The role of ATP-binding cassette (ABC) transporters located in the cell membrane, including P-glycoprotein and the multidrug resistance protein 1 (MRP1), is the removal of xenobiotics from the cell. In normal cells, this is a physiological process, an element of the so-called third line of tissue protection. In cancer cells, the ABC transporters, acting by the same principle, reduce the effectiveness of treatment by lowering the concentration of the anticancer drug inside the cell. This is effected by active transport, proceeding against the concentration gradient and using the energy derived from ATP hydrolysis (11). Both the ABC transporters and their inhibitors exhibit substrate specificity (12), however, most cytostatic agents may be translocated by different types of transporter molecules. This is the case of vincristine (VCR), a Vinca alkaloid widely used in the treatment of solid tumors, which may be translocated by ABCB1 (P-glycoprotein), as well as by ABCC1 (MRP1). Vincristine transport by the MRP1 molecule is affected by the intracellular GSH level (11, 13), and in cells in which the cytostatic agent translocation is MRP1-mediated, a lowering of the GSH level results in VCR accumulation. Notably, such cells include melanoma cells (14, 15). We have previously shown that extracts of defatted O. paradoxa seeds considerably lowered the GSH level in melanoma cells of HTB-140 line (6).
The aim of the present study was to compare the effect of standardized 60% ethanolic extract of defatted O. paradoxa seeds (EPE) with the activity of individual constituents of this extract, including PGG, gallic acid, (+)-catechin and the procyanidin rich fraction. Next, the combined effect of EPE and VCR in HTB-140 cells expressing the gene encoding the MRP1 transporter (16) and in cells of the HepG2 hepatoma line expressing mainly the MDR1 gene encoding P-glycoprotein (17) was investigated. In HTB-140 cells, the above combined effect was assessed in the absence or presence of an MRP1 inhibitor (indomethacin), while in the HepG2 cells - in the absence or presence of P-glycoprotein inhibitor (verapamil).
Chemicals
Vincristine sulfate salt (VCR), indomethacin (IND), (±)-verapamil hydrochloride (VRP) were purchased from Sigma-Aldrich Chemie (Germany). Gallic acid (GA) and (+)-catechin (CAT) were purchased from Serva Feinbiochemica (Germany). Pentagalloylglucose (PGG) was isolated and identify as described previously (18).
Plant material
Dried 60% ethanolic extract of defatted seeds of Oenothera paradoxa obtained from Agropharm SA (Poland) contains 745.5±8.8 mg/g of total polyphenols, 2.18±0.08 mg/g of gallic acid, 30.41±1.48 mg/g of (+)-catechin and 16.75±0.64 mg/g of pentagalloylglucose, as determined previously (6). Before each experiment the tested substance (EPE, PGG, GA, CAT or PA fraction) was dissolved in water and sterilized by filtration. Thus prepared working solutions were diluted in the cellular medium.
Preparation of polymeric procyanidin (PA) rich fraction
Accurately weighted 20 g of 60% ethanolic extract was dissolved in water (200 mL) and extracted with ethyl acetate (3x200 mL). The aqueous residue was lyophilized (giving 16.2 g).
High-performance liquid chromatography (HPLC) analysis
The phenolics of extract and polymeric procyanidin rich fraction were analyzed by HPLC as described originally (6), chromatograms were acquired at 280 nm. Samples were dissolved in MeOH + 2.5% CH3COOH (1:1) to the concentration of 10 mg/mL. The chromatograms of EPE and PA fraction are shown in Fig. 1A and 1B.
 |
Fig. 1. HPLC chromatograms of (A) EPE (B) polymeric procyanidin rich fraction. Gallic acid (GA), (+)-catechin (CAT), pentagalloylglucose (PGG), procyanidins (PA). |
Cell culture
Human metastatic melanoma cells, line Hs 294T (HTB-140) and human hepatoma cells, line HepG2 were purchased from American Type Culture Collection (USA) and incubated in DMEM (Lonza Inc., USA) as described previously (6).
Cell viability assay
The viability of both cell types was assessed with the use of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma-Aldrich, Germany) as described ref. 6. Cells were incubated with the tested substance (EPE, PGG, GA, CAT or PA fraction) for 24 hours and then with the MTT solution for 1 hour. In the case of pre-incubation with verapamil (100 µM) or indomethacin (700 µM), after 15 min vincristine (1 µM) was added for 2 hours, then the cells were rinsed with PBS (phosphate buffered saline, Biomed, Poland) and incubated for the next 24 hours with the extract.
