In attempts to enhance esthetics and to facilitate technical processing, other materials besides titanium have been used in the marginal, transmucosal part of the implant. A good long-term prognosis of implant therapy is related to sustained osseointegration and a proper mucosal/implant barrier protecting the bone tissue against factors released from the oral environment (3-5). A carefully insertion technique and sufficient stability of the implants are essential factors to correct healing of the soft tissues. An insufficient primary stability causes bad healing and the premature loss of the implant.
The gingival and the periimplant mucosa are covered by a keratinized oral epithelium, which is continuous with the JE with a length 2 mm and a connective tissue (CT) zone with a height 1 to 2 mm (6-8).
The JE of the periimplant mucosa is attached to the implant surface via hemidesmosomes and basal lamina (8). In case of cementum layer absence most fibers in the CT run in a parallel direction to the implant surface (1, 6), as well as in the coronal-apical direction or circumferentially oriented (2). Owing to the absence of the extrinsic fiber cementum on the implant surface, no fiber attachment at implants is possible.
Smooth surface has been shown to guarantee decent soft tissue attachment on material surface. Such fibers appear to be more prominent on microtextured compared to smooth transmucosal surfaces (2). The thickness of the CT depends on the implant material and the stage of healing (9-11). Although a direct connective tissue contact (CTC) was observed commonly for machined, roughly sandblasted, and plasma-sprayed implant surfaces, the collagen fibers don’t show any signs of perpendicular insertion to the respective surfaces (1, 6, 8).
However, as previous studies have also demonstrated, the surface texture significantly influences fibroblast and epithelial cell attachment, it was suggested that a certain roughness surface is needed for an optimal soft tissue sealing (12).
Proper dimension and function of the soft tissue seal around dental implants is considered to be a pre-requisite for achieving long term stable peri-implant conditions (6). Accordingly extensive research has been performed to investigate the biological soft tissue seal at different types, materials and roughness of dental implants (6, 8, 13). The biocompatibility of the material used in the transmucosal part of the implant may, therefore, be a factor of importance for treatment success. Therefore, the aim of the present study is to investigate the soft tissue integration of non-submerged titanium implants types in pig models.
In this study two different NobelBiocare implants types (NobelReplace® Tapered Groovy 4.3 X 10mm and Replace® Select Tapered TiU RP 4.3 X 10mm) were inserted respectively into the right and left mandibles of pigs (Sus scrofa domestica) according to a uniform protocol. Eight animals from 15 to 16 months old and average weight of 80 kg were used.
The protocol of this study was approved by the Ethical Committee for Animal Research LVL MV/TSD/7221.3-1.1-037/05. Mandibular premolars P2-P4 were extracted bilaterally. After 3 months of healing, the screws titanium implants were fixed between the canines and molars (a total of two implants per animal). All implants were inserted by a standard surgical technique of implant system. The implants shoulders were at the ridge crest level after placement. In one of the eight animals used in this research the implant Replace® Select Tapered TiU was not installed due to preexistence local gingival inflammation.
All implants were carried out by one person to reduce the application fault. The implants were immediately provided with a ceramic crown (Ceramic Copping Easy AbutmentTM, NobelBiocare, Germany). Then the Resonance Frequency Analysis (RFA) of each implant was measured to analyze and evaluate the implant-bone stability. The Implant Stability Quotient (ISQ) values were measured by OsstellTM (Integration Diagnostics, Goteborgsvagen, Sweden). The analysis results show the stability in scale from 0 to 100 Hz. The RFA is based on abutment of implant to evaluate the clinical implications. This method is used for evaluating healing and quantitative measurement of the osseointegration.
The surgical procedures were accomplished in the animals under general anesthesia (i.v. injection into v. auricularis caudalis) of ketamine hydrochloride 150-200 mg (Ketamin, Belapharm, Vercha, Germany), droperidole 4.0-7.0 mg (Droleptan, Janssen Pharmaceutical, Oxon, UK) and Faustan 4.0 mg (Temmler Pharma, Marburg, Germany). In addition, approximately 1-2 ml of 2% Xylocaine®/Epinephrine 1:50.000 was injected at the surgical site to reduce bleeding. No plaque control program was used in this study.
To assess the repair process and bone remodeling, during the healing period, intravenous fluorochrome substances injections were used. Intravital fluorochrome staining was performed on days 14 (2% tetracycline, 10 mg/kg bodyweight (yellow)), and 28 (1% calcein, 5 mg/kg (light green)). 56 (6% xylenol, 1.5 mg/kg (orange)).
Two soft tissue parameters were assessed: sulcus depth (SDI) and junctional epithelium (JE). Periodontal probe was realized with a periodontal milimetric metallic instrument, calibrated in 0.5 mm (PCPUNC-156, HU-friedy®). Standard t-test was used to calculate the significance of data differences (p£0.05) in the SDI and primary stability of the implants. In order to achieve minimum error in measurement, probe was carried out twice in all SDI by the same operator and the average was used in the test.
After 70 days postoperatively, the animals were killed with an intracardiac injection of a high dose (5 mg/kg) of pentobarbital natrium (Eutha 77, Essex-Pharma, München, Germany). The implants/bone complex blocks were removed, fixed in formalin 4% and embedding with Technovit 9100 New® (Kulzer & Co, Wehrhein, Germany). The histological preparations were processed in agreement with the technique established by Donath (14).
The results of Resonance Frequency showed no differences in stability (primary and in the end of investigation) between both kinds of implants. The standard and adequate primary stability of the implants obtained after surgical installation allowed an equilibrated healing of soft tissues. Since the first to the last week soft tissue parameters were assessed. All investigated implants showed clinically a light inflammable mucous hypertrophy and low plaque accumulation.
The data in Fig. 1 represented in numbers the weekly application of Resonance Frequency in the NobelReplace® Tapered Groovy and Replace® Select Tapered TiU implants until the animals sacrifice. The implant stability quotient (ISQ) results are showed in scale from 0 to 100 Hz. The distribution of ISQ index showed the primary stability to the both implants in the day of insertion (average 80 Hz). In the following three weeks it was observed a decline of ISQ index to 60Hz. From this point on the ISQ index presented variations otherwise they were kept between 50 and 60 Hz until the end of the 70 days experiment. The implants comparison did not present a significant difference (p
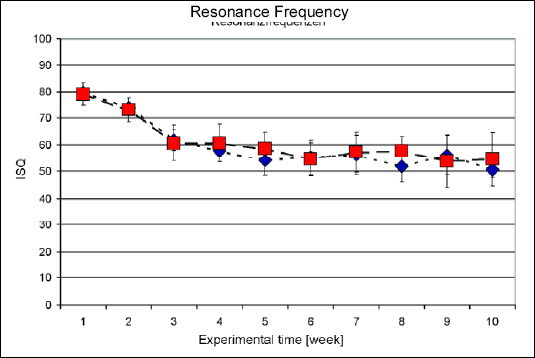 |
| Fig. 1. Results of Resonance Frequency analysis (t-test). Red color= NobelReplace® Tapered Groovy and blue color = Replace® Select Tapered TiU. The implant stability quotient (ISQ) results show the stability in scale from 0 to 100 Hz. |
The data obtained from 1st to 9th week probing of the SDI are showed in Fig. 2. The SDI of gingival in the mandibles of pigs remained in the average of 1.5 and 2.5 mm to the both implants. Although there was a small difference in the first 3 pos-operatory weeks, probably due to the tissue cicatrisation process, the final resultant remained slightly below 2 mm. The TiU superficial treatment until the top of NobelReplace® Tapered Groovy implant did not present significant statistic differences when compared to the one without treatment, during gingival sulcus measurement. The numeric result of the weekly gingival evaluation of each installed implant was submitted to t-test analysis. The results demonstrated medium values from 2.4 mm until 1.8 mm, however without statistics difference
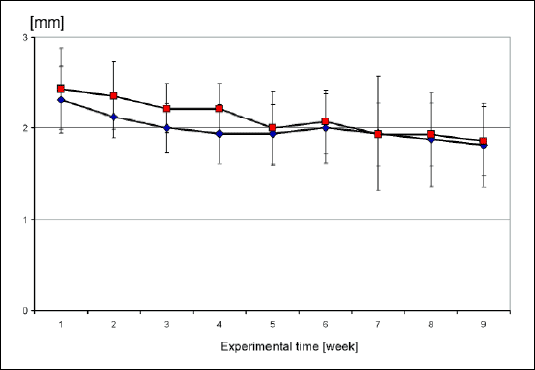 |
| Fig. 2. Results of SDI measurement analysis (t-test) in millimeters. Red color= NobelReplace® Tapered Groovy and blue color = Replace® Select Tapered TiU |
During the 70 days after implantation, no gingival ressection was observed. Between vestibular gingival sulcus and width of inserted gingival nothing was observed. The cervical section of the implant showed sufficient esthetic solution under point of view of biological functions.
The histomorphometry of gingival SDI was also obtained through images using a Universalmikroskop Axioplan Zeiss (Oberkochen, BRD) connected to a high-resolution video camera Colorview II SIS (Olympus Europa GmbH, Hambrug, BRD) and one SIS, Software Image analysis 3.1 (Soft Imaging System GmbH, Münster, BRD), for each implant inserted. The numeric results of gingival SDI under t-test of the studied implants showed no significant differences on its final average data. The results represented in the graphic (Fig. 3) showed under this analysis, a numeric balance comparing the two implants necks.
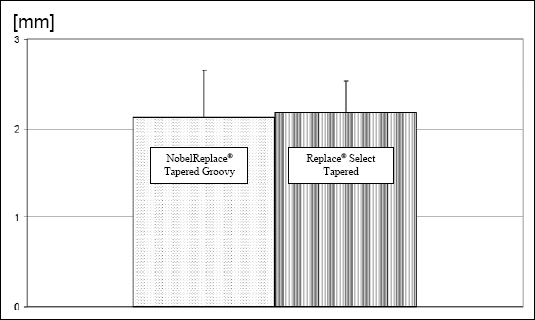 |
| Fig. 3. Representative histomorphometry (t-test) of gingival SDI results to the implants NobelReplace® Tapered Groovy (2.13 mm) and Replace® Select Tapered TiU (2.179 mm) obtained by light microscopy |
The histological evaluation of the implant-soft tissue interface exhibited no morphologic and substancial differences between the two implant types. Histomorphometry was possible in all speciment (Figs. 4, 5, 6).
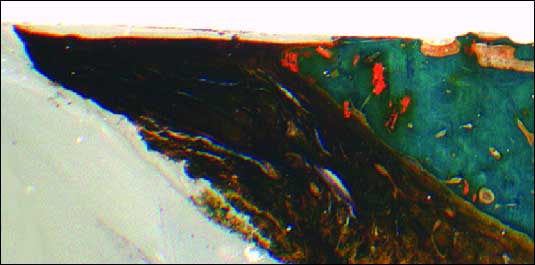 |
| Fig. 4. General view of undecalcified implant neck region (NobelReplace® Tapered Groovy) and bone / gingiva structure. Morphological condition was observed with aldehyde–fuchsin–Masson–Goldner staining. Destructive changes of the peri-implant bone were not found. Histologically, peri-implant gingiva showed similar structure to that of the gingiva around natural teeth. |
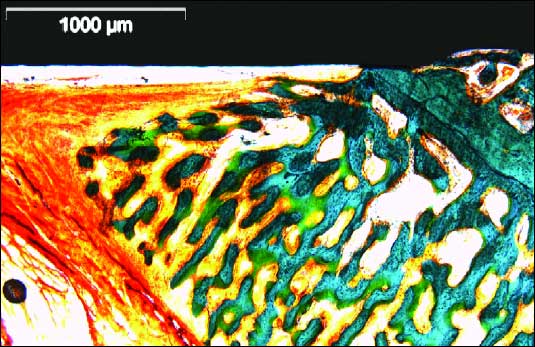 |
| Fig. 5. Photomicrography under light microscopy. Aldehyde–fuchsin–Masson–Goldner staining. Bone levels with bone to implant contact. The sample demonstrated a similar distance from the bone to implant like in healthy tooth. Morphological condition was observed with aldehyde–fuchsin–Masson–Goldner staining. |
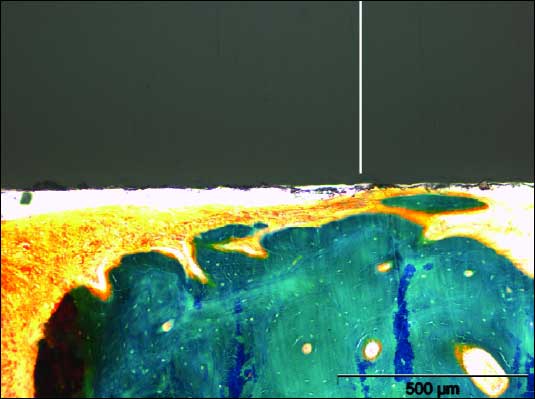 |
| Fig. 6. Photomicrography under light microscopy. Region of implant neck Replace® Select Tapered with mineralized new bone formation. Starting point of sulcus depth measurement. White line shows the beginning of gingival anchorage to implant. Morphological condition was observed with aldehyde–fuchsin–Masson–Goldner staining. |
The bone markers under fluorescence microscopy showed intense bone deposition and well organized lamellar formation (Fig. 7).
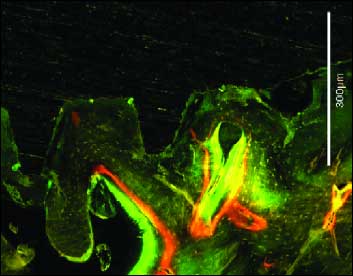 |
Fig. 7. Photomicrografy under fluorescence microscopy. Intense contact areas of new bone with implant surface. Tetracycline – yellow, Calcein – green, Xylenol – orange, Bar -300 µm |
Longitudinal section in polarized light of implant with TiU surface showed functionally oriented collagen fibrils in SDI (Fig. 8). Thick horizontal and vertical collagen fibers were found. The vertical fibers were running from the periosteum and the alveolar crest towards the oral epithelium (Fig. 8)
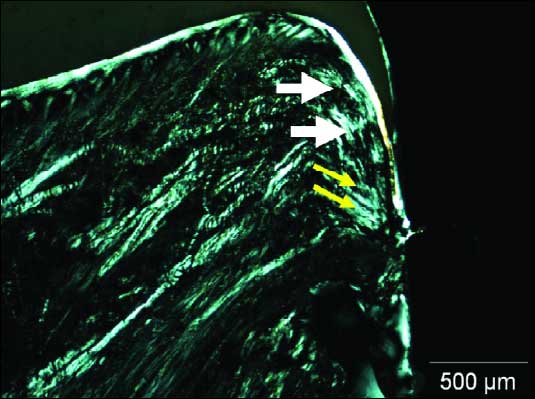 |
| Fig. 8. Photomicrografy under polarized microscopy. Parallel collagen fibers between the implant surface in JE (white arrows). Oblique fibers in direction to bone crest (yellow arrows). Bar - 500µm. |
Collagen fibers bundles were noted following parallel to the crown in vertical plane (white arrows). Close to the abutment surface, collagen fibers changed their direction into a oblique orientation to the bone crest (yellow arrows). In all specimens the mucosa was covered by keratinized oral epithelium that was continuous with the parakeratinized sulcular epithelium that lined the lateral surface of the periimplant SDI (Fig. 9). The sulcular epithelium was continuous providing an epithelial union between the implant and surrounding periimplant mucosa. The different implants did not demonstrate bone crest lost on the neck level (Fig. 9). The morphological characteristic of bone tissue observed in the two different surfaces neck did not allow the penetration of the tip of periodontal probe beyond this level.
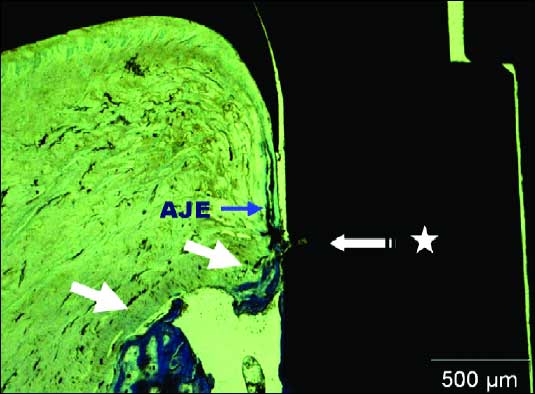 |
| Fig. 9. Photomicrografy under light microscopy. Bone crestal level (white arrows). AJE covered to parakeratinized tissue (blue arrow). Region between implant neck and abutment (star). Toluidine blue. Bar - 500µm. |
One of the primordial factors to long time osteointegration implants success is the health maintenance of surrounding tissues.
This study was originally designed to compare the structure of peri-implants tissues between surface-treated and non-treated neck implants. The mechanism for the termination of the epithelial migration along the surface of dental implants is not yet known. It may be argued, therefore, that differences may occur in the soft tissue attachment to implant transgengival or abutments designed with smooth or rough surfaces. The ceramic material used in the abutment portion of the implant was of decisive importance for the epithelial-connective tissue attachment in this study. Studies in vitro and in vivo (15-17) showing that the orientation and proliferation of epithelial and CT cells on titanium surfaces are influenced by the topography of the material surface. According to recent research, the adherence and spreading of epithelial cells on metallic surfaces, such as titanium and gold alloy, are good, especially if the surface is smooth (6). As a consequence of the fact that rough surfaces accumulate and retain more plaque than smooth surfaces, nowadays, most implant systems use a highly polished titanium in the transmucosal part (18). A polished implant collar may provoke crestal bone loss associated with “nonload” factor, but, similarly to microgap, bone loss can be avoided by leaving smooth implant neck above the bone level (19, 20). According a study from Buser et al. (2) the authors speculated that plasma spray titanium surface promote a better attachment of CT fibers.
The sulcular epithelium must remain stretchable to compensate the movements of soft tissue. It is a strategically important interface to seal the underlying tissues and is the most important tissue for peripheral defense against the repeated or continual bacterial challenge. In the study of Schupbach and Glauser (21), the autor concluded that the junctional epithelium (JE) serves as a seal to restore mucosal continuity and to form a compartment for peripheral defense.
JE and CTC are important parameters in providing information about the location of the apical extension of JE cells, the crestal bone height and extent of bone-implant contact. Some authors suggest that certain height of periimplant mucose is needed to allow appropriate conjunctive and epithelium insertion. If this soft tissue dimension is not adequate bone resorption can occur to ensure the “biological width” (6). The average of bone loss to osteointegrated implants reaches less that 1.5mm in the first year, continuing <0.2mm in the following three and five years (19).
The periimplant health monitoring and maintenance are extremely dependent on trusty diagnostic tests. Therefore, it is indispensable the use of clinical diagnostic parameters that correctly identify inflammatory alteration, even in its initial level. As periodontal and peiimplant tissues are apparently similar, there is a tendency to use periodontal parameters to evaluate clinical state of the implant surrounding tissues. According Lang et al. (22), probing consists a good indicator to evaluate healthy or unhealthy status of periimplant tissues.
The use of Osstell® permits the non-invasive measurement of quality of implantation. Furthermore, it is possible to measure the increase or decrease in implant stiffness through initial assessment of stability (made immediately after placement), and thus to monitor the healing process. The implant stability is subdivided into primary (immediately after implantation) and secondary (heal up) stability. The primary stability is largely a mechanical parameter. Two parameters are particularly useful when evaluating implant stability. The first one is the location in the bone. The second one is the stiffness of the implant in the surrouding tissue. The stiffness can be viewed in three ways: first of all the stiffness of the implant components themselves. Here the geometry and material composition for implants play the important part. In a second view is the stiffness of the implant/bone interface (bond between the surface of the implant and the surrounding bone) and in the third view is the stiffness of the bone itself (determined by the trabecular/cortical bone ratio and the importance of length and type of used implants and the preparation technique depending on the bone quality and quantity (23). The used of t-test on histomorphometry of the implant/bone samples permitted better quantification of primary stability after the surgical procedure than resonance frequency.
The results of this research demonstrate accentuate decline of ISQ index during the pos-operatory first 3 weeks. Implants installed in dense bone tissue show decreasing stability during osteointegration process. This reduce in anchorage may correspond to the remodel phase of necrotic tissue, followed by a bone apposition phase. According to Ueda et al. (24), a high compression of bone tissue can cause cellular death (necrosis), leading to bone cortical resorption. Other authors showed direct correlation between high stress regions and bone reabsortion process Huiskes and Nunamaker (25). When a local crestal bone resorption occurs, it is expected a reduce in AFR values in the subsequent weeks.
Moreover, it was observed in this study a relative equilibrium in AFR index after the 3rd week. After a long term, the implants reach stabile values non depending the bone density presented at the act of installation. The absence of loading over implants may have determined a stable phase between bone resorption and deposition performed by osteoclasts and osteoblasts during remodeling process; and in sequence the equilibrium in AFR index after the 3rd week pos-operatory.
Gingival evaluation in the present study was done by manual instruments and histological parameter. Probing procedures are frequently used for periimplants disease diagnostics, so that special attention must be given to periimplantar probing depth (PPd) that is considered a crucial parameter to determine tissue conditions surrounding implants. Such conditions may influence the exam exactness.
Some scientific studies relate that perimplant mucose resistance may be lower due to the configuration and orientation of tissue fibers that surround implants (26). The collagen fibers of conjunctive tissue are paralely arranged to the implant surface (1, 8) differing of the situation founded in teeth (Fig.10) where there are various fibers groups running from cement to conjunctive tissue and alveolar bone (26). The periodontal probe tends to penetrate near to bone crest, apically to the JE in titanium implants (6, 8).
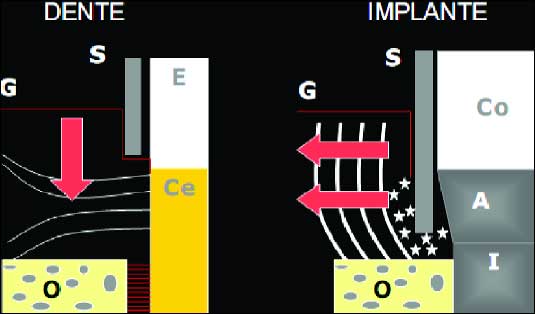 |
| Fig. 10. Periodontal probe effect in teeth (left side) and in implant region (right side).On the left side of diagram, the metallic probe do not penetrate into JE because of the perpendicular fibers towards to cement. On the right side, besides penetrating into JE, the metallic probe keeps away perimplant mucose to lateral direction. (G) gengival; (O) alveolar bone; (S) periodontal probe; (Co) protetic crown; (E) enamel; (A) abutment; (Ce) cementum; (I) titanium implant. Source: Lindhe (32). |
Ericsson and Lindhe (27) observed that probing resistance offered by dental gingive is bigger than that offered by periimplant mucose. Berglundh et al. (8), related that at both tooth and implant sites, the apical cells of the epithelium terminated approximately 1.0mm to 1.5mm coronal to the alveolar bone crest. Christensen et al. 14 found depth probing in the average of 2.18mm to 2.28 in implants and 1.65 to 1.82 in teeth. In a recent comparative review about biologic width around teeth and implant, the authors found values nearly by 1.5 mm (26). It is evident that the peri-implant seal around implants tends to be longer than around teeth (26). The findings in this research showed gingival SDI and JE varying from 1.8 mm to 2.4 mm for both implant types. These results are very similar to those found by Christensen et al. (28). Other study about unloaded implants showed values of 0.49 mm for SDI and 1,16 mm for JE after three months (1). In a comparative research about different implants surfaces, the authors found that length of the JE was higher on machined (mean 2.9 mm) than on the acid-etched (mean 1.4 mm) and oxidized surface (mean 1.6 mm) (29). The authors supposed that the smooth surface may allow more pronounced epithelial downgrowth as was reported for rough surfaces. Neverthless, the results of the present research did not demonstrate significant difference in the two implant necks. The probably stability of the alveolar bone crest observed in this work can be a difference mark towards the Glauser’s work (29).
The penetration of the probe apparently deflects the soft tissue from the implant surface and the tip of the probe reaches the JE apically. The present study showed in both surfaces that the most fibers at the interface run in a direction more or less parallel to the implant surface. This result is in accordance with the literature (1, 6, 21).
It was observed a small alteration of soft tissue with easy inflammable mucous hypertrophy. The existence of peri-mucositis was not confirmed through pathological test, but clinical examination. According to Lang et al. (22) the density of periimplant tissues in vivo influences in the probe penetration. In the presence of inflammatory tissue, the probe penetrated next to the crestal bone going through CT. However, in healthy tissue or with mucositis identified the supra crestal level. It was presented in this study that clinical healthy periimplant tissues showed a more rigid attachment and offered more resistance against probe penetration when compared with periimplantitis area. This way, probing means a reliable clinical procedure to monitor periimplant tissues fast reading and repeatable (22). Nevertheless, Smith and Zarb (30) relate that PPd is not a good predictor to periimplant bone alterations.
According Hermann et al. (31) dynamic changes were shown to occur within the components (SDI, JE and CTC) that constitute this stable structure, and their dimensions were found to be dependent on the presence/absence of microgap (interface) between the implant and abutment, and the location of the microgap (interface) in relation to the bone crest. A firm attachment of the mucosa to a tooth or an implant is a pre-requisite for peripherical defense and hemidesmossomes have a definite role in providing this attachment.
The soft tissue parameters revealed no significant differences between the two implant types. The peri-implant soft tissues appear to behave similarly in both implant types. The kind of implantation and implant design play a decisive role for good primary stability. The aesthetic demands are of particular importance for the patients. However, these results need to be verified also in loaded conditions in clinical setting.
Acknowledgements: The research was partly funded by a fellowship from the Alexander von Humboldt Foundation (BRA / 1115625) and Nobel Biocare.
Conflicts of interest statement: None declared.
- Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol 1997; 68: 186-198.
- Buser D, Weber HP, Donath K, Fiorellini JP, Paquette DW, Williams RC. Soft tissue reactions to non-submerged unloaded titanium implants in beagle dogs. J Periodontol 1992; 63: 225-235.
- Czesnikiewicz-Guzik M, Lorkowska B, Zapala J et al. NADPH oxidase and uncoupled nitric oxide synthase are major sources of reactive oxygen species in oral squamous cell carcinoma. Potential implications for immune regulation in high oxidative stress conditions. J Physiol Pharmacol 2008; 59: 139-152.
- Czesnikiewicz-Guzik M, Konturek SJ, Loster B, Wisniewska G, Majewski S. Melatonin and its role in oxidative stress related diseases of oral cavity. J Physiol Pharmacol 2007; 58: 5-19.
- Slomiany BL, Slomiany A. Role of endothelin-1-dependent up-regulation of leptin in oral mucosal repair. J Physiol Pharmacol 2005; 56: 531-541.
- Abrahamsson I, Berglundh T, Wennström J, Lindhe J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res 1996; 7: 212-219.
- Arvidson K, Fartash B, Hilliges M, Köndell PA. Histological characteristics of peri-implant mucosa around Brånemark and single-crystal sapphire implants. Clin Oral Implants Res 1996; 7: 1-10.
- Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res 1991; 2: 81-90.
- Anderson JM. Inflammation, wound healing, and the foreign body response. In: Davies JE (ed.) The bone-biomaterial interface, Toronto, University of Toronto Press, 1996, pp 165-173.
- Proff P, Bayerlein T, Rottner K, Mai R, Fanghänel J, Gedrange T. Effect of bone conditioning on primary stability of FRIALIT-2 implants. Clin Oral Implants Res 2008; 19: 42-47.
- Gedrange T, Boening K, Harzer W. Orthodontic implants as anchorage appliances for unilateral mesialization: a case report. Quintessence Int 2006; 37: 485-491.
- Mustafa K, Silva Lopez B, Hultenby K, Wennerberg A, Arvidson K. Attachment and proliferation of human oral fibroblasts to titanium surfaces blasted with TiO2 particles. A scanning electron microscopic and histomorphometric analysis. Clin Oral Implants Res 1998; 9: 195-207.
- Gedrange T, Bourauel C, Kobel C, Harzer W. Simulation of bone strain by orthodontic implants using the finite element method. Biomed Tech (Berl) 2003; 48: 287-290.
- Donath, K. Die Treen-Dünnschliff-Technik zur Herstellung histologischer Präparate von schneidbaren Geweben und Materialen. Der Präparator 1988; 34: 197–206.
- Chehroudi B, Soorany E, Black N, Weston L, Brunette DM. Computer-assisted three-dimensional reconstruction of epithelial cells attached to percutaneous implants. J Biomed Mater Res 1995; 29: 371-379.
- Gedrange T, Kobel C, Harzer W. Hard palate deformation in an animal model following quasi-static loading to stimulate that of orthodontic anchorage implants. Eur J Orthod 2001; 23: 349-354.
- Gedrange T, Bourauel C, Kobel C, Harzer W. Three-dimensional analysis of endosseous palatal implants and bones after vertical, horizontal, and diagonal force application. Eur J Orthod 2003; 25: 109-115.
- Schwarz F, Sculean A, Romanos G et al. Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig 2005; 9: 111-117.
- Chou CT, Morris HF, Ochi S, Walker L, DesRosiers D. AICRG, Part II: Crestal bone loss associated with the Ankylos implant: loading to 36 months. J Oral Implantol 2004; 30: 134-143.
- Mack F, Samietz SA, Mundt T et al. Prevalence of single-tooth gaps in a population-based study and the potential for dental implants—data from the Study of Health in Pomerania (SHIP-0). J Craniomaxillofac Surg 2006; 34: 82-85.
- Schupbach P, Glauser R. The defense architecture of the human periimplant mucosa: a histological study. J Prosthet Dent 2007; 97: 15-25.
- Lang NP, Wetzel AC, Stich H, Caffesse RG. Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin Oral Implants Res 1994; 5: 191-201.
- Gedrange T, Hietschold V, Mai R, Wolf P, Nicklisch M, Harzer W. An evaluation of resonance frequency analysis for the determination of the primary stability of orthodontic palatal implants. A study in human cadavers. Clin Oral Implants Res 2005; 16: 425-431.
- Ueda M, Matsuki M, Jacobsson M, Tjellström A. Relationship between insertion torque and removal torque analyzed in fresh temporal bone. Int J Oral Maxillofac Implants 1991; 6: 442-447.
- Huiskes R, Nunamaker D.Local stresses and bone adaption around orthopedic implants. Calcif Tissue Int 1984; 36: 110-117.
- Linkevicius T, Apse P. Biologic width around implants. An evidence-based. Stomatologija 2008; 10: 27-35.
- Ericsson I, Lindhe J. Probing depth at implants and teeth. An experimental study in the dog. J Clin Periodontol 1993; 20: 623-627.
- Christensen MM, Joss A, Lang NP. Reproducibility of automated periodontal probing around teeth and osseointegrated oral implants. Clin Oral Implants Res 1997; 8: 455-464.
- Glauser R, Schunbach P, Gottlow J, Hämmerle CH. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: a light-microscopic overview and histometric analysis. Clin Implans Dent Relat Res 2005; 7: 44-51.
- Smith DE. Zarb GA. Criteria for success of osseointegrated endosseous implants. J Prosthetic Dentistry (St. Louis)1989; 62: 567-572.
- Hermann JS, Buser D, Schenk RK, Schoolfield JD, Cochran DL. Biologic width around one- and two-piece titanium implants. Clin Oral Implants Res 2001; 12: 559-571.
- Lindhe J. Tratado de periodontia clinica e implantodontia oral. 4th Ed. Rio de Janeiro, Guanabara-Koogan, 2005.