GHRELIN ACCELERATES THE HEALING OF ORAL ULCERS
IN NON-SIALOADENECTOMIZED AND SIALOADENECTOMIZED RATS
2Second Department of General Surgery, Jagiellonian University Medical College, Cracow, Poland
INTRODUCTION
Saliva is a fluid of the oral cavity produced by salivary glands. The functions of saliva are diverse. It cleans the mouth, protects the mucosal surfaces of the oral cavity from desiccation, dissolves food chemical ingredients so that they can be tasted, moistens food and aids in bolus formation, and digests starchy foods. Saliva is the body's first barrier against infections. It contains a myriad of proteins and peptides that protect against injury evoked by microbial, mechanical or chemical agents (1). A number of growth factors, such as nerve growth factor (NGF), fibroblast growth factors (FGFs) and especially epidermal growth factor (EGF) have been found in saliva (1). Studies, performed mainly on rodents, have shown that sialoadenectomy, among others, increases ethanol- or stress-induced gastric ulceration (2, 3), delays healing of oral wounds (4, 5), and duodenal and gastric ulcers (6), as well as decreases liver regeneration (7). These detrimental effects of sialoadenectomy can be reversed by administration of exogenous EGF (2-7). EGF concentration in human saliva is around 100,000 fold lower than in rodent saliva and the study performed by Oudhoff et al. (8) has suggested that histatins, rather than EGF, are the major wound-closure stimulating factors in human saliva.
Xerostomia, the subjective feeling of the dry mouth, is mainly caused by a reduction of in saliva production. This is itself caused by salivary glands hypofunction, which results in a decrease in the amount of saliva and composition alterations. Reduced saliva flow rate may lead to the development of mouth inflammation and high rate of caries followed by increased risk of tooth loss. This effect has been shown in animal and clinical studies (9-11). Moreover, animal studies have shown that desalivation causes a long-term delay in tooth sockets healing and inhibits healing of palatal wounds (12, 13).
Ghrelin, a peptide predominantly produced in the stomach, exhibits numerous physiological functions, including stimulation of growth hormone release, food intake and gastric empting, and regulation of energy expenditure (14). Previous studies have shown that ghrelin exhibits protective and healing-promoting effects in different organs, including the gut. Pretreatment with ghrelin reduces gastric mucosal damage in experimental ulcers induced by ethanol (15, 16), stress (17) or alendronate (18), as well as accelerates the healing of gastric ulcers evoked by acetic acid (19). Protective and therapeutic effect of ghrelin has been also found in the course of cysteamine-induced (20) and acetic-acid-induced (19) duodenal ulcers. In the pancreas, ghrelin promotes survival of human islets and cultured cells (21), inhibits the development of acute pancreatitis evoked by cerulein (22), ischemia followed by reperfusion (23) or taurocholate (24). Also, ghrelin exhibits therapeutic effect in the course of cerulein-induced pancreatitis (25, 26). There are clinical (27, 28) and animal (29, 30) studies suggesting protective effect of ghrelin in the liver.
Influence of ghrelin administration on colitis is controversial. Some studies have shown that administration of ghrelin ameliorates the severity of the trinitrobenzene sulfonic acid (TNBS)-induced colitis, as well as increases survival of experimental animals and accelerates the healing in this model of colitis (31, 32).
Opposite effects of ghrelin on the course of colitis has been found by De Smet et al. (33). They have reported that the development of dextran sodium sulphate (DSS)-induced colitis is attenuated in ghrelin knockout mice; whereas treatment of non-inbred mice with exogenous ghrelin enhances the severity of inflammation in this model of colitis.
In the oral cavity, ghrelin has been found in salivary glands, saliva, teeth, taste buds of the tongue and gingival epithelium (34-37). Concentration of ghrelin in saliva is similar to that observed in serum or plasma (38, 39). Also, there are clinical studies showing that psychosocial stress enhances salivary level of ghrelin and this effect is more pronounced in symptomatic patients with bulimia nervosa (40). The role of ghrelin in the healing of oral mucosa ulcers is unknown. Therefore, the aim of present study was to examine the influence of ghrelin administration on oral mucosa integrity and healing of oral ulcers in rats with intact salivary gland and sialoadenectomized rats.
MATERIALS AND METHODS
Animals and treatment
Studies were performed on 120 male Wistar rats weighing 150–170g and were conducted following the experimental protocol approved by the Local Commission of Ethics for the Care and Use of Laboratory Animals. Animals were housed in normal room temperature (22±1°C) and a 12-h light-dark cycle.
Studies were conducted on rats with intact salivary gland or rats with reduced salivary secretion evoked by sialoadenectomy. Rats were divided into twelve experimental groups as shown in Table 1. Experiments were repeated and observations were made on ten rats in each experimental group.
