COMPARISON OF ANTI-INFLAMMATORY PROPERTIES OF PEROXISOME
PROLIFERATOR-ACTIVATED RECEPTOR GAMMA AGONISTS ROSIGLITAZONE
AND TROGLITAZONE IN PROPHYLACTIC TREATMENT
OF EXPERIMENTAL COLITIS
INTRODUCTION
Non-specific inflammatory bowel disease (IBD), including ulcerative colitis and Crohn`s disease, is a chronic non-infectious inflammation of unknown etiology. Its incidence is increasingly high, particularly in the developed countries, and it is a serious clinical problem. Our knowledge regarding the pathogenesis, course and treatment of IBD is increasing. Genetic, environmental and immunological factors are known to be involved in the complex etiopathogenesis of IBD. The individual stages of the inflammatory process in non-specific IBD have already been well described; however, the major IBD causative factor is still to be conclusively determined. Research on the mechanisms of IBD development is being continued.
Recently, thiazolidinediones (glitazones) - PPAR-γ (peroxisome proliferator-activated receptors gamma) agonists have been implicated to effectively control the inflammatory processes of the gastrointestinal tract (1-5). Three subtypes of peroxisome proliferator-activated receptors (PPARs) were identified in humans, i.e. α, β (also called δ or NUC-1) and g. They belong to the superfamily of nuclear steroid hormone receptors, which act as the transcription factors regulating the expression of genes (6-8). PPARs-g are characterized by the widest spectrum of action and are present in many organs, e.g. the heart, liver, kidneys, bone marrow, pancreas, small and large intestine. PPARs-g affect the body’s immune response, mainly the course of inflammatory reactions. Thiazolidinediones such as rosiglitazone and troglitazone show extremely high specificity and act selectively through PPARs-g (9-11). High hopes are associated with the use of PPARs-γ to control the inflammatory processes; therefore, numerous ongoing studies focus on application of their agonists for the treatment of such diseases as asthma, atherosclerosis, rheumatoid arthritis, pancreatitis and non-specific inflammatory bowel diseases (3).
The effects of PPAR- γ agonists on the course of IBD are very intriguing. Although still not fully elucidated, they are hoped to improve the quality of life of millions of people worldwide. In the literature available, studies regarding the prophylactic use of thiazolidinediones are less numerous than those describing their therapeutic effects. Since the study findings about the effects of PPAR-γ agonists on colitis are very promising, the potential benefits associated with their prophylactic use seem worth considering. The present study was to analyse the prophylactic effects of PPAR-γ agonists rosiglitazone and troglitazone administered 2 weeks before and during development of colitis on inflammatory reactions in rats.
Research studies on effective methods of IBD treatment have used various experimental animal models of colitis, which imitate the processes occurring in the inflammation-affected human large intestine. In the two most commonly used models colitis is induced by intrarectal administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS) or by adding dextran sodium sulfate (DDS) to the drinking water. The above two models were earlier compared by Celinski et al. (12) in their study concerning novel methods to control IBD pathogenesis. In the present study, the 1.5% DDS model was applied. Previous studies by Celinski et al. (13) focused on therapeutic properties of thiazolidinediones administered to rats in several different doses after the induction of colitis. The present study considered the prophylactic use of PPAR-γ agonists at a concentration of 0.01% administered in food 14 days before and on induction of colitis.
The aim of present the study was to analyze and compare possible prophylactic effects of PPAR-γ agonists, rosiglitazone and troglitazone administered 2 weeks before and during 1.5% DSS administration on increased resistance of colonic tissues to the damaging factor. Moreover, to assess their effects on inflammatory processes in a more detailed way, intestinal specimens were microscopically evaluated and concentrations of Th1- (IL-2, INF-γ) and Th2-dependent (IL-4, IL-10) cytokines were determined in the serum and intestinal homogenates.
MATERIAL AND METHODS
Animals
Experiments followed the protocol approved by the local Animal Ethics Committee in Lublin no. 23/2008 and was carried out in Wistar male rats weighing 200–220 g. Animals were assigned randomly to study or control groups and examined simultaneously. Colitis was induced with 1.5% DDS administered in the drinking water ad libitum for 14 days. In various studies available in literature, experimental colitis was induced by administering DDS for several to twenty days in concentrations of 1% to 5%, or even 9% (14-23).