Cellular membrane integrity assessment
To assess cellular membrane integrity at 24 hours of incubation of HTB-140 or HepG2 cells with the tested substance (EPE, PGG, GA, CAT or PA fraction) the activity of lactate dehydrogenase (LDH) released from cytosol of damaged cells to the supernatant was measured using the Cytotoxicity Detection Kit (Roche Diagnostics, Germany) as described previously (6). In the case of pre-incubation with verapamil or indomethacin cells were treated as in the viability assay.
ATP level measurement
ATP level of HTB-140 cells was measured using the ViaLight Plus Assay Kit (Lonza Inc., USA) as described ref. 6. In the case of pre-incubation with indomethacin cells were treated as above.
Morphology
Changes in morphology were assessed with the use of the Eclipse TS100F microscope (Nikon, USA) in visual light and, in the case of HTB-140 cells, also in fluorescence after their prior dyeing with calcein-AM and propidium iodide (MoBiTec, Germany).
Statistical analysis
The data were compared with the use of one-way analysis of variance (ANOVA) with the Tukey's posthoc test, using the Statistica 8.0 software (StatSoft, Poland). Significance was determined at: p<0.05, p<0.001.
Comparison of the EPE effect with the activity of individual constituents of the extract
In HTB-140 cells, both EPE and PGG (Table 1), as well as the procyanidin rich fraction (Fig. 2), caused a significant reduction in viability, with the observed IC50 (fifty percent growth inhibition concentration) values after a 24-hour incubation of 72.4 µg/mL, 47.4 µM and 78.7 µg/mL for EPE, PGG and PA fraction, respectively. Incubation of HTB-140 cells with PGG (>50 µM) or with PA fraction (>50 µg/mL) resulted in the LDH release reduction. In light microscopy, the cells were markedly swollen, with loss of cell membrane integrity, suggesting the cells have entered the necrotic death pathway (19). After 24-hour incubation of HepG2 cells with EPE (>50 µg/mL) or PGG (>50 µM), a reduction in viability and an increase in cell death were noted, although the IC50 values were not achieved within the range of concentrations studied. In both cell types, neither gallic acid nor (+)-catechin exhibited a statistically significant cytotoxic effect (5-200 µM, p>0.05). The combined administration of PGG, gallic acid and (+)-catechin did not increase PGG cytotoxicity towards HTB-140 cells, causing only a slight drop in cell viability (data not shown).
| Table 1. MTT and LDH release assay results from HTB-140 and HepG2 cells treated with EPE or PGG. |
 |
| Cells were incubated with EPE (5-200 µg/mL) or PGG (5-200 µM/940 g/mol) for 24 hours. Data are expressed as means±S.D.from three independent experiments performed in triplicate. * p<0.05; ** p<0.001 refer to the control (untreated cells). |
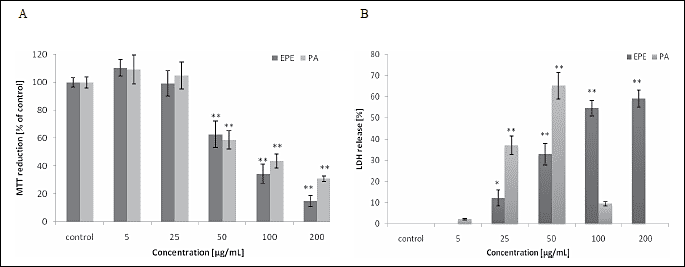 |
| Fig. 2. MTT (A) and LDH (B) release assay results from HTB-140 cells treated for 24 h with EPE or PA. Data are expressed as means ±SD from three independent experiments performed in triplicate. Statistically significant differences ( *p < 0.05, ** p < 0.001) refer to the control (untreated cells). |
Assessment of the co-administration of EPE and vincristine
Addition of EPE (25-200 µg/mL) to HTB-140 cells after their incubation with vincristine at 1 µM (the concentration showing approx. 10% cytotoxicity for the cells of both lines) resulted in a significant reduction in cell viability and an increase in cell death. Significant cytotoxicity in HepG2 cells was also obtained at 100 µg/mL and higher EPE concentrations, however, the effect was statistically significantly lower than that achieved in HTB-140 cells (p<0.001) (Fig. 3). In light microscopy, significant changes in cell morphology were observed after incubation with either vincristine alone (Fig. 4D) or with vincristine and the extract (Fig. 4G). Fluorescence microscopy revealed differences in cell viability following staining with calcein-AM and propidium iodide. Incubation with vincristine and the extract resulted a significant reduction in the number of cells stained with calcein (Fig. 4H) and an increase in the number of PI-positive cells (Fig. 4I). Furthermore, strong hyperfluorescence of cells incubated with vincristine only and stained with calcein-AM was observed (Fig. 4E), which might be connected with a vincristine-mediated reduction in calcein efflux (20).
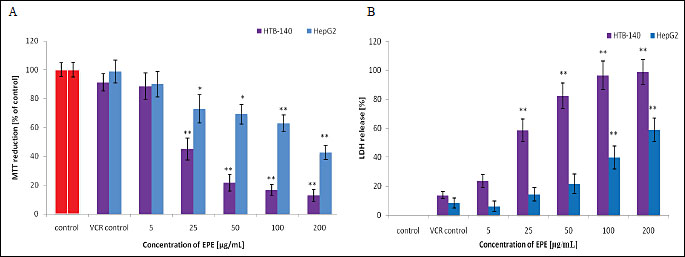 |
| Fig. 3. MTT and LDH release assay results from HTB-140 and HepG2 cell lines. Cells were incubated with vincristine (1 µM) for 2 hours, rinsed with PBS and then incubated for next 24 hours with the indicated concentrations of EPE. Data are expressed as means±S.D. from three independent experiments performed in triplicate. Statistically significant differences: * p<0.05; ** p<0.001 refer to the VCR control (cells incubated with vincristine alone). |
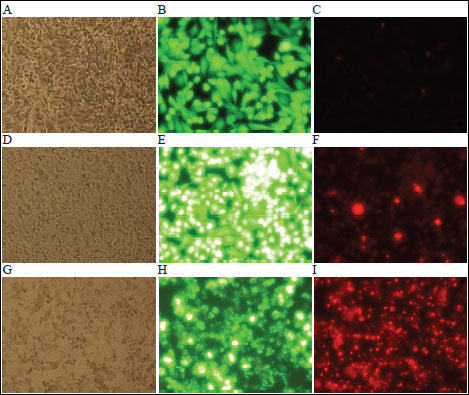 |
Fig. 4. Morphologic changes of HTB-140 cells. A. Untreated cells observed in the light microscope (100x); B. in fluorescence (200x) after calcein-AM staining; C. in fluorescence (200x) after PI staining; D. Cells treated with vincristine (1 µM) for 2 hours observed in the light microscope; E and F in fluorescence after calcein-AM or PI staining, respectively; G. Cells treated with vincristine (1 µM) for 2 hours, rinsed with PBS and incubated for additional 24 hours with EPE (25 µg/mL) observed in the light microscope; H and I in fluorescence after calcein-AM or PI staining, respectively. The calcein in viable cells emits green fluorescence, while PI emits red fluorescence only in dead cells. |
Impact of MRP1 and P-glycoprotein inhibitors on the co-administration of EPE and VCR
Incubation of HTB-140 cells with EPE (25 µg/mL, the lowest concentration resulting in a statistically significant reduction in cell viability), VCR (1 µM) and MRP1 inhibitor (indomethacin at the concentration of 700 µM, reducing the cell viability by approx. 10% as compared to cells exposed to vincristine alone) did not produce any changes in viability or mortality of cells as compared to cells incubated with EPE and VCR; cell death remained at the level of approx. 60% irrespective of the presence of an inhibitor (Fig. 5A and 5B). The addition of indomethacin had no effect either on the intracellular ATP level which was lowered more than 7-fold as a result of incubation of HTB-140 cells with EPE (25 µg/mL) and VCR (1 µM) as compared to cells exposed to vincristine alone (Fig. 6). In the case of HepG2 cells, addition of the P-glycoprotein inhibitor (verapamil at the concentration of 100 µM, reducing cell viability by approx. 10% as compared to cells exposed to vincristine alone) caused a statistically significant reduction in viability and an increase in cytotoxicity as compared to cells incubated with EPE (25 µg/mL) and VCR (1 µM) without the inhibitor (Fig. 5C and 5D).
 |
Fig. 5. Graphs present MTT and LDH release assay results from HTB-140 (A/B) and HepG2 (C/D) cell lines. Cells preincubated for 15 min with verapamil (100 µM) or indomethacin (700 µM) then incubated with inhibitors and vincristine (1 µM) for 2 hours, rinsed with PBS and incubated for additional 24 hours with EPE (25 µg/mL). Data are expressed as means±S.D. from three independent experiments performed in triplicate. Statistically significant differences: * p<0.05; ** p<0.001 refer to the VCR control (cells incubated with vincristine alone). |
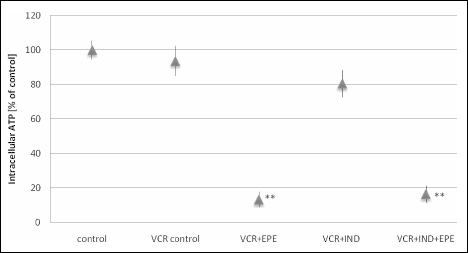 |
Fig. 6. Changes in ATP levels of HTB-140 cells preincubated for 15 min with indomethacin (700 µM) then incubated with inhibitor and vincristine (1 µM) for 2 hours, rinsed with PBS and incubated for additional 24 hours with EPE (25 µg/mL). Data are presented as means±S.D. from two independent experiments performed in triplicate. Statistically significant differences (** p<0.001) refer to the VCR control (cells incubated with vincristine alone). |
Both the ethanolic extract of defatted O. paradoxa seeds (EPE) as well as PGG and procyanidins presented in this extract displayed significant cytotoxicity towards human melanoma cells (HTB-140). Incubation with higher concentrations of PGG (>50 µM) and procyanidins (>50 µg/mL) caused damage to cellular structures and made the cells enter the necrotic death pathway, as evidenced by the analysis of cell morphology and the drop in LDH release with a concomitant reduction in cell viability. It is known that the half-life of LDH released from cells is approximately 9-10 hours, therefore, after 24-hour incubation a strongly cytotoxic substance may produce low LDH release (21). On the other hand, neither gallic acid nor (+)-catechin caused a drop in cell viability and an increase in cell death within the investigated concentration range. This might indicate that it is mainly PGG and procyanidins that are responsible for the potency of EPE action, all the more so because PGG exhibits stronger pro-oxidant properties and higher cytotoxicity towards cancer cells than gallic acid (22), as well as procyanidins, oligomerized flavanols, have been reported to be more cytotoxic than monomer flavanols, including (+)-catechin, in a variety of human cell lines (23). HepG2 cells are much more resistant to the action of both EPE and EPE with VCR than melanoma cells, what may be associated with the level of intracellular GSH. Our experience indicates that metastatic melanoma HTB-140 cells have a higher baseline GSH value than those of HepG2 line, and it is the former that are more sensitive to the fluctuations in this molecule level. These observations correspond with the findings that highly metastatic melanoma cells, as compared with the low metastatic, have elevated levels of GSH (24-26). Therefore depletion of GSH content might sensitize metastatic melanoma cells to chemotherapy (26). Previously, we have demonstrated that EPE (25-200 µg/mL) significantly lowered the GSH level in HTB-140 cells (6). This cell line expresses MRP1 (16), and vincristine efflux is dependent on the GSH level, the drop in which most probably results in intracellular VCR accumulation and intensification of its anticancer action. This may explain the almost 3-fold higher cytotoxicity of EPE towards HTB-140 cells than towards HepG2 cells which express mainly MDR1 encoding P-glycoprotein (17). The lowering of the intracellular GSH level below the capacity of the antioxidant system results in the reactive oxygen species accumulation, destruction of the electron transport chain complexes and in ATP synthesis inhibition (27). The co-administration of EPE and VCR reduced ATP synthesis in HTB-140 cells more than 7-fold.
In conclusion, EPE, PGG and the procyanidin fraction exhibited potent cytotoxic effect against human melanoma cells. Incubation of HTB-140 cells with EPE and VCR reduced the viability, increased mortality and limited the synthesis of intracellular ATP, while addition of an MRP1 inhibitor did not produce any further cytotoxicity changes. HepG2 cells were much less sensitive to both EPE alone and EPE with VCR. In future, EPE might prove a component increasing the effectiveness of therapy for certain types of cancer, including melanoma, but further studies on cell lines resistant to cytostatics as well as in vivo studies are still needed.
Conflict of interests: None declared.
- Newall CA, Anderson LA, Phillipson JD. Herbal Medicines, a Guide for Health-Care Professionals. London, The Pharmaceutical Press, 1996, pp. 110-113.
- Peschel W, Dieckmann W, Sonnenschein M, Plescher A. High antioxidant potential of pressing residues from evening primrose in comparison to other oilseed cakes and plant antioxidant. Ind Crops Prod 2007; 25: 44-54.
- Kapiszewska M, Soltys E, Visioli F, Cierniak A, Zajac G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J Physiol Pharmacol 2005; 56: 183-197.
- Hoelzl C, Bichler J, Ferk F, et al. Methods for the detection of antioxidants which prevent age related diseases: a critical review with particular emphasis on human intervention studies. J Physiol Pharmacol 2005; 56: 49-64.
- Patra SK, Rizzi F, Silva A, Rugina DO, Bettuzzi S. Molecular targets of (-)-epigallocatechin-3-gallate (EGCG): specificity and interaction with membrane lipid rafts. J Physiol Pharmacol 2008; 59(Suppl. 9): 217-235.
- Jaszewska E, Kosmider A, Kiss AK, Naruszewicz M. Pro-oxidative and pro-apoptotic action of defatted seeds of Oenothera paradoxa on human skin melanoma cells. J Agric Food Chem 2009; 57: 8282-8289.
- Zhang J, Li L, Kim SH, Hagerman AE, Lu J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res 2009; 26: 2066-2080.
- Ho LL, Chen WJ, Lin-Shiau SY, Lin JK. Penta-O-galloyl-b-D-glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase-9 through down-regulation of activator protein-1. Eur J Pharmacol 2002; 453: 149-158.
- Oh GS, Pae HO, Oh H, et al. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett 2001; 174: 17-24.
- Kitagawa S, Nabekura T, Nakamura Y, Takahashi T, Kashidawa Y. Inhibition of P-glycoprotein function by tannic acid and pentagalloylglucose. J Pharm Pharmacol 2007; 59: 965-969.
- Borst P, Elfernik RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem 2002; 71: 537-592.
- Teisseyre A, Duarte N, Ferreira MJ, Michalak K. Influence of the multidrug transporter inhibitors on the activity of Kv1.3 voltage-gated potassium channels. J Physiol Pharmacol 2009; 60: 69-76.
- Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res 2001; 6: 1798-1804.
- Depeille P, Cuq P, Mary S, et al. Glutathione S-transferase M1 and multidrug resistance protein 1 act in synergy to protect melanoma cells from vincristine effects. Mol Pharm 2004; 65: 897-905.
- Depeille P, Cuq P, Passagne I, Evrard A, Vian L. Combined effects of GSTP1 and MRP1 in melanoma resistance. Br J Cancer 2005; 93: 216-223.
- Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res 2004; 64: 4912-4918.
- Zhai BJ, Shao ZY, Zhao CL, Hu K, Wu F. Development and characterization of multidrug resistant human hepatocarcinoma cell line in nude mice. World J Gastroenterol 2006; 41: 6614-6619.
- Kiss AK, Derwinska M, Dawidowska A, Naruszewicz M. Novel biological properties of Oenothera paradoxa defatted seed extracts. Effects on the metallopeptidases activity. J Agric Food Chem 2008; 56: 7845-7852.
- Lemasters JJ. Dying a thousand deaths: redundant pathways from different organelles to apoptosis and necrosis. Gastroenterology 2005; 129: 351-360.
- Feller N, Broxterman HJ, Wahrer DCR, Pinedo HM. ATP-dependent efflux of calcein by multidrug resistance protein (MRP): no inhibition by intracellular glutathione depletion. FEBS Lett 1995; 368: 385-388.
- Riss TL, Moravec RA, O'Brien MA, Hawkins EM, Niles A. Homogeneous Multiwell Assays for Measuring Cell Viability, Cytotoxicity, and Apoptosis. In Handbook of Assay Development in Drug Discovery, LK Minor (ed). Boca Raton FL, CRC Press, 2006, pp. 392-395.
- Abdelwahed A, Bouhlel I, Skandrani I, et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem Biol Interact 2007; 165: 1-13.
- Actis-Goretta L, Romanczyk LJ, Rodriguez CA, Kwik-Uribe C, Keen CL. Cytotoxic effects of digalloyl dimer procyanidins in human cancer cell lines. J Nutr Biochem 2008; 19: 797-808.
- Ortega A, Ferrer P, Carretero J, et al. Down-regulation of glutathione and Bcl-2 synthesis in mouse B16 melanoma cells avoids their survival during interaction with the vascular endothelium. J Biol Chem 2003; 278: 39591-39599.
- Benlloch M, Ortega A, Ferrer P, et al. Acceleration of glutathione efflux and inhibition of g-glutamyltranspeptidase sensitize metastatic B16 melanoma cells to endothelium induced cytotoxicity. J Biol Chem 2005; 280: 6950-6959.
- Mena S, Benlloch M, Ortega A, et al. Bcl-2 and glutathione depletion sensitizes B16 melanoma to combination therapy and eliminates metastatic disease. Clin Cancer Res 2007; 13: 2658-2666.
- Hsu M, Srinivas B, Kumar J, Subramanian R, Andersen J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: implications for Parkinson's disease. J Neurochem 2005; 92: 1091-1103.