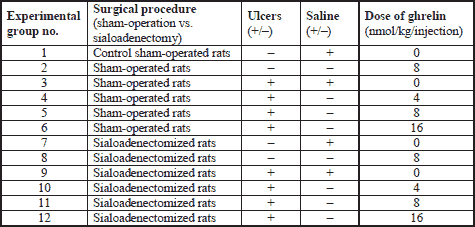
As shown on Fig. 1, sialoadenectomy was performed two weeks before induction of oral ulcers. In rats, submandibular and sublingual glands, in contrast to parotid glands, are a main source of growth factors in saliva (41). Moreover, previous study has shown that wound licking with submandibular and sublingual saliva promotes healing of cutaneous wounds; whereas parotid saliva does not exhibit any effect in treatment of dermal injury (42). For this reason we decide to remove submandibular and sublingual salivary glands without resection of parotid salivary glands. Intact parotid salivary glands have prevented excessive reduction of saliva secretion and allowed sialoadenectomized rats to masticate and swallow. Animals before sialoadenectomy were fasted for 16 hours, with the unlimited access to water. At the day of operation, rats were anesthetized with ketamine (50 mg/kg, intraperitoneally (i.p.), Bioketan, Vetoquinol Biowet, Gorzów Wielkopolski, Poland). To remove the submandibular and sublingual salivary glands, a 15 mm incision of the skin was made below the mandible. After the exposition of salivary glands, salivary ducts and blood vessels were ligated, and submandibular and subligual glands were removed.
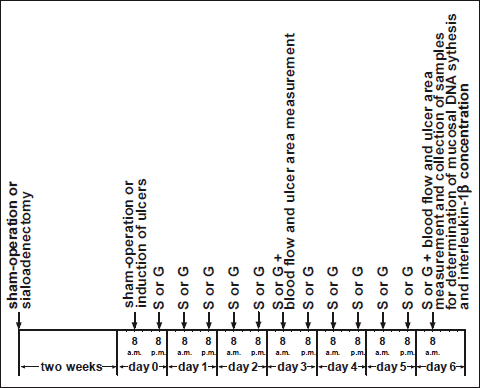 |
Fig. 1. Study design describing time relationship between sham-operation, induction of ulcers and treatment with saline or ghrelin. S or G means saline or ghrelin administration. |
The rest of animals was sham-operated. In these rats, incision of the skin and mobilization of salivary glands was performed without ligation of any salivary duct or blood vessel.
Two weeks after sialoadenectomy or sham operation, rats were fasted for 16 hours and reanesthetized with ketamine. Gingival and lingual ulcers were performed by modification of method previously described by Konturek et al. (6). Briefly, in the case of gingival ulcers, about 70 µl of 100% acetic acid was applied for 15 s through the plastic tube (4 mm inner diameter) to frontal surface of gingival mucosa of the maxilla above incisors. In the case of lingual ulcers, we used the same amount and concentration of acetic acid and the same time of exposition, but plastic tube had bigger inner diameter (5 mm). Acetic acid was applied to upper surface of the tongue.
After induction of ulcers, rats were treated intraperitoneally twice a day with saline (0.9% NaCl) or ghrelin (given at the dose of 4, 8 or 16 nmol/kg/dose) for six days. Ghrelin has been synthesized in Yanaihara Institute Inc. from Shizuoka in Japan.
Animals without induction of ulcers were treated intraperitoneally twice a day with saline or ghrelin (given at the dose of 8 nmol/kg/dose) for six days before the end of study. Ghrelin was diluted in saline immediately before administration. Final volume of saline or ghrelin solution was 0.3 ml. Drugs have been given intraperitoneally through frontal part of abdominal wall in conscious immobilized rats.
Determination of mucosal blood flow and measurement of ulcer area
Three and six days after induction of gingival and lingual ulcers, rats were anesthetized again with ketamine. Blood flow in the gum mucosa of the maxilla and in the mucosa of upper surface of the tongue was measured using laser Doppler flowmeter (PeriFlux 4001 Master monitor, Perimed AB, Järfälla, Sweden). Blood flow was measured in five areas of the gum mucosa and lingual mucosa, and mean value of five recordings was presented as percent of mucosal blood flow measured in saline-treated rats with intact salivary glands and without induction of ulcers (control group). After measurement of mucosal blood flow, the area of ulcerated mucosa was measured, using computerized planimeter (Morphomat, Carl Zeiss, Berlin, Germany) as described previously (43).
Biochemical analysis
Six days after induction of oral ulcers, at the end of experiment, after measurement of mucosal blood flow, biopsy samples from the gingival and lingual mucosa (one biopsy per each type of mucosa per rat) were taken for determination of mucosal DNA synthesis and mucosal concentration of pro-inflammatory interleukin-1β.
DNA synthesis, an index of cell proliferation, was determined by measurement of [3H]thymidine incorporation ([6-3H]-thymidine, 20-30 Ci/mmol, Institute for Research, Production and Application of Radioisotopes, Prague, Czech Republic) into mucosal DNA as described previously (43, 44). The incorporation of labeled thymidine into DNA was determined by counting 0.5 ml DNA-containing supernatant in a liquid scintillation system. DNA synthesis was expressed as tritium disintegrations per minute per g DNA (dpm/ g DNA).