Animals received the food with 0.01% rosiglitazone or troglitazone for 4 weeks (during the final two weeks, 1.5% DDS was additionally administered in the drinking water). Rats were decapitated using a guillotine and the blood was sampled for clotting testing; the large intestine was retrieved, longitudinally sectioned and faecal residues were removed. The intestinal specimens for histopathological evaluation were fixed in 10% formalin and the samples for enzyme-linked immunosorbent assay (ELISA) were homogenized and frozen at –80°C. The levels of IL-2, IL-4, IL-10 and INF-γ were determined in the serum and large intestine homogenates. The division into groups (8 rats each) and methods of administration of the substances used were presented in Table 1.

Histopathologic evaluation
The large intestine was divided into segments: S1 - the rectum up to 2 cm, S2 – 2 to 5 cm, S3 – 5 to 10 cm, S4 – from 10 cm to the caecum, S5 – the caecum. From each segment, sections for microscopic evaluation were collected from the places with visible lesions (ulcerations, erosions) or every 1 cm on average when macroscopic lesions were invisible. Tissues were fixed in 10% buffered formalin, pH 7.4 for 3 days and rinsed for 4 hours. The sections were dehydrated by passing through a series of alcohols of incremental concentrations, equilibrated in xylene for 10–15 min and embedded in paraffin; paraffin blocks were cut into about 2-micrometer sections using a microtone. The prepared specimens were stained with haematoxylin and eosin according to the Baginski and Masson method (fibrosis) or with mucicarmine (mucus).
The following scales were used for microscopic evaluation of individual parameters: (24-26).
– Extent of inflammation, atrophy of crypts, ulceration: 0 – lack, 1 – focal, 2 – segment-confined, 3 – involvement of more than 1 segment.
– Depth of injury: 0 – lack, 1 – superficial, 2 – moderate (involving the muscularis mucosae), 3 – severe (involving the intestinal muscularis).
– Severity of inflammation (mononuclear cells and multi-segment cells): 0 – lack, 1 – slight, focal, 2 – moderate, 3 – marked, diffuse.
– Lymph follicles: 0 – lack, 1 – sporadic, not in all segments, 2 – sporadic, in all segments, 3 – intensified, distinct, in all segments.
The histological specimens were evaluated using the light microscope Axiostar plus (ZEISS).
Enzyme-linked immunosorbent assay (ELISA)
Immediately after decapitation, the blood, about 10ml, was sampled for clotting testing. After centrifugation at 5000 rpm, the serum was frozen at –80°C. The large intestine parts for immunoenzymatic assay were homogenized by adding 2 ml of phosphate-buffered saline (PBS) without Ca2+ and Mg2+ ions (Biomed Lublin, Poland). The solution was centrifuged at 2000 rpm, and the supernatant was frozen at –80°C. The concentrations of IL-2, IL-4, IL-10 and INF-γ were determined in the individual groups in the serum and intestinal homogenates. The results were read using the ELISA Victor 3 reader (PERKIN ELMER USA). The protein concentrations in the samples tested were calculated with KCJR software.
Statistical analysis
The Kolmogorov-Smirnov, Student’s t, Cochran-Cox and Levene`s tests were used for calculations of descriptive statistics, i.e. a mean, variance and standard deviation (S.D.).
The results were quantitative, thus the variables were described using an arithmetic mean and standard deviation (S.D.) as the numerical data had normal distribution (checked with the Kolmogorov-Smirnov test). The intergroup comparisons of parameters were carried out (groups A-L) once intergroup variances were checked using the Levene’s test. In cases of equal variances, the Student’s t test was used (“t” denoting the test function); otherwise, the Cochran-Cox test was applied (“C” denoting the test function).
The error risk was assumed at 5%, which means that the tested hypothesis was rejected at p>0.05 and the alternative hypothesis was true (–). The intergroup differences were considered significant at p<0.05 (*); at p<0.01, the differences were more significant (**) whereas at p<0.001 highly significant (***). The calculations were performed using Statistica 9.0 software.
