In the present study, we sought to reveal the potential additional effects of two EDCs: bisphenol A (BPA) and isobutylparaben (IBP). BPA is produced worldwide for use in a variety of industrial and consumer products, such as epoxy resins used to line food cans, polyester-styrene, and polycarbonate plastics, which are used in some baby bottles and other containers (5). BPA in food and beverage packaging is likely the source of most human oral exposure, and dermal and inhaled exposure may occur from other sources (6). Biochemical assays have investigated the kinetics of BPA binding to estrogen receptors (ERs) and have found that BPA binds to both estrogen receptor-alpha (ER
Parabens are widely used as preservatives in cosmetics, foods and drugs (12, 13). The estrogenicity of these compounds has been examined in vitro and in vivo. In some screening tests, parabens showed estrogenic activity, such as ligand binding to ERs and the proliferation of MCF-7 cells, and the reported in vivo effects include increased uterine weight and male reproductive tract effects (14, 15). Isobutylparaben (IBP) has relatively high estrogenic activity among the parabens (16). Like other EDCs, IBP can bind to ERs, stimulating an ER-dependent response and influencing the expression of estrogen-responsive genes, including ERa and PR (17).
We have chosen BPA and IBP among EDCs since they are both estrogenic. In addition, human have more opportunity to be exposed to these chemicals from many plastic wares and cosmetic products (18, 19). To demonstrate the combined effects of BPA and IBP, we designed an in vitro experiment using the calbindin-D9k (CaBP-9k) gene as a biomarker induced by xenoestrogen exposure (20, 21). Other recent studies have also used reliable biomarkers to evaluate and characterize the estrogenicity of EDCs. The potency of EDCs was determined by assays monitoring the response of biomarkers to EDC exposure, illuminating the additional effects of these two chemicals (17, 22-24).
CaBP-9k is a member of large family of intracellular calcium binding proteins that have high affinity for calcium. CaBP-9k has been proposed as a new biomarker to detect EDCs. CaBP-9k has been shown to be expressed in several mammalian tissues, including kidney, uterus, and intestine (25-30). The estrogen responsive element (ERE) and progesterone responsive element (PRE) are present in the CaBP-9k promoter and mediate transcriptional regulation of CaBP-9k in the rat uterus (31). The ERE in the rat CaBP-9k gene is located at nucleotide +51 (transcriptional initiation site = +1) (32) and oligonucleotides containing the minimal ERE for the CaBP-9k promoter (nucleotides +51 to +61) is able to bind to ER
The GH3 cell line is a well-established pituitary cell line sensitive to estrogenic stimulation (34) and dependent on estrogen for proliferation in culture (35). We employed GH3 cells in this study to examine additional effects of BPA and IBP, since GH3 cells express CaBP-9k gene regulated by E2 and EDCs (17, 36). In a previous study, we used GH3 cells as an in vitro model to examine the estrogenicity of parabens (17, 36). In the present study, GH3 cells were treated with various concentrations of BPA and IBP, and the transcription and translation of CaBP-9k and PR were analyzed by molecular techniques. In addition, we utilized ICI182,780 treatment to investigate the possible involvement of ERs in EDC-induced CaBP-9k and PR expression.
Reagents and chemicals
17ß-estradiol (E2) and bisphenol A (BPA) were purchased from Sigma Chemical Company (St. Louis, MO). Isobutyl p-hydroxybenzoate (IBP) (minimum 99.0% purity) was obtained from Tokyo Kasei Kogyo Co. LTD (Tokyo, Japan) and ICI182,780 (also known as flaslodex or fulvestrant) was purchased from Tocris (Ellisville, MO). All chemicals were dissolved in 100% dimethyl sulfoxide (DMSO; Sigma-Aldrich Company, Ayrshire, UK) and stored as a stock solution at –20°C to avoid contamination. Rabbit CaBP-9k and goat-anti rabbit antibodies were provided by Swant (Bellsinzona, Switzerland). Anti-PR antibodies and horseradish peroxidase (HRP)-conjugated anti-mouse IgG and anti-rabbit antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Cell culture and treatment
GH3 cells were obtained from The Korean Cell Line Bank (Seoul, Korea). Cells were grown as monolayer cultures in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, Grand Island, NY), supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY), 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco BRL, Grand Island, NY) at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. GH3 cells were plated on 6-well plastic tissue culture dishes (NUNC™; Roskilde, Denmark) at a density of 3×106 cells/well and grown until 70–80% confluent. The media was replaced with phenol red-free DMEM supplemented with 5% charcoal dextran-stripped FBS and 100 U/ml penicillin-streptomycin for 7 days to ensure the depletion of steroid hormones in the cells. The cells used in these experiments were grown normally throughout the study. After 7 days, the cells were exposed to a single dose of BPA (at 10–7, 10–6, or 10–5M), IBP (at 10–7, 10–6, or 10–5M), or each combination of these doses. Each chemical was dissolved in DMSO and added to phenol red-free DMEM-5% FBS-CD (starvation media) with the final DMSO concentration being 0.1%. Starvation media with 10–9 M E2 was used as a positive control, and starvation media with DMSO only was used as a negative control (vehicle). GH3 cells were harvested 24 hours after treatment to measure mRNA and protein levels. To examine the mechanism of CaBP-9k induction by these EDCs, cells were pre-treated with 10–7 M ICI182,780 for 30 min prior to EDC exposure (37). After ICI182,780 treatment, cells were treated with high-dose BPA (10–5 M) in combination with 10–7, 10–6 and 10–5M IBP. These combination doses were administered both in the presence and in the absence of ICI182,780. The concentrations of BPA and IBP tested were those that produced the highest response in GH3 cells in a dose-response experiment. After 24 hours, whole cells were harvested for mRNA and Western blot analysis. All experiments were performed in triplicate.