After biopsy, samples of mucosa, taken from the gum and tongue for determination of interleukin-1β content, were homogenized in ice-cold phosphate-buffered saline (PBS, 20 mM, pH 7.4). Homogenate was centrifuged at 1500 g for 10 min at 4°C. Content of interleukin-1β in the supernatant was measured using the BioSource Cytoscreen rat IL-1β kit (BioSource International, Camarillo, California, USA) based on ELISA. Concentration of interleukin-1β in gingival and lingual mucosa was expressed as ng per 1 g of protein.
Statistical analysis
Results were expressed as mean ± S.E.M. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test using GraphPadPrism (GraphPad Software, San Diego, CA, USA). Differences were considered to be statistically significant when P was less than 0.05.
RESULTS
In animals with intact salivary glands without induction of oral ulcers, treatment with ghrelin given at the dose of 8 nmol/kg/dose for 3 or 6 days was without significant effect on blood flow in gingival (Fig. 2 and 3) and lingual mucosa (Fig. 4 and 5). Also, in this group of animals, treatment with ghrelin for 6 days was without significant effect on DNA synthesis (Fig. 6 and 7) and concentration of interleukin-1β (Fig. 8 and 9) in gingival and lingual mucosa.
Topical application of acetic acid on the mucosa of the gum or tongue has produced gingival and lingual ulcers. These ulcers have undergone spontaneous healing. In rats with intact salivary glands, three days after induction of ulcers, the lesion area of gingival (Fig. 10) and lingual (Fig. 11) ulcers reached 11.2±0.3 and 19.2±0.4 mm2, respectively. Three days later, in this group of animals, the area of gingival (Fig. 12) and lingual (Fig. 13) ulcers was 1.4±0.1 and 1.5±0.1 mm2, respectively. Induction of oral ulcers has affected blood flow, DNA synthesis and interleukin-1β in gingival and lingual mucosa. Three days after induction of ulcers, blood flow in mucosa of the gum (Fig. 2) and tongue (Fig. 4) has been significantly reduced by around 43 and 27%, respectively. After next three days, mucosal blood flow in the gum and tongue was similar to that observed in control rats with intact salivary gland and without induction of ulcers (Fig. 3 and 5). Six days after induction of ulcer, in saline-treated rats with intact salivary glands, DNA in mucosa of the gum (Fig. 6) and tongue (Fig. 7) was significantly increase by 19 and 21%, respectively above values observed in control salivary gland-intact rats without induction of ulcers. interleukin-1β concentration in the mucosa of the gum (Fig. 8) and tongue (Fig. 9) in salivary glands-intact rats with oral ulcers has been increased above a control value by 114 and 66%, respectively.
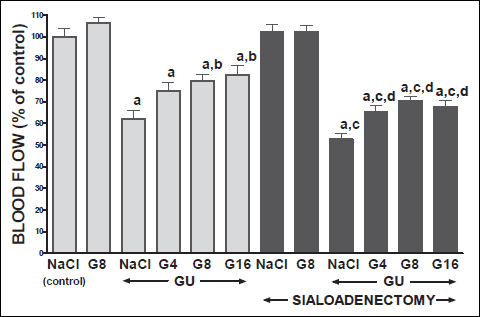 |
Fig. 2. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on gingival mucosal blood flow in salivary glands-intact or sialoadenectomized rats at the third day after induction of gingival ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of gingival ulcers (GU); c P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; d P<0.05 compared to saline-treated sialoadenectomized rats with induction of gingival ulcers (GU). |
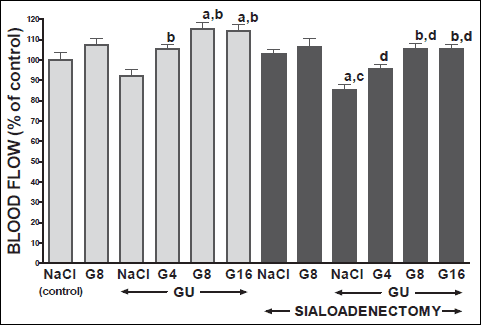 |
Fig. 3. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on gingival mucosal blood flow in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of gingival ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M.. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of gingival ulcers (GU); c P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; d P<0.05 compared to saline-treated sialoadenectomized rats with induction of gingival ulcers (GU). |
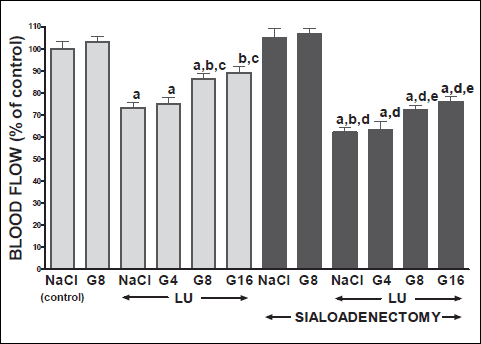 |
Fig. 4. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on lingual mucosal blood flow in salivary glands-intact or sialoadenectomized rats at the third day after induction of lingual ulcers (LU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of lingual ulcers (LU); c P<0.05 compared to salivary glands-intact rats with lingual ulcers and treated with ghrelin at the dose of 4 nmol/kg/dose (G4); d P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; eP<0.05 compared to saline-treated sialoadenectomized rats with induction of lingual ulcers (LU). |
In salivary glands-intact rats, intraperitoneal administration of ghrelin has significantly accelerated the healing rate of both, gingival (Figs. 10 and 12) and lingual (Figs. 11 and 13) ulcers and this effect was observed at the 3rd and 6th day after ulcer induction. Maximal therapeutic effect was observed after ghrelin used at the dose of 8 and 16 nmol/kg/dose. This result was associated with a significant increase in blood flow (Figs. 2-5) and DNA synthesis (Figs. 6 and 7) in gingival and lingual mucosa, as well as with a significant decrease in mucosal concentration of pro-inflammatory interleukin-1β (Figs. 8 and 9).