RESULTS
In the control group, the microscopic picture of large intestine sections was normal. The architecture and structure of intestinal mucosal crypts were preserved with the proper amount of mucus in the lumen. A few mononuclear cells, mainly lymphocytes, were observed in the lamina propria whereas dispersed, fine lymph follicles were found in the submucosa. The epithelium did not show defects and was covered with the mucous layer. The submucosal, muscular and serous membranes of the large intestine were normal. (Fig. 1)
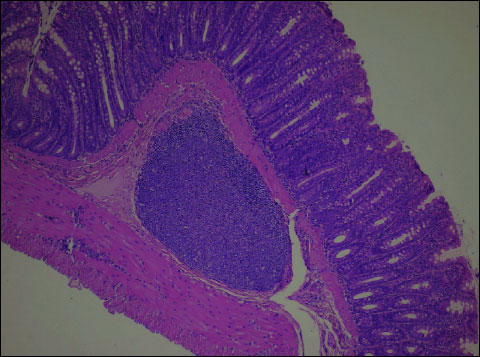 |
Fig. 1. Control group. Normal bowel wall; mucosal, submucosal and muscular layers. A single lymphatic follicle visible in the submucosa. H+E × 200. |
In the study groups receiving 0.01% rosiglitazone (group I) and 0.01% troglitazone (group J), the microscopic picture was similar to that in the control group. Occasionally, slightly enhanced focal mucosal oedema was observed. The structure of intestinal mucosal crypts was preserved with the proper amount of mucus. Dispersed, fine lymph follicles typical of the large intestine were observed in the lamina propria. The epithelium did not show defects and was covered with the mucus. The submucosal, muscular and serous membranes of the large intestine were normal.
In all animals of the group receiving 1.5% DSS (group B), the microscopic picture revealed changes in all segments of the large intestine (Fig. 2). Numerous ulcerations and full-wall thickness inflammation of moderate and marked severity, in the form of inflammatory infiltrations consisting of mononuclear cells, such as lymphocytes and plasmocytes (although less abundant), were present in all intestinal segments. The mononuclear cells were observed in the mucosal, submucosal (focally), muscular and serous membranes of the large intestine. The inflammation was characterized by substantial activity with the presence of neutrophils. The oedema of the mucosa and submucosa was noted in all intestinal segments in all animals.
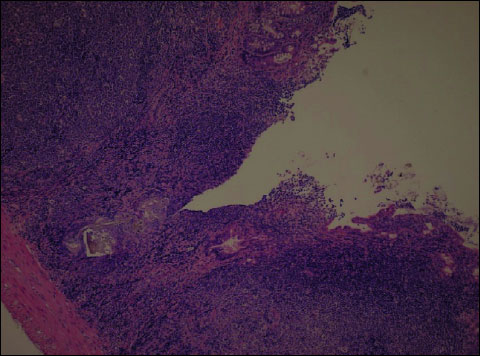 |
Fig. 2. DSS (Group B). Ulceration, oedema and active inflammation of the mucosa reaching the muscular layer of colon. In the inflammatory infiltration - numerous neutrophils with crypt abscesses. H+E ´ 200. |
In the majority of animals, the mucosal crypts were atrophied in all segments and the amount of mucus in their lumen was reduced. In 5/8 animals, ulcerations and erosions of the mucosa were visible in several segments, most pronounced in the distal intestine (S1). In 3/8 animals of this group, ulcerations affected the intestinal lamina propria and muscularis mucosae. In the remaining 5/8 animals, ulcerations were focally full-walled involving also the intestinal muscularis mucosae. Focally, the mucosal and submucosal lamina propria was characterized by slight thickening and slight fibrosis at the site of ulcerations. In all animals receiving 1.5% DSS, dispersed, yet distinct lymph follicles were found in all colon segments; in one animal, they were distinct and numerous.
Both rosiglitazone and troglitazone administered prophylactically before the induction of colitis enhanced the colonic tissue resistance to the damaging factor, which was confirmed by histopathological findings. Histopathological evaluation of intestinal sections from the group K – animals receiving 0.01% rosiglitazone for 4 weeks plus 1.5% DSS for the last 14 days (Fig. 3) and those from group L – administered 0.01% troglitazone for 4 weeks plus 1.5% DSS for the last 14 days (Fig. 4) revealed comparable microscopic lesions of slight severity.
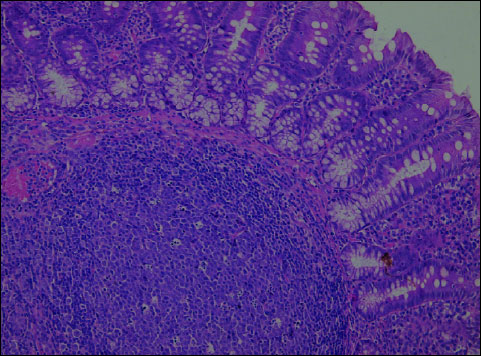 |
Fig. 3. Rosiglitazone + DSS (Group K). In the colonic mucosa - focal, moderate inflammatory infiltrations with lymphocytes, plasmocytes and dispersed neutrophils. The lymphatic follicles visible in the submucosa. H+E ´ 200. |
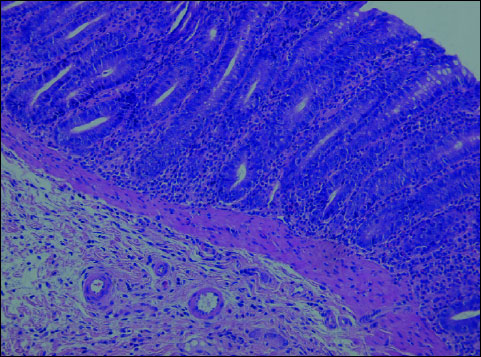 |
Fig. 4. Troglitazone + DSS (Group L). In the colonic mucosa - focal, slight inflammatory infiltrations with lymphocytes, plasmocytes and dispersed neutrophils. H+E ´ 200. |
In both (K+L) groups, the following changes were observed: segmental slight oedema confined to the intestinal mucosa, focally increased number of mononuclear cells in the lamina propria (compared to controls), focally dispersed, not numerous neutrophils in the lamina propria. Moreover, the architecture was disturbed; intestinal crypts were focally and slightly atrophied. There were no ulcerations or defects of the mucosal epithelium. Lymph follicles in the intestinal wall were observed in all animals. The epithelium was covered with the layer of mucus. (Fig. 5, 6).
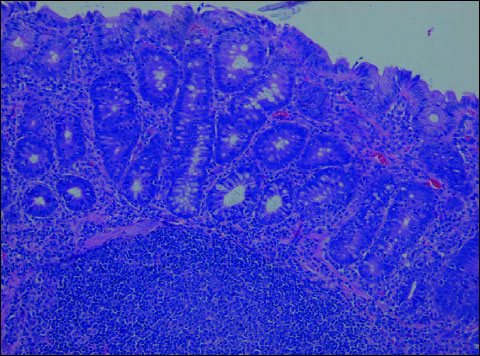 |
Fig. 5. Rosiglitazone + DSS (Group K). In the colonic mucosa - slight oedema and moderate inflammatory infiltration with lymphocytes, plasmocytes and few neutrophils. Architecture of intestinal crypts slightly distorted. H+E ´ 200. |
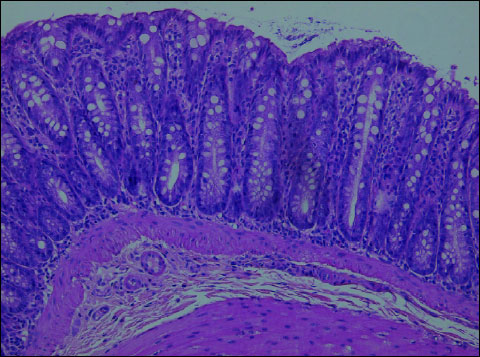 |
Fig. 6. Troglitazone + DSS (Group L). In the colonic mucosa - slight inflammatory infiltration with mononuclear cells consisting of lymphocytes, plasmocytes and focally dispersed neutrophils. The epithelium covered with the layer of mucus. H+E ´ 200. |
The levels of IL-2, IL-4, IL-10 and INF-γ were determined in the serum and intestinal homogenates using ELISA. The comparison of serum and homogenate concentrations in individual groups was presented in figures (Fig. 7-10).
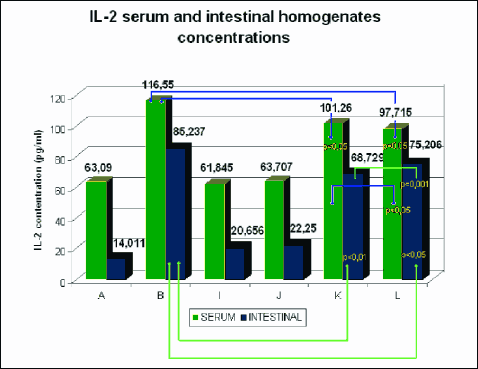 |
Fig. 7. Mean serum and intestinal homogenate concentrations of IL-2 in pg/ml in study (8 animals each) groups: control group (A), DSS (B), rosiglitazone (I), troglitazone (J), rosiglitazone + DSS (K), troglitazone + DSS (L). p<0.05 – significant differences; p<0.01 – more significant differences; p<0.001 – highly significant differences. |
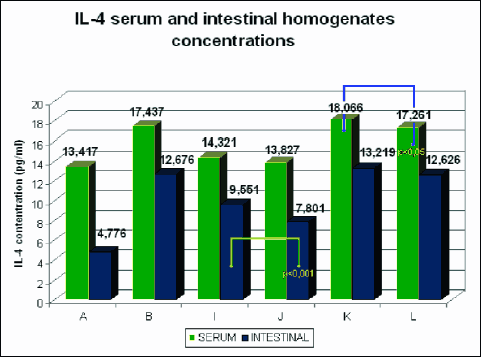 |
Fig. 8. Mean serum and intestinal homogenate concentrations of IL-4 in pg/ml in study (8 animals each) groups: control group (A), DSS (B), rosiglitazone (I), troglitazone (J), rosiglitazone + DSS (K), troglitazone + DSS (L). p<0.05 – significant differences; p<0.01 – more significant differences; p<0.001 – highly significant differences. |
The rats receiving 0.01% rosiglitazone and 0.01% troglitazone showed significantly reduced levels of IL-2 and increased levels of anti-inflammatory IL-4 and IL-10 in intestinal homogenates (IL-10 was also increased in serum) compared to the control group. In groups receiving both rosiglitazone and troglitazone for 2 weeks before the administration of DSS and together with DSS for another two weeks, concentrations of pro-inflammatory cytokines (IL-2, INF-γ) were markedly lower (Fig. 7, 10), whereas concentrations of anti-inflammatory cytokines (IL-4, IL-10) were substantially higher in comparison to controls, both in the intestinal homogenate and serum. (Fig. 8, 9).
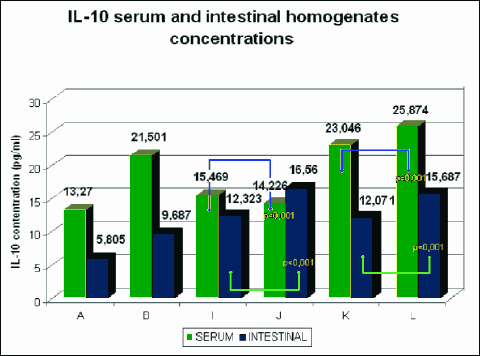 |
Fig. 9. Mean serum and intestinal homogenate concentrations of IL-10 in pg/ml in study (8 animals each) groups: control group (A), DSS (B), rosiglitazone (I), troglitazone (J), rosiglitazone + DSS (K), troglitazone + DSS (L). p<0.05 – significant differences; p<0.01 – more significant differences; p<0.001 – highly significant differences. |
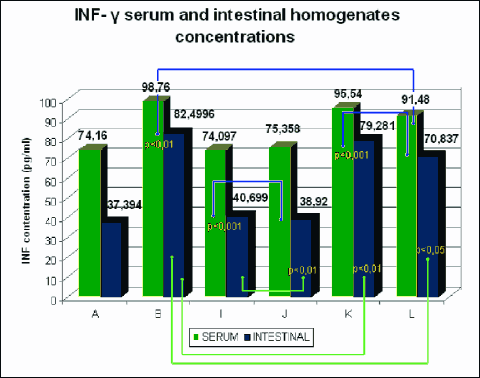 |
Fig. 10. Mean serum and intestinal homogenate concentrations INF-g in pg/ml in study (8 animals each) groups: control group (A), DSS (B), rosiglitazone (I), troglitazone (J), rosiglitazone + DSS (K), troglitazone + DSS (L). p<0.05 – significant differences; p<0.01 – more significant differences; p<0.001 – highly significant differences. |
The comparison of groups receiving only 0.01% rosiglitazone and 0.01% troglitazone with those given rosiglitazone and troglitazone for 2 weeks and DSS for the last 14 days demonstrated higher levels of Th1 and Th2-dependent cytokines in the serum and intestinal homogenates in the latter groups.
Compared to the group receiving 0.01% rosiglitazone and DSS for the last two weeks, the group given prophylactic troglitazone with DSS for the last 14 days had significantly lower levels of IL-2 in the serum and of INF-γ in the serum and intestinal homogenate. The concentration of IL-2 in the intestinal homogenate, however, was significantly lower in the group receiving rosiglitazone. In those groups, the effects of troglitazone on the expression of IL-10 in the serum and intestinal homogenate were stronger as compared to rosiglitazone. The level of IL-4 was slightly higher in animals receiving rosiglitazone and DSS for the last 14 days.
DISCUSSION
This study confirms the usefulness of prophylactic, prior-colitis administration of PPARγ agonists for attenuating the macroscopic and histopathological features of colitis. Most of the available studies are concerned with therapeutic use of PPAR-γ ligands after induction of colitis and only a few discuss the prophylactic use of thiazolidinediones. Given the promising results of the studies evaluating the effects of PPAR agonists on the course of colitis, the potential benefits resulting from their prophylactic use are worth considering. The present paper analysed and compared the effects of PPAR-γ ligands, rosiglitazone and troglitazone, on the resistance of colonic tissues to the damaging factor. The results of immunoenzymatic determinations confirm the suppressive effects of activated PPAR-γ receptors on Th1-dependent processes, with the shift of immune system activity toward the activation of Th2-dependent immune processes. Our paper confirms earlier findings by Saubermann et al. (27). In their experimental murine model of colitis induced by 2.5% DSS administered in the drinking water, they used rosiglitazone, pioglitazone and troglitazone. The authors demonstrated beneficial inhibitory effects of PPAR-γ receptors on the large intestine inflammatory reaction manifested in reduced concentrations of pro-inflammatory cytokines TNF-α and INF-γ as well as increased levels of Th2-depedent anti-inflammatory cytokines IL-10 and IL-4. The use of PPAR-γ agonist resulted in decreased concentrations of Th1-dependent pro-inflammatory cytokines (TNF-α and INF-γ). However, the authors emphasize that this decrease was accompanied by increased activity of Th2-dependent processes, evidenced by enhanced expression of GATA-3, the key transcription factor of Th2-dependent immune response. Moreover, concentrations of Th2-dependent IL-10 and IL-4 after the activation of PPAR-γ receptor were found to be increased.
The results of the experiment carried out by Sanchez-Hidalgo et al. (26) were similar to our findings. In their experimental model of colitis, rosiglitazone was used in the doses of 4 and 8 mg/kg b.w. Colitis was induced by intrarectal administration of TNBS and rosiglitazone given in three doses before induction of colitis and in a single daily dose after TNBS administration. The intestinal specimens of animals receiving TNBS revealed substantial injury to the mucosa, epithelial necrosis, focal ulcerations and diffuse infiltration with inflammatory cells. In the group receiving rosiglitazone 8 mg/kg b.w., there were no inflammatory cells in the lamina propria, ulcerations were small, not numerous, showing the features of re-epithelialisation, and the epithelium was partially intact.
Furthermore, the potentially therapeutic, anti-inflammatory action of thiazolidinedione drugs was confirmed by Takaki et al. (25). In their experiment divided into the prophylactic and therapeutic parts, 1% DSS in the drinking water was used to induce colitis and PPAR-γ agonists pioglitazone and netoglitazone in the doses of 10, 50 and 150 mg/kg/day were administered. In the prophylactic part, pioglitazone and netoglitazone were given two days before the use of DSS. In the therapeutic schedule, the PPAR-γ agonist was administered two days after DSS administration. The evaluation of effects of thiazolidinediones activating PPAR-γ was based on the clinical scale (weight loss, faeces consistency and gastrointestinal bleedings), histology (severity and extent of inflammatory reactions, injuries to the intestinal mucosae) and the large intestine length. The findings demonstrated significant anti-inflammatory action of both substances, more potent in the case of pioglitazone. The best anti-inflammatory effects, both in the therapeutic and prophylactic model, were exerted by pioglitazone in the dose of 150 mg/kg/day.
In recent years, the first attempts were undertaken to use PPAR-γ ligands for the treatment of non-specific inflammatory bowel diseases in humans (28-30). The results of the trials raise hopes that in the nearest future PPAR-γ ligands will be used for therapy of non-specific inflammatory bowel diseases in clinical practice. Moreover, the results of the present study about prophylactic use of PPAR agonists are promising. However, the studies on this issue are not numerous and possible uses of rosiglitazone and/or troglitazone for IBD prevention require further research, in particular, their possible cardio- and hepatotoxic effects (31, 32).
Rosiglitazone and troglitazone administered prophylactically exerted beneficial effects on the course of colitis markedly down-regulating the macroscopic inflammatory parameters. Rosiglitazone and troglitazone administered before the induction of colitis comparably and significantly reduced the concentrations of pro-inflammatory cytokines and enhanced the expression of inflammation-limiting cytokines. Results of immunoenzymatic determinations of cytokine levels in individual groups confirm down-regulatory effects of activated PPAR-γ receptors on Th1-dependent processes, with the shift of the immune system activity towards the activation of Th2-dependent immune processes. In future, thiazolidinedione drugs, such as rosiglitazone and troglitazone used in our experiment, are likely to be used for the treatment and prevention of non-specific inflammatory bowel diseases. However, the prophylactic use of PPAR ligands requires further studies and observations.
Conflict of interests: None declared.
REFERENCES
- Celinski K, Dworzanski T, Korolczuk A, et al. Effects of peroxisome proliferator-activated receptors-gamma ligands on dextran sodium sulphate - induced colitis in rats. J Physiol Pharmacol 2011; 62: 347-356.
- Celinski K, Madro A, Prozorow-Krol B, et al. Rosiglitazone, a peroxisome proliferator-activated receptor gamma (PPAR-γ)-specific agonist, as a modulator in experimental acute pancreatitis. Med Sci Monit 2009; 15: 21-29.
- Celinski K, Dworzanski T, Prozorow-Krol B, Korolczuk A. The role of PPAR-γ receptors in gastrointestinal inflammation diseases. Gastroenterol Pol 2009; 16: 51-56.
- Andersen V, Christensen J, Ernst A, et al. Polymorphisms in NF-kB, PXR, LXR, PPARg and risk of inflammatory bowel disease. World J Gastroenterol 2011; 17: 197-206.
- Hontecillas R, Horne WT, Climent M, et al. Immunoregulatory mechanisms of macrophage PPAR-γ in mice with experimental inflammatory bowel disease. Mucosal Immunol 2011; 4: 304-313.
- Saraf N, Sharma PK, Mondal SC, Garg VK, Singh AK. Role of PPARg2 transcription factor in thiazolidinedione-induced insulin sensitization. J Pharm Pharmacol 2012; 64: 161-171.
- Xiang GQ, Tang SS, Jiang LY, et al. PPARg agonist pioglitazone improves scopolamine-induced memory impairment in mice. J Pharm Pharmacol 2012; 64: 589-596.
- Ma JJ, Zhang T, Fang N, Zou Y, Gong QH, Yu LM, Chen DX. Establishment of a cell-based drug screening model for identifying agonists of human peroxisome proliferator-activated receptor gamma (PPARγ). J Pharm Pharmacol 2012; 64: 719-726.
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995; 270: 12953-12956.
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferators activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998; 391: 79-82.
- Willson TM, Cobb JE, Cowan DJ, et al. The structure activity relationship between peroxisome proliferator- activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 1996; 39: 665-668.
- Celinski K, Dworzanski T, Korolczuk A, et al. Comparison of main models of experimental colitis essential for studies on novel therapies of inflammatory bowel disease. Gastroenterol Pol 2010; 17: 195-202.
- Celinski K, Dworzanski T, Fornal R, Korolczuk A, Madro A, Slomka M. Comparison of the anti-inflammatory and therapeutic actions of PPAR-γamma agonists rosiglitazone and troglitazone in experimental colitis. J Physiol Pharmacol 2012; 63: 631-640.
- Shizuma T, Ishiwata K, Nagano M, Mori H, Fukuyama N. Protective effects of Kurozu and Kurozu Moromimatsu on dextran sulfate sodium-induced experimental colitis. Dig Dis Sci 2011; 56: 1387-1392.
- Nakashima T, Maeda T, Nagamoto H, Kumakura T, Takai M, Mori T. Rebamipide enema is effective for treatment of experimental dextran sulfate sodium induced colitis in rats. Dig Dis Sci 2005; 50(Suppl. 1): S124-S131.
- Li H, Wu WK, Li ZJ, et al. 2,3’,4,4’,5’-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br J Pharmacol 2010; 160: 1352-1361.
- Kumar GK, Dhamotharan R, Kulkarni NM, Honnegowda S, Murugesan S. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol 2011; 11: 724-731.
- Cancado GG, Fiuza JA, de Paiva NC, et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm Bowel Dis 2011; 17: 2275-2286.
- Vasina V, Broccoli M, Ursino MG, et al. Non-peptidyl low molecular weight radical scavenger IAC attenuates DSS-induced colitis in rats. World J Gastroenterol 2010; 16: 3642-3650.
- Van Dop WA, Marengo S, te Velde AA, et al. The absence of functional PI3Kgamma prevents leukocyte recruitment and ameliorates DSS-induced colitis in mice. Immunol Lett 2010; 131: 33-39.
- Yamamoto K, Ninomiya Y, Iseki M, et al. 4-Hydroxy-docosahexaenoic acid, a potent peroxisome proliferator-activated receptor gamma agonist alleviates the symptoms of DSS-induced colitis. Biochem Biophys Res Commun 2008; 367: 566-572.
- Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 2000; 62: 240-248.
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993; 69: 238-249.
- Ramakers JD, Verstege MI, Thuijls G, te Velde AA, Mensink RP, Plat J. The PPARgamma agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J Clin Immunol 2007; 27: 275-283.
- Takaki K, Mitsuyama K, Tsuruta O, Toyonaga A, Sata M. Attenuation of experimental colonic injury by thiazolidinedione agents. Inflamm Res 2006; 55: 10-15.
- Sanchez-Hidalgo M, Martin AR, Villegas I, Alarcon de la Lastra C. Rosiglitazone, a PPARg ligand, modulates signal transduction pathways during the development of acute TNBS-induced colitis in rats. Eur J Pharmacol 2007; 562: 247-258.
- Saubermann LJ, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis 2002; 8: 330-339.
- Lewis JD, Lichtenstein GR, Stein RB, et al. An open-label trial of the PPAR-γamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol 2001; 96: 3323-3328.
- Liang HL, Ouyang Q. A clinical trial of combined use of rosiglitazone and 5-aminosalicylate for ulcerative colitis. World J Gastroenterol 2008; 14: 114-119.
- Lewis JD, Lichtenstein GR, Deren JJ, et al. Rosiglitazone for Ulcerative Colitis Study Group. Rosiglitazone for active ulcerative colitis: a randomized placebo controlled trial. Gastroenterology 2008; 134: 688-695.
- Guo L, Zhang L, Sun Y, et al. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol Divers 2006; 10: 349-360.
- Tolman KG. The safety of thiazolidinediones. Expert Opin Drug Saf 2011; 10: 419-428.
A c c e p t e d : September 10, 2013