Quantitative real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRI reagent (Ambion, Austin, TX, USA) according to the methods outlined in the protocol, and the concentration of total RNA was determined by measuring the absorbance at 260 nm. One microgram of total RNA was reverse transcribed into first-strand cDNA using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and 9-mer random primers (Takara Bio, Otsu, Shiga, Japan). Quantitative RT-PCR was performed using a real-time PCR system 7300 (Applied Biosystems, Foster City, CA, USA) with 1 µl of cDNA template added to 10 µl of 2× SYBR Premix Ex Taq (TaKaRa Bio Inc.) containing specific primers at a concentration of 10 pM each. Reactions were carried out for 40 cycles. The cycling parameters were as follows: denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. Fluorescence intensity was measured at the end of the extension phase of each cycle. The threshold value for the fluorescence intensity of all the samples was set manually. The reaction cycle at which PCR products exceeded this fluorescence intensity threshold in the exponential phase of PCR amplification was taken as the threshold cycle (CT). The PCR product of cytochrome c oxidase subunit 1 (1A, a ubiquitously expressed housekeeping gene) (38) was used as a control for mRNA concentrations in the RT-PCR reactions. The relative expression level of each gene was quantified using RQ software (Applied Biosystems). The amount of transcript present was inversely related to the observed CT and, for every two-fold dilution in the amount of transcript, CT was expected to increase by 1. Relative expression (R) was calculated using the equation R=2- [
Western blot analysis
Protein samples were extracted with Pro-prep solution (iNtRON Biotechnology, Seoul, Korea) following the manufacturer’s protocol. Forty micrograms of cytosolic protein per lane was size-fractionated by 7.5% and 12.5% SDS-PAGE and transferred to a nitrocellulose membrane (Millipore, Bedford, MA, USA). The membranes were then blocked for 2 hours with 5% skim milk (Difco™, Sparks, MD) in phosphate-buffered saline containing 0.05% Tween-20 (PBS-T). Primary and secondary antibodies were applied to the membranes in 5% skim milk in PBS-T for 1 hour each at room temperature. Antibodies were used against rat CaBP-9k (diluted 1:2000, CB9, Swant, Bellinzona, Switzerland) and PR (diluted 1:500, sc-538, Santa Cruz Biotech). HRP-conjugated anti-rabbit and anti-mouse secondary antibodies (diluted 1:3000) and Western blotting luminol reagent (Santa Cruz Biotechnology Inc., CA) were used to assess immunoreactivity. Each immunoblot was stripped with 2% SDS and 100 mM mercaptoethanol in 62.5 mM Tris-HCl, pH 6.8, for 30 min at 50–60°C; membranes were then washed in PBS-T (twice, for 5 min each), blocked for 1 hour in 5% skim milk (39), and re-probed with an antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (diluted 1:2000, CSA-335, Assay Designs Inc., Ann Arbor, MI). Immunoreactive proteins were visualized by exposure to X-ray film. Protein bands were quantified by image scanning, and optical density was measured using a Gel Doc EQ system (Bio-rad Laboratories Inc.) after the data were corrected by background subtraction and normalized using GAPDH as an internal control.
Construction of a reporter plasmid and transient transfection
Three copies of the ERE [p(ERE)3] sequence were inserted into the pGL3-promoter vector (Promega, USA) upstream of the SV40 promoter. ERE oligomers were synthesized containing MluI and XhoI restriction sites at both termini. The ERE sequence was 5’-AGG TCA CTG TGA CCC TGG GTC ACT GTG ACC CTG GGT CAC TGT GAC C-3’. For transient transfection, 3×105 cells/well were plated onto a 6-well dish and transfected 18 hours later with a luciferase plasmid by transfection using lipofectamine™ 2000 (Invitrogen Corporation) according to the manufacturer’s directions. Four micrograms of DNA and 10 µl of lipofectamine™ 2000 reagent were used per well. The control plasmid RSV-lacZ (0.5 µg) (Clontech, USA) was co-transfected to monitor transfection efficiency. After 4 hours of incubation, the transfection mixtures were removed and replaced with normal growth medium or hormone-supplemented medium. Following an additional 24 hours of culture, the cells were harvested and their luciferase activity was determined by a Dual Luciferase assay (Promega, USA). The activity was normalized for transfection efficiency after determining the ß-galactosidase activity of each sample. Each transfection was carried out in triplicate, and experiments were repeated at least four times.
Statistical analyses
Results are presented as means ±standard error of the mean (S.E.M.); p values were calculated using one-way analysis of variance, followed by Tukey’s test for multiple comparisons of columns. Data were considered statistically significant at p<0.05.
Effects of single or combined administration of bisphenol A and isobutylparaben on estrogen responsive element activity
Luciferase activity was induced by E2, BPA and IBP in GH3 cells transfected with the ERE-luciferase construct. Transiently transfected GH3 cells were incubated with single or combination doses of BPA and IBP for 24 hours. As shown in Fig. 1, increased luciferase activity was observed with both single and combination treatments (BPA single doses: 10–7, 10–6 and 10–5 M; IBP single doses: 10–7, 10–6 and 10–5 M; combined doses: 10–7, 10–6 and 10–5 M for each) and was dose-dependent. In addition, luciferase activity was significantly higher with a combination of the highest dose of BPA (10–5 M) with the lowest (10–7 M) or middle dose of IBP (10–6 M) compared to a single dose exposure respectively. However, other combination doses did not induce a significant increase in luciferase activity, and the effects were masked with higher EDC doses.
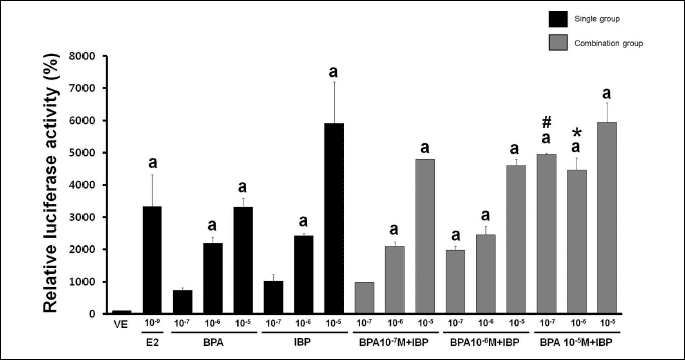 |
| Fig. 1. Effects of single or combined treatment with BPA and IBP on ERE activity. GH3 cells transfected with p(ERE)3 constructs were treated with 0.1% DMSO (VE) as a negative control, E2 at 10–9 M as a positive control, BPA alone (10–7, 10–6, and 10–5 M), IBP alone (10–7, 10–6, and 10–5 M), or a combination of BPA (10–7, 10–6, and 10–5 M) and IBP (10–7, 10–6, and 10–5 M). An expression vector encoding RSV-lacZ was co-transfected to normalize transfection efficacy. Luciferase activity is represented as percent induction after being normalized to ß-galactosidase compared to cells transfected with pGL3-promoter, which was set as 100%. Data represent the means ±S.E.M. of triplicate experiments. a, p<0.05 compared to vehicle; #, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–7 M); *, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–6 M). |
Combined effects of bisphenol A and isobutylparaben on the expression of CaBP-9k mRNA and protein
The effects of BPA and IBP on the expression of CaBP-9k were assessed by RT-PCR and Western blot analysis. As shown in Fig. 2, dose-dependent effects were observed 24 hours after single or combined EDC exposure. A significant increase in the expression of CaBP-9k mRNA was observed with a mixture of the lowest concentration of BPA (10–7 M) and IBP (10–7 M) or the highest concentrations of BPA (10–5 M) and IBP (10–5 M), as seen in Fig. 2A. In addition, the expression of CaBP-9k protein was significantly increased with the lowest dose of BPA (10–7 M) and the highest dose of IBP (10–5 M), and with the highest dose of BPA (10–5 M) and IBP (10–5 M) combined compared to single exposures with these EDCs (Fig. 2B). Additional effects were not observed with other combined doses of these EDCs. Effects on CaBP-9k gene expression were masked with relatively higher doses. Expression of CaBP-9k mRNA was 462-fold higher and protein expression was 141-fold higher in the positive control (E2, 10–9 M) than a negative control. To determine the biological pathways involved in the regulation of CaBP-9k expression by combined BPA and IBP in GH3 cells, we pre-treated cells with ICI182,780. GH3 cells were treated with single or combination doses of BPA and IBP in the absence or presence of ICI182,780 (10–7 M) treatment 30 min prior to chemical exposure. As shown in Fig. 3A, ICI182,780 pre-treatment completely attenuated the transcription and translation of CaBP-9k induced by EDCs. This result indicates that the effects of BPA and IBP on the induction of CaBP-9k expression involve ER-mediated pathway in GH3 cells.
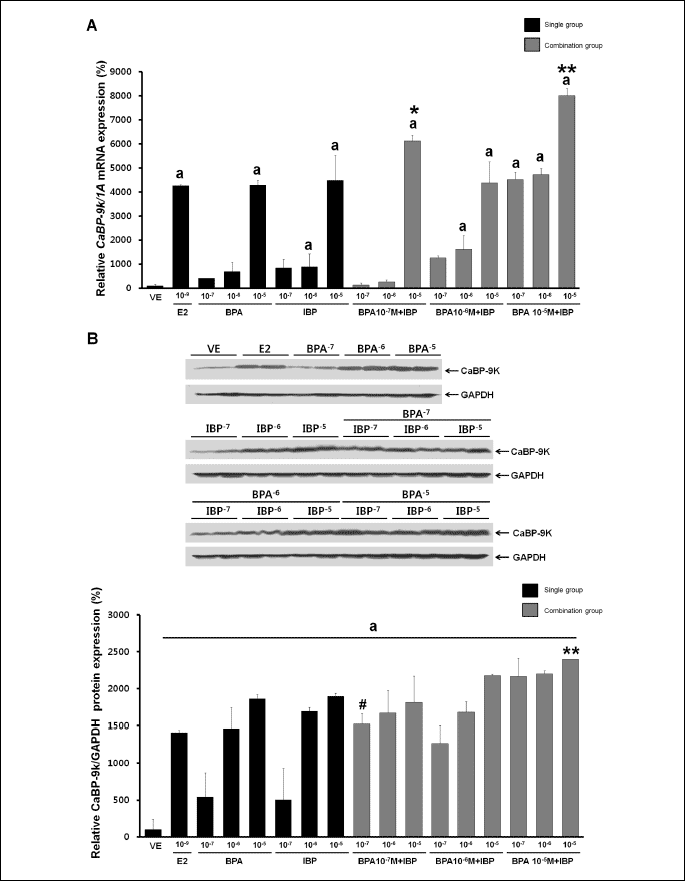 |
| Fig. 2. Effects of single or combined BPA and IBP treatment on CaBP-9k mRNA and protein expression. Cells were treated with DMSO alone as a vehicle (VE); with E2 at 10–9 M as a positive control; or with BPA alone (10–7, 10–6, and 10–5 M), IBP alone (10–7, 10–6, and 10–5 M), or a combination of BPA (10–7, 10–6, and 10–5 M) and IBP (10–7, 10–6, and 10–5 M). (A) CaBP-9k mRNA expression was determined by RT-PCR. (B) CaBP-9k protein expression was determined by Western blot analysis. Data represent the means ±S.E.M. of triplicate experiments. CaBP-9k gene expression was normalized to that of an internal control gene (1A for mRNA and GAPDH for protein). a, p<0.05 compared to vehicle; *, p<0.05 compared to BPA alone (10–7 M) and IBP alone (10–5 M); **, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–5 M); #, p 0.05 compared to BPA alone (10–7 M) and IBP alone (10–7 M). |
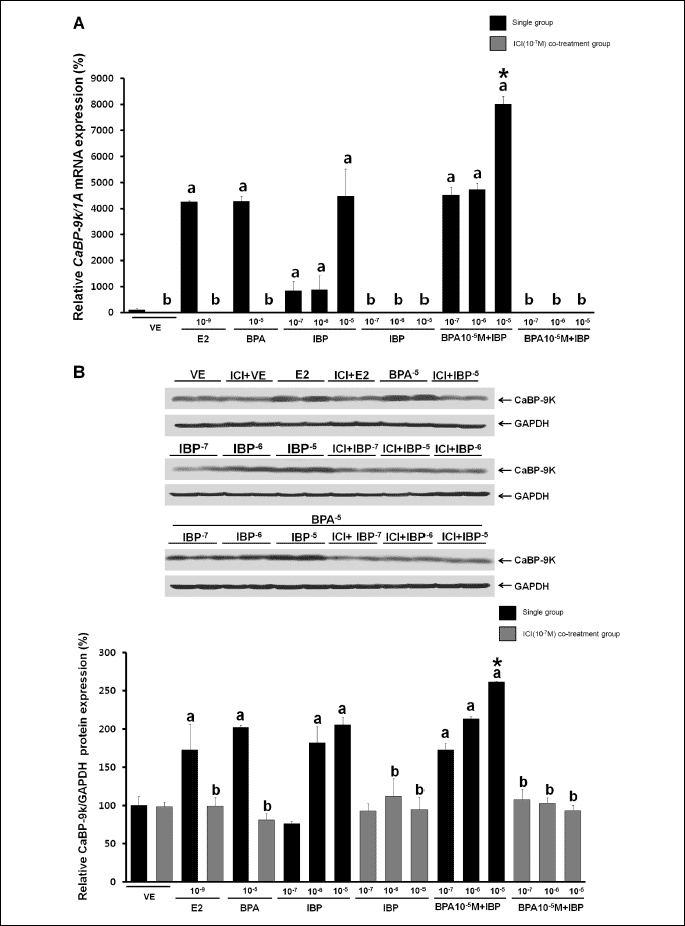 |
| Fig. 3. Effects of ICI182,780 on the expression of CaBP-9k mRNA and protein. (A) Expression of CaBP-9k mRNA was determined by RT-PCR. (B) Expression of CaBP-9k protein was determined by Western blot analysis. Data represent the means ±S.E.M. of triplicate experiments. CaBP-9k expression was normalized to that of an internal control (1A for mRNA and GAPDH for protein). a, p<0.05 compared to vehicle; b, p<0.05 compared to EDC only; *, p<0.05 compared to BPA alone (10–5 M) and IBP (10–5 M) alone. |
Combined effects of bisphenol A and isobutylparaben on the expression of progesterone receptor mRNA and protein in GH3 cells
We next assessed PR expression following single and combined treatment with BPA and IBP (BPA single doses: 10–7, 10–6 and 10–5 M; IBP single doses: 10–7, 10–6 and 10–5 M; combinations: each BPA dose combined with each IBP dose). After 24 hours, PR mRNA and protein expression had increased in a dose-dependent manner (Fig. 4). As shown in Fig. 4A, a strong increase in expression of PR mRNA was observed after combined treatment with the highest dose of BPA (10–5 M) and the middle dose of IBP (10–6 M) compared to a single exposure to each of these doses. The expression of PR protein, however, showed the greatest increase with a combination of the highest dose of BPA (10–5 M) with a middle (10–6 M) or highest dose (10–5 M) of IBP (Fig. 4B). Compared to a vehicle, the expression of PR mRNA was increased 189-fold, and protein expression was increased 26.4-fold by the positive control (E2, 10–9 M). No other additional effects on PR expression were observed.
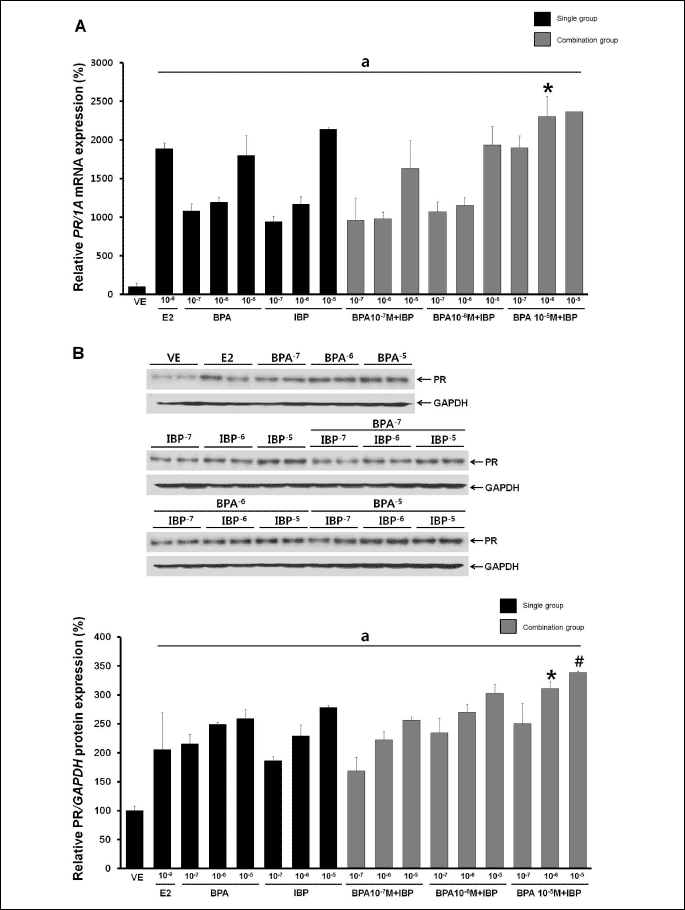 |
| Fig. 4. Effects of single or combined BPA and IBP treatment on the regulation of PR mRNA and protein. (A) PR mRNA expression was determined by RT-PCR. (B) PR protein expression was determined by Western blot analysis. Data represent the means ±S.E.M. of triplicate experiments. PR expression was normalized to that of an internal control (1A for mRNA and GAPDH for protein). a, p<0.05 compared to vehicle; *, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–6 M); #, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–5 M). |
To investigate whether the ER pathway mediates expression of PR, GH3 cells were treated with ICI182,780 30 min prior to EDC treatment. GH3 cells were treated with a single treatment of the highest dose of BPA (10–5 M), with IBP (10–7, 10–6, or 10–5 M), or with a combination of BPA (10–5 M) and IBP (10–7, 10–6, or 10–5 M). After 24 hours of EDC treatment, IBP was observed to up-regulate PR expression in a dose-dependent manner and this effect was completely abolished by ICI182,780 treatment (Fig. 5). These results provide evidence that BPA and IBP exposure in GH3 cells increases PR gene expression. In addition, these results further imply that an ER-mediated pathway is involved in the up-regulation of PR mRNA and protein.
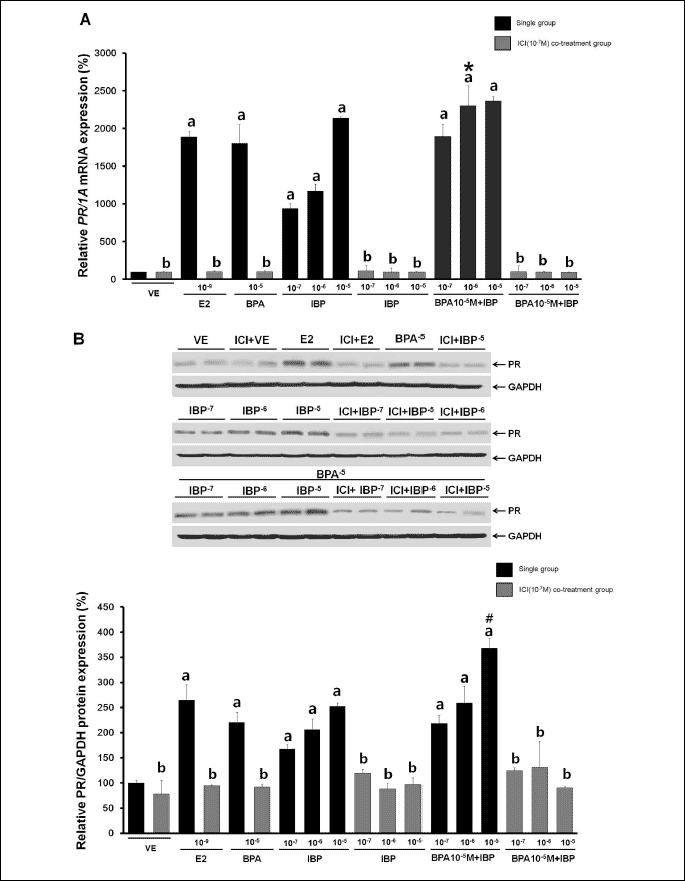 |
| Fig. 5. Effects of ICI182,780 on the regulation of PR mRNA and protein expression. Cells were treated with DMSO alone as a vehicle (VE) or with E2 at 10–9 M as a positive control or with BPA alone (10–7, 10–6, and 10–5 M), IBP alone (10–7, 10–6, and 10–5 M), or a combination of BPA (10–7, 10–6, and 10–5 M) and IBP (10–7, 10–6, and 10–5 M) in the presence or absence of 30 min pretreatment with ICI182,780 (10–7 M). (A) Expression of PR mRNA was determined by RT-PCR. (B) Expression of PR protein was determined by Western blot analysis. Data represent the means ±S.E.M. of triplicate experiments. PR gene expression was normalized to that of an internal control (1A for mRNA and GAPDH for protein). a, p<0.05 compared to vehicle; b, p<0.05 compared to EDC only; *, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–6 M); #, p<0.05 compared to BPA alone (10–5 M) and IBP alone (10–5 M). |
EDCs exert their effects either by binding to hormone receptors or through direct action on cell signaling pathways and can have effects even at very low dose (40). Previous evidence suggests that the combined effects of EDCs from the same category, i.e., estrogenic, anti-androgenic, or thyroid-disrupting agents, may act together through dose addition (41). The topic of combined exposure to EDCs has been considered important because environmental EDC exposure results from many chemicals and not from single chemicals. More complicated interactions may take place if two chemicals act on related targets and, in some cases, there may be additional effects (4).