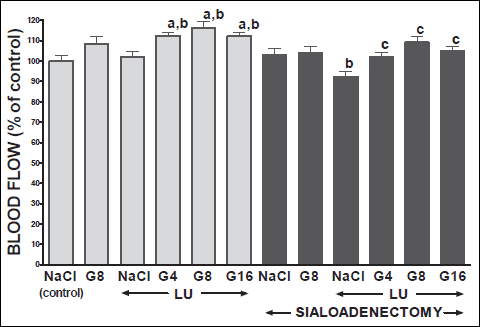 |
Fig. 5. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on lingual mucosal blood flow in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of lingual ulcers (LU) and/or the start of saline or ghrelin administration. Mean ± S.E.M.. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of lingual ulcers (LU); cP<0.05 compared to saline-treated sialoadenectomized rats with induction of lingual ulcers (LU). |
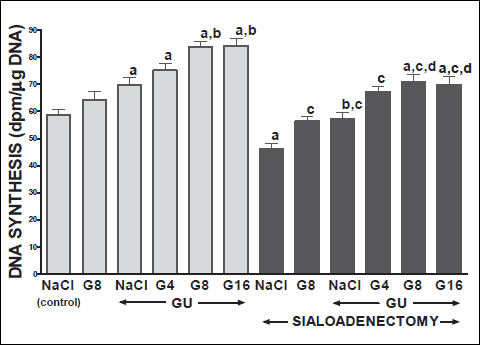 |
Fig. 6. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on DNA synthesis in gingival mucosa in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of gingival ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of gingival ulcers (GU); c P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; d P<0.05 compared to saline-treated sialoadenectomized rats with induction of gingival ulcers (GU). |
Sialoadenectomy alone, without induction of ulcers, has reduced DNA synthesis, an index of cell proliferation (Figs. 6 and 7) and increased concentration of pro-inflammatory interleukin-1β (Figs. 8 and 9) in oral mucosa. In these rats, administration of ghrelin reversed the sialoadenectomy-induced reduction in cell proliferation (Figs. 6 and 7) and decreased the sialoadenectomy-induced increase in concentration of pro-inflammatory intereleukin-1β (Figs. 8 and 9) in gingival and lingual mucosa. Blood flow in gingival (Figs. 2 and 3) and lingual (Figs. 4 and 5) mucosa was not affected by sialoadenectomy applied alone or in combination with ghrelin administration in rats without induction of oral ulcers.
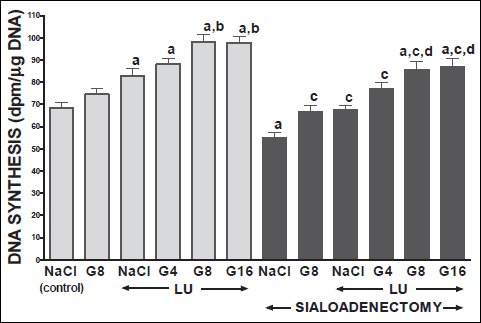 |
Fig. 7. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on DNA synthesis in lingual mucosa in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of lingual ulcers (LU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of lingual ulcers (LU); c P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; d P<0.05 compared to saline-treated sialoadenectomized rats with induction of lingual ulcers (LU). |
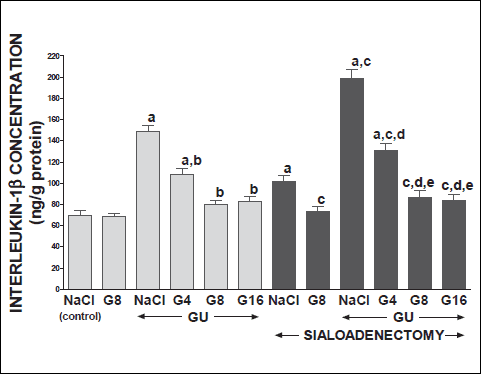 |
Fig. 8. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on concentration of interleukin-1 in gingival mucosa in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of gingival ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of gingival ulcers (GU); c P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; d P<0.05 compared to saline-treated sialoadenectomized rats with induction of gingival ulcers (GU); eP<0.05 compared to sialoadenectomized rats with induction of gingival ulcers (GU) and treated with ghrelin at the dose of 4 nmol/kg/dose (G4). |
Extirpation of sublingual and submandibular salivary glands has decreased the healing rate of gingival (Figs. 10 and 12) and lingual (Figs. 11 and 13) ulcres. This effect was associated with maximal reduction in blood flow (Figs. 2-5) and DNA synthesis (Figs. 6 and 7), as well as with maximal increase in concentration of pro-inflammatory interleukin-1β in oral mucosa (Figs. 8 and 9), when compared to values observed in other groups of animals.
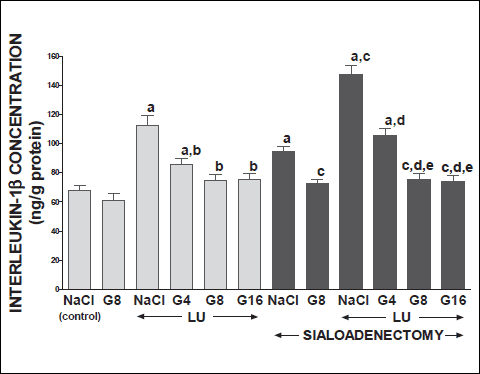 |
Fig. 9. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on concentration of interleukin-1β in lingual mucosa in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of lingual ulcers (LU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to saline-treated rats with intact salivary glands, without induction of ulcers; b P<0.05 compared to saline-treated rats with intact salivary glands and induction of lingual ulcers (LU); c P<0.05 compared to saline-treated sialoadenectomized rats without induction of ulcers; d P<0.05 compared to saline-treated sialoadenectomized rats with induction of lingual ulcers (LU); e P<0.05 compared to sialoadenectomized rats with induction of lingual ulcers (LU) and treated with ghrelin at the dose of 4 nmol/kg/dose (G4). |
 |
Fig. 10. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on the area of gingival ulcers in salivary glands-intact or sialoadenectomized rats at the third day after induction of gingival ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to rats with intact salivary glands and treated with saline after induction of ulcers; b P<0.05 compared to sialoadenectomized rats and treated with saline after induction of ulcers; c P<0.05 compared to sialoadenectomized rats with induction of gingival ulcers (GU) and treated with ghrelin at the dose of 4 nmol/kg/dose (G4). |
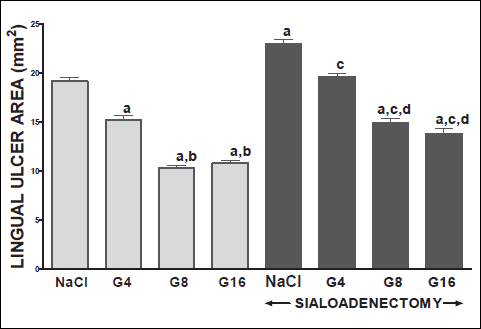 |
Fig. 11. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on the area of lingual ulcers in salivary glands-intact or sialoadenectomized rats at the third day after induction of lingual ulcers (LU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to rats with intact salivary glands and treated with saline after induction of ulcers; b P<0.05 compared to rats with intact salivary glands and treated with ghrelin at the dose of 4 nmol/kg/dose (G4); c P<0.05 compared to sialoadenectomized rats treated with saline after induction of ulcers; dP<0.05 compared to sialoadenectomized rats with induction of gingival ulcers (GU) and treated with ghrelin at the dose of 4 nmol/kg/dose (G4). |
Treatment with ghrelin has reversed deleterious effect of sialoadenectomy. Ghrelin given at the dose of 8 or 16 nmol/kg/dose increased the healing rate of oral ulcers above a value observed in treated with saline rats with intact salivary glands (Figs. 10-13). This effect was associated with a significant improvement of mucosal blood flow (Figs. 2-5) and DNA synthesis (Figs. 6 and 7), as well as with a reduction in concentration of pro-inflammatory interleukin-1β (Figs. 8 and 9) in oral mucosa.
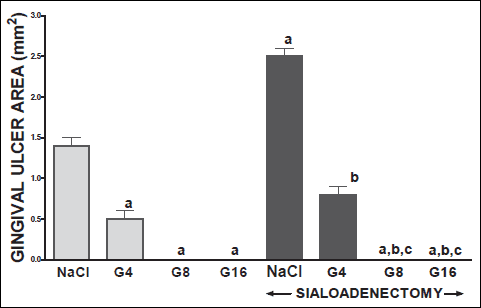 |
Fig. 12. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on the area of gingival ulcers in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of gingival ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to rats with intact salivary glands and treated with saline after induction of ulcers; b P<0.05 compared to sialoadenectomized rats and treated with saline after induction of ulcers; cP<0.05 compared to sialoadenectomized rats with induction of gingival ulcers (GU) and treated with ghrelin at the dose of 4 nmol/kg/dose (G4). |
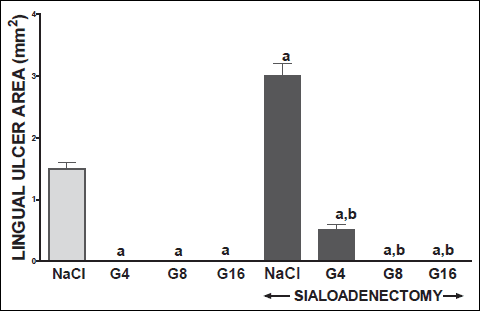 |
Fig. 13. Influence of saline (NaCl) or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose (G4, G8 or G16) on the area of lingual ulcers in salivary glands-intact or sialoadenectomized rats at the sixth day after induction of lingual ulcers (GU) and/or the start of saline or ghrelin administration. Mean ± S.E.M. N=10 in each group of animals. a P<0.05 compared to rats with intact salivary glands and treated with saline after induction of ulcers; b P <0.05 compared to sialoadenectomized rats and treated with saline after induction of ulcers. |
DISCUSSION
Our present study has brought several important findings concerning the influence of ghrelin administration on the integrity of oral mucosa and healing of gingival and lingual ulcers. First of all, we have found that treatment with ghrelin accelerates healing of oral ulcers in rats with intact salivary glands. Therapeutic effect of ghrelin was associated with improvement of mucosal cell proliferation and blood flow, and reduction in mucosal concentration of proinflammatory interleukin-1β. Integrity of the mucosa is maintained by dynamic equilibrium between cell proliferation and cell loss through exfoliation of surface cells. Cells are produced by mitosis in the deepest layer and subsequently are moved to surface where they are shed (45). Insufficient cell production or increased cell loss may lead to atrophy and ulceration. On the other hand, increased cell proliferation or reduced cell loss may result in hyperplasia. DNA synthesis, measured by incorporation of labeled thymidine into DNA, is an index of cell proliferation (46). Our present study has shown that spontaneous healing of oral ulcers is associated with a stimulation of DNA synthesis in gingival and lingual mucosa. Administration of ghrelin has led to an additional increase in mucosal DNA synthesis. This last finding indicates that therapeutic effect of ghrelin in the healing of oral ulcers involves stimulation of cell renewal.
Mucosal blood flow plays an important role in the protection and healing of mucosa in the gastrointestinal tract (47-49). Numerous experimental studies have shown that exposure of gastric mucosa to potentially noxious factors results in little or no damage, as long as adequate blood flow is maintained, whereas reduction in mucosal blood flow leads to severe gastric injury (47). Blood flow contributes to gastric mucosa protection by supplying the mucosa with oxygen, bicarbonate and nutritious substances, and by removing of carbon dioxide, hydrogen ions and other toxic agents diffusing from the gastric lumen and local improvement of blood flow reduces damage of gastric mucosa (47-49). The same protective and healing-promoting effect of blood flow has been also shown in the mucosa of other parts of gastrointestinal tract such as the esophagus (50), duodenum (19, 20, 51) or colon (52). Our present study has shown that induction of gingival and lingual ulcers causes initial reduction of mucosal blood flow followed by subsequent increase in this parameter during spontaneous healing of these ulcers. Administration of ghrelin in rats with intact salivary glands significantly improved mucosal blood, leading to faster healing of gingival and lingual ulcers. These data indicate that improvement of mucosal blood flow is involved in therapeutic effect of ghrelin in treatment of oral ulcers. This concept is additionally supported by observation that ghrelin stimulates sprouting new capillary blood vessels and deficiency of ghrelin is a major cause of the aging-related impairment of angiogenesis (53). Mechanism of mucosal blood flow improvement evoked by treatment with ghrelin may be a result of direct or indirect (e.g. though IGF-1) action of ghrelin on blood vessels or/and a result of oral mucosa recovery. Previous studies in the stomach have shown that an increase in mucosal blood flow can be a cause or/and a result of improvement of mucosal condition (46-49). An increase in mucosal blood flow reduces mucosal damage and accelerates ulcer healing, but simultaneously a reduction in mucosal damage improves mucosal blood flow. The same relationship is observed between condition of mucosa and a rate of DNA synthesis. DNA synthesis is an index of cell proliferation, but also an index of cell vitality. Stimulation of DNA synthesis leading to an increase in cell proliferation, accelerates ulcer healing, but on the other hand, an improvement of mucosal condition increases mucosal DNA synthesis (19, 20, 46).
Another finding of our present study is observation concerning the influence of induction of oral ulcers and ghrelin administration on mucosal concentration of interleukin-1β. Interleukin-1β is a well known mediator of acute inflammation and plays a crucial role in the induction of systemic acute phase response and in the release of other members of the pro-inflammatory cytokine cascade (54). This interleukin stimulates production and release next mediators of inflammation such as tumor necrosis factor, platelet activating factor, prostaglandins and pro-inflammatory interleukins (54). Numerous studies have shown that administration of interleukin-1 receptor antagonist prevents the rise in serum concentration of interleukin-6 and TNF-α , and decreases severity of systemic inflammation (55-58). Our study has shown that induction of oral ulcer leads to initiation of inflammatory process in gingival and lingual mucosa, and increases mucosal concentration of pro-inflammatory intereukin-1b. On the other hand, administration of ghrelin has reduced mucosal concentration of interleukin-1β in the gum and tongue in rats with oral ulcers. These data indicate that ghrelin reduces local inflammatory response in the acetic acid-induced oral ulcers and this effect seems to be another mechanism involved in ghrelin's therapeutic effect in oral mucosa. Anti-inflammatory effect of ghrelin is probably dependent on local improvement of mucosal condition through acceleration of cell proliferation and recovery of mucosa, as well as on direct influence of ghrelin on immune system. Several immune cells, including human leukemic B, T and myeloid cell lines, human peripheral lymphocytes and neutrophils, as well as mouse splenic T cells, exhibit the presence of receptors for ghrelin, growth hormone secretagogue receptor (GHS-R) (59-60). Biological action of ghrelin, on the immune system, includes attenuation of septic shock, promotion of thymopoiesis during aging in mice, and inhibition of expression of pro-inflammatory cytokines by human monocytes and T lymphocytes (61-63). Administration of ghrelin has been also found to reduce phagocytic activity of peritoneal macrophages in rats exposed to cold-restraint stress (64).
The important findings our present study are observations concerning rats with sialoadenectomy. Extirpation of submandibular and sublingual salivary glands, without induction of oral ulcers, has reduced DNA synthesis, an index of cell proliferation, and increased concentration of pro-inflammatory interleukin-1β in gingival and lingual mucosa. In macroscopic examination, oral mucosa was dry and red, animals had problem with mastication and swallowing. In control rats, around 0.8 g of food per rat per day was not swallowed and in shredded form was found on the cage bottom. In sialoadenectomized rats, amount of uneaten and shredded foot was fivefold higher (data not shown on separate figure). These findings are consistent with clinical manifestation of xerostomia and indicate that sialoadenectomized rat can be used as experimental model of this disease.
In sialoadenectomized rats, administration of ghrelin has reversed the reduction in oral mucosa cell proliferation and decreased mucosal concentration of interleukin-1β to a level observed in control, salivary glands-intact rats. This result suggests that treatment with ghrelin can be useful in the protection of oral mucosa against damage in the case of xerostomia. On the other hand, it should be pointed out that every kind of therapy exhibits beneficial and side-effects. Our study has shown that ghrelin stimulates cell proliferation in oral mucosa and accelerates healing rate of oral ulcers. Growth-promoting factors regulate essential biological processes, such as cell proliferation, survival, apoptosis, migration and differentiation. Previous studies have shown that growth factors and growth factor signaling pathways are involved in carcinogenesis (65-67). Ghrelin acts on tissues directly though GHS-R and indirectly by release of growth hormone and insulin-like growth factor-1 (IGF-1). Several endocrine and non-endocrine neoplasms express ghrelin and GHS-R at both mRNA and protein levels (68, 69). Study performed by Tian et al. (70) has shown that ghrelin promotes the oncogene CDK6 gene expression, upregulates the metastasis factor MMP2 expression and represses the tumor suppressor gene P53 gene expression in human gastric carcinoma cell lines. This result strongly suggests that treatment with ghrelin should be avoided in gastric cancer patients. In contrast to that, there are also studies showing that low baseline concentration of serum ghrelin is associated with a statistically significant increase in the risk of gastric noncardia adenocarcinoma and esophagogastric junctional adenocarcinoma (71).
In sialoadenectomized rats, ulcers of gingival and lingual mucosa have undergone spontaneous healing, but this process was significantly reduced when compared with healing of ulcers in salivary glands-intact rats. Moreover, restoration of mucosal blood flow and cell renewal was delayed, whereas mucosal concentration of pro-inflammatory interleukin-1β was increased.
In sialoadenectomized rats, administration of ghrelin has accelerated healing of gingival and lingual ulcers. Treatment with ghrelin given at the dose 4 nmol/kg/dose has restored healing rate of ulcers to a level observed in rats with intact salivary glands and this effect was associated with a similar reduction in mucosal concentration of interleukin-1β. Mucosal blood flow and mucosal DNA synthesis have reached values comparable to values observed in control saline-treated rats with intact salivary glands and without induction of ulcers. Ghrelin given at the higher doses, 8 and 16 nmol/kg/dose, caused strong and similar for both doses therapeutic effect in sialoadenectomized rats. Healing rate of ulcers was higher than in salivary glands-intact rats treated with saline and this effect was associated with significant improvement of mucosal cell proliferation and mucosal blood flow, and significant reduction in mucosal concentration of interleukin-1β.
It is well known that ghrelin enhances food intake (14) and food intake is the most important physiological stimulator of saliva secretion (72). Saliva exhibits protective and healing promoting effects on oral mucosa and these effects are most likely related to the presence of growth factors and other biologically active peptides in saliva (1). Moreover salivary glands secrete ghrelin and amount of ghrelin in saliva increases during meal intake (39). These data suggest that therapeutic effect of exogenous ghrelin in rats with intact salivary glands may be partly related to stimulation of saliva secretion. However this mechanism of therapeutic effect of ghrelin in oral ulcer healing can not be involved in sialoadenectomized rats. The main physiological function of ghrelin found by Kojima, a discoverer of ghrelin, is stimulation of growth hormone release from the anterior pituitary (73). In next step of hormonal axis, growth hormone stimulates release of insulin-like growth factor1 (IGF-1) and hepatocyte growth factor (HGF) (74-76). For this reason, therapeutic effect of ghrelin in oral ulcers may be direct action through ghrelin's receptor, GHS-R and/or indirect influence through IGF-1 or HGF receptor.
In summary, the treatment with ghrelin accelerates healing of oral ulcers in salivary glands-intact rat, as well as in rats with sialoadenectomy. Mechanisms of therapeutic effect of ghrelin administration involve an increase in mucosal blood flow, and cell proliferation, as well as a reduction in local inflammation.
Acknowledgement: Results presented in this paper were based in part on Ph.D. thesis of Dr. P. Kownacki from Outpatient Dental Clinic „Kowdent“, Cracow, Poland.
Conflict of inerests: None declared.
REFERENCES
- Nauntofte B, Jensen JL. Salivary secretion. In: Textbook of Gastroenterology. Yamada T, Alpers D, Laine L, Owang C, Powell DW (eds), Philadelphia, Lippincott, Wiliams & Wilkins Publishers, 1999, pp. 263-278.
- Leitch GJ. Role of the salivary glands in protecting the stomach against ethanol. Alcohol Alcohol 1985; 20: 305-311.
- Konturek SJ, Brzozowski T, Konturek PK, Majka J, Dembinski A. Role of salivary glands and epidermal growth factor (EGF) in gastric secretion and mucosal integrity in rats exposed to stress. Regul Pept 1991; 32: 203-215.
- Noguchi S, Ohba Y, Oka T. Effect of salivary epidermal growth factor on wound healing of tongue in mice. Am J Physiol 1991; 260: E620-E625.
- Bodner L, Dayan D, Pinto Y, Hammel I. Characteristics of palatal wound healing in desalivated rats. Arch Oral Biol 1993; 38: 17-21.
- Konturek SJ, Dembinski A, Warzecha Z, Brzozowski T, Gregory H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology 1988; 94: 1300-1307.
- Lambotte L, Saliez A, Triest S, et al. Effect of sialoadenectomy and epidermal growth factor administration on liver regeneration after partial hepatectomy. Hepatology 1997; 25: 607-612.
- Oudhoff MJ, Bolscher JG, Nazmi K, et al. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J 2008; 22: 3805-3812.
- Bowen WH, Pearson SK, Young DA. The effect of desalivation on coronal and root surface caries in rats. J Dent Res 1988; 67: 21-23.
- Kaplan I, Zuk-Paz L, Wolff A. Association between salivary flow rates, oral symptoms, and oral mucosal status. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: 235-241.
- Caplan DJ, Hunt RJ. Salivary flow and risk of tooh loss in an elderly population. Community Dent Oral Epidemiol 1996; 24: 68-71.
- Bodner L, Dayan D, Pinto Y, Hammel I. Characteristics of palatal wound healing in desalivated rats. Arch Oral Biol 1993; 38: 17-21.
- Bodner L, Kaffe I, Cohen Z, Dayan D. Long-term effect of desalivation on extraction wound healing: a densitometric study in rats. Dentomaxillofac Radiol 1993; 22: 195-198.
- Warzecha Z, Dembinski A. Protective and therapeutic effects of ghrelin in the gut. Curr Med Chem 2012; 19: 118-125.
- Konturek PC, Brzozowski T, Pajdo R, et al. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol 2004; 55: 325-336.
- Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology 2003; 144: 353-359.
- Brzozowski T, Konturek PC, Konturek SJ, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept 2004; 120: 39-51.
- Iseri SO, Sener G, Yuksel M, et al. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol 2005; 187: 399-406.
- Ceranowicz P, Warzecha Z, Dembinski A, et al. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J Physiol Pharmacol 2009; 60: 87-98.
- Warzecha Z, Ceranowicz D, Dembinski A, et al. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med Sci Monit 2012; 18: BR181-BR187.
- Granata R, Settanni F, Biancone L, et al. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3',5'-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology 2007; 148: 512-529.
- Dembinski A, Warzecha Z, Ceranowicz P, et al. Ghrelin attenuates the development of acute pancreatitis in rats. J Physiol Pharmacol 2003; 54: 561-573.
- Dembinski A, Warzecha Z, Ceranowicz P, et al. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF Res 2006; 16: 348-356.
- Zhou X, Xue C. Ghrelin inhibits the development of acute pancreatitis and nuclear factor kappaB activation in pancreas and liver. Pancreas 2009; 38: 752-757.
- Ceranowