled breath condensate and in serum of patients with tuberculosis and sarcoidosis. Pol Merk Lek 2005; 9: 37-40.
- Mackevin NI, Patel K, Rayanade RJ et al. Distinct classes of chaperoned IL-6 in human blood: differential immunological and biological availability. J Immunol 1998; 160: 494-501.
- Scheideler L, Manke HG, Schwulera U, Inacker O, Hammerle H. Detection of nonvolatile macromolecules in breath. a possible diagnostic tool? Am Rev Respir Dis 1993; 148: 778-784.