We investigated the possible additional interaction of BPA and IBP. BPA and IBP are estrogenic agents that are able to evoke a response similar to that of E2, such as uterine cell proliferation (42). They appear to exhibit estrogenicity interacted with ERs resulting in the activation of estrogen-dependent gene expression (7, 8). BPA, known as an endocrine disruptor, is manufactured worldwide for use in various industrial and consumer products (5, 6). BPA is often used in food and beverage containers, including baby bottles, and is also used as an additional in other plastics (6). BPA was initially regarded as a weak xenoestrogen based on its relative affinity for the classical nuclear receptors ERa and ERb which was estimated to be at least 1,000–10,000-fold lower than that of E2 (8). However, the latest studies have determined that BPA can stimulate cellular responses at very low concentrations. For instance, it was reported that BPA stimulated PKA and PKC pathways via a G-protein-coupled nonclassical membrane ER (GPER) at very low concentration (10–9 to 10–12 M) (43). Also, picomolar concentration of xenoestrogens including BPA has been known to trigger calcium influx (44) and ERK phosphorylation in rat pituitary cells (45). In some cases, BPA has been shown to be equivalent in potency to E2 in mice and rats (46, 47). Early BPA exposure has been reported to influence female reproduction, and altered estrous cycles, which have been reported in BPA-exposed females, can serve as the first indicator of disruption of the hypothalamic-pituitary-ovarian axis (48). BPA has been shown to affect the proliferation of estrogen-dependent MCF-7 cells in a dose-dependent manner, influence the proliferation of both primary anterior pituitary cells and GH3 cells, and induce estrogenic changes in the uterus of CD-1 mouse, similar to its action on MCF-7 cells (10, 11, 49, 50).
Parabens are widely used as preservatives in underarm cosmetics. Although parabens are generally considered safe, recent reports suggest that they show estrogenicity in a variety of in vitro tests, including the proliferation of MCF-7 and ZR-75-1 human breast cancer cell lines, and in vivo tests such as uterotrophic assays in both rat and mouse (15, 51-54). The estrogenic effect of parabens is approximately 1000 times less than that of E2, but parabens can bind to ERs stimulating an ER-dependent response (55). Parabens have effects on the regulation of estrogen response elements, including ER
In this study, we used the CaBP-9k gene as a biomarker to evaluate the estrogenic potential of BPA and IBP (21, 25). Potency of single and combined EDCs was assessed using biomarker induction assays to reveal additional effects of the two chemicals. The ERE and PRE are located in the CaBP-9k promoter and mediate transcriptional regulation of CaBP-9k in the rat uterus (31). Interaction between the ERE and ER on the CaBP-9k gene promoter results in the regulation of gene expression, and ER mediates estrogen responsiveness in the pituitary cells (58). We investigated the effects of BPA and IBP on luciferase activity using a plasmid containing three copies of an ERE sequence to determine if ER-mediated pathways mediate EDC-induced expression of CaBP-9k and PR. We monitored transiently transfected cells with a plasmid containing ERE luciferase reporter gene. We showed that luciferase activity increased in a concentration-dependent manner following single or combined doses of BPA and IBP. Also, an additional effect on luciferase activity was observed with some combination doses, including a mixture of the highest dose of BPA (10–5 M) with the lowest dose of IBP (10–7 M). This finding implies that the estrogenic effects of BPA and IBP on CaBP-9k and PR gene expression are induced through ERs, and these EDCs have additional effects at certain doses.
Measurement of CaBP-9k mRNA and protein induced by BPA and IBP in GH3 cells showed dose-dependent up-regulation of CaBP-9k gene induction after 24 hours, similar to the effects of BPA and IBP on luciferase activity. Several doses of BPA and IBP showed probable additional effects on CaBP-9k mRNA and protein expression. Interestingly, CaBP-9k transcription and translation exhibited additional effects with the same combination dose (the highest dose of both BPA and IBP). We also observed that the expression of CaBP-9k was masked by relatively higher doses of BPA or IBP in the combined groups that did not show additional effect. For example, the expression of CaBP-9k mRNA induced by a combination of BPA (10–5 M) and IBP (10–6 M) was similar to that induced by a single dose of BPA (10–5 M). The lack of addition in CaBP-9k induction may be attributable to saturation of ER in these cells. On the other hand, high doses of hormonal agents revealed less responses than as expected, suggesting that its receptors may be down-regulated by the ligand (59).
To examine the biological pathway involved in the induction of CaBP-9k mRNA and protein, GH3 cells were pretreated with ICI182,780 30 min before EDC treatment. As expected, the estrogenic effects of BPA and IBP were diminished by pre-treatment with ICI182,780. This suggests that ER-mediated pathway is involved in the up-regulation of CaBP-9k expression by BPA and IBP. The PR gene is one of the most widely studied ER-regulated genes and its expression indicates functioning ER pathway (60). Therefore, we measured PR mRNA and protein expression after BPA and IBP exposure to better comprehend the mechanism of CaBP-9k induction by these estrogenic chemicals. Interestingly, the patterns of PR mRNA and protein expression were highly similar to those of CaBP-9k. Induction of PR expression increased in a dose-dependent manner, and additional effects were observed with some combination doses. ICI 182 780, an antagonist against ERs including ER
In the present study, we demonstrated that the expression of the estrogenic biomarkers, CaBP-9k and PR gene, were additionally affected by combined EDCs. Furthermore, we showed that the combined estrogenic effect of BPA and IBP was mediated by ER-associated signaling pathway. These findings suggest that there are also possibilities of additive or synergistic influence of other EDCs in our body. Although the accumulated concentration of these chemicals are low, it is highly possible that combination of EDCs including BPA and IBP can bring more unanticipated severe adverse effects in the reproductive organs in both males and females. Because combination effects can result from agents present at or even below their effect thresholds, further analysis should be conducted to determine the concentrations needed for the additional effects of combined EDCs, and to understand the levels and characteristics of EDCs present in the environment and in human tissue.
Acknowledgements: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0011433).
Drs. Kim and Jung equally contributed to this work.
Conflict of interests: None declared.
- Ozen S and Darcan S. Effects of environmental endocrine disruptors on pubertal development. J Clin Res Pediatr Endocrinol 2011; 3: 1-6.
- Kortenkamp A, Altenburger R. Synergisms with mixtures of xenoestrogens: a reevaluation using the method of isoboles. Sci Total Environ 1998; 221: 59-73.
- Kortenkamp A, Altenburger R. Approaches to assessing combination effects of oestrogenic environmental pollutants. Sci Total Environ 1999; 233: 131-140.
- Carpenter DO, Arcaro K, Spink DC. Understanding the human health effects of chemical mixtures. Environ Health Perspect 2002; 110(Suppl. 1): 25-42.
- Cantonwine D, Meeker JD, Hu H, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health 2010; 9: 62.
- Barrett JR. The Pharmacokinetics of BPA: similarities in human and animal metabolism suggest higher exposure than thought. Environ Health Perspect 2011; 119: a177.
- Gould JC, Leonard LS, Maness SC, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol 1998; 142: 203-214.
- Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998; 139: 4252-4263.
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24: 139-177.
- Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 1997; 138: 1780-1786.
- Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect 2001; 109: 55-60.
- Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol 2008; 28: 561-578.
- Cashman AL, Warshaw EM. Parabens: a review of epidemiology, structure, allergenicity, and hormonal properties. Dermatitis 2005; 16: 57-66; quiz 55-56.
- Golden R, Gandy J, Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol 2005; 35: 435-458.
- Vo TT, Jeung EB. An evaluation of estrogenic activity of parabens using uterine calbindin-d9k gene in an immature rat model. Toxicol Sci 2009; 112: 68-77.
- Morohoshi K, Yamamoto H, Kamata R, Shiraishi F, Koda T, Morita M. Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays. Toxicol in vitro 2005; 19: 457-469.
- Vo TT, Jung EM, Choi KC, Yu FH, Jeung EB. Estrogen receptor alpha is involved in the induction of calbindin-D(9k) and progesterone receptor by parabens in GH3 cells: a biomarker gene for screening xenoestrogens. Steroids 2011; 76: 675-681.
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005; 43: 985-1015.
- Erler C, Novak J. Bisphenol a exposure: human risk and health policy. J Pediatr Nurs 2010; 25: 400-407.
- Jung EM, An BS, Yang H, Choi KC, Jeung EB. Biomarker genes for detecting estrogenic activity of endocrine disruptors via estrogen receptors. Int J Environ Res Public Health 2012; 9: 698-711.
- Choi KC, Jeung EB. Molecular mechanism of regulation of the calcium-binding protein calbindin-D9k, and its physiological role(s) in mammals: a review of current research. J Cell Mol Med 2008; 12: 409-420.
- Dang VH, Nguyen TH, Lee GS, Choi KC, Jeung EB. in vitro exposure to xenoestrogens induces growth hormone transcription and release via estrogen receptor-dependent pathways in rat pituitary GH3 cells. Steroids 2009; 74: 707-714.
- Dang VH, Choi KC, Jeung EB. Membrane-impermeable estrogen is involved in regulation of calbindin-D9k expression via non-genomic pathways in a rat pituitary cell line, GH3 cells. Toxicol in vitro 2010; 24: 1229-1236.
- Jung EM, An BS, Choi KC, Jeung EB. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol Lett 2012; 208: 142-148.
- An BS, Choi KC, Kang SK, Hwang WS, Jeung EB. Novel calbindin-D(9k) protein as a useful biomarker for environmental estrogenic compounds in the uterus of immature rats. Reprod Toxicol 2003; 17: 311-319.
- Armbrecht HJ, Boltz M, Strong R, Richardson A, Bruns ME, Christakos S. Expression of calbindin-D decreases with age in intestine and kidney. Endocrinology 1989; 125: 2950-2956.
- Mathieu CL, Burnett SH, Mills SE, Overpeck JG, Bruns DE, Bruns ME. Gestational changes in calbindin-D9k in rat uterus, yolk sac, and placenta: implications for maternal-fetal calcium transport and uterine muscle function. Proc Natl Acad Sci USA 1989; 86: 3433-3437.
- An BS, Choi KC, Kang SK, et al. Mouse calbindin-D(9k) gene expression in the uterus during late pregnancy and lactation. Mol Cell Endocrinol 2003; 205: 79-88.
- An BS, Choi KC, Hong EJ, Jung YW, Manabe N, Jeung EB. Differential transcriptional and translational regulations of calbindin-D9k by steroid hormones and their receptors in the uterus of immature mice. J Reprod Dev 2004; 50: 445-453.
- Choi KC, An BS, Yang H, Jeung EB. Regulation and molecular mechanisms of calcium transport genes: do they play a role in calcium transport in the uterine endometrium? J Physiol Pharmacol 2012; 62: 499-504.
- Lee KY, Oh GT, Kang JH, et al. Transcriptional regulation of the mouse calbindin-D9k gene by the ovarian sex hormone. Mol Cells 2003; 16: 48-53.
- Darwish H, Krisinger J, Furlow JD, Smith C, Murdoch FE, DeLuca HF. An estrogen-responsive element mediates the transcriptional regulation of calbindin D-9K gene in rat uterus. J Biol Chem 1991; 266: 551-558.
- Lee GS, Kim HJ, Jung YW, Choi KC, Jeung EB. Estrogen receptor alpha pathway is involved in the regulation of calbindin-D9k in the uterus of immature rats. Toxicol Sci 2005; 84: 270-277.
- Fujimoto N, Igarashi K, Kanno J, Inoue T. Identification of estrogen-responsive genes in the GH3 cell line by cDNA microarray analysis. J Steroid Biochem Mol Biol 2004; 91: 121-129.
- Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol 2005; 239: 27-36.
- Kim YR, Jung EM, Choi KC, Jeung EB. Synergistic effects of octylphenol and isobutyl paraben on the expression of calbindin-D(9)k in GH3 rat pituitary cells. Int J Mol Med 2012; 29: 294-302.
- Ao A, Morrison BJ, Wang H, Lopez JA, Reynolds BA, Lu J. Response of estrogen receptor-positive breast cancer tumorspheres to antiestrogen treatments. PLoS One 2011; 6: e18810.
- Tinnanooru P, Dang VH, Nguyen TH, Lee GS, Choi KC, Jeung EB. Estrogen regulates the localization and expression of calbindin-D9k in the pituitary gland of immature male rats via the ERalpha-pathway. Mol Cell Endocrinol 2008; 285: 26-33.
- Kaufmann SH, Ewing CM, Shaper JH. The erasable Western blot. Anal Biochem 1987; 161: 89-95.
- Ozen S, Darcan S. Effects of environmental endocrine disruptors on pubertal development. J Clin Res Pediatr Endocrinol 2011; 3: 1-6.
- Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 2007; 115(Suppl 1): 98-105.
- Charles GD, Gennings C, Zacharewski TR, Gollapudi BB, Carney EW. An approach for assessing estrogen receptor-mediated interactions in mixtures of three chemicals: a pilot study. Toxicol Sci 2002; 68: 349-360.
- Bouskine A, Nebout M, Brucker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect 2009; 117: 1053-1058.
- Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect 2005; 113: 431-439.
- Jeng YJ, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells. Environ Health Perspect 2011; 119: 104-112.
- Alonso-Magdalena P, Ropero AB, Carrera MP, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 2008; 3: e2069.
- Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology 2005; 146: 5388-5396.
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 2001; 109: 675-680.
- Suzuki T, Ide K, Ishida M. Response of MCF-7 human breast cancer cells to some binary mixtures of oestrogenic compounds in-vitro. J Pharm Pharmacol 2001; 53: 1549-1554.
- Han D, Tachibana H, Yamada K. Inhibition of environmental estrogen-induced proliferation of human breast carcinoma MCF-7 cells by flavonoids. in vitro Cell Dev Biol Anim 2001; 37: 275-282.
- Harvey PW. Parabens, oestrogenicity, underarm cosmetics and breast cancer: a perspective on a hypothesis. J Appl Toxicol 2003; 23: 285-288.
- Darbre PD, Byford JR, Shaw LE, Horton RA, Pope GS, Sauer MJ. Oestrogenic activity of isobutylparaben in vitro and in vivo. J Appl Toxicol 2002; 22: 219-226.
- Yamasaki K, Noda S, Imatanaka N, Yakabe Y. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol Lett 2004; 146: 111-120.
- Shaw J, deCatanzaro D. Estrogenicity of parabens revisited: impact of parabens on early pregnancy and an uterotrophic assay in mice. Reprod Toxicol 2009; 28: 26-31.
- Hossaini A, Larsen JJ, Larsen JC. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem Toxicol 2000; 38: 319-323.
- Okubo T, Yokoyama Y, Kano K, Kano I. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERalpha and PR. Food Chem Toxicol 2001; 39: 1225-1232.
- Robert NJ, Martin L, Taylor CD, et al. Nuclear binding of the estrogen receptor: a potential predictor for hormone response in metastatic breast cancer. Breast Cancer Res Treat 1990; 16: 273-278.
- Nguyen TH, Lee GS, Ji YK, Choi KC, Lee CK, Jeung EB. A calcium binding protein, calbindin-D9k, is mainly regulated by estrogen in the pituitary gland of rats during estrous cycle. Brain Res Mol Brain Res 2005; 141: 166-173.
- Calabrese EJ. Hormesis is central to toxicology, pharmacology and risk assessment. Hum Exp Toxicol 2010; 29: 249-261.
- Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast 2005; 14: 458-465.
- Holm A, Baldetorp B, Olde B, Leeb-Lundberg LM, Nilsson BO. The GPER1 agonist G-1 attenuates endothelial cell proliferation by inhibiting DNA synthesis and accumulating cells in the S and G2 phases of the cell cycle. J Vasc Res 2011; 48: 327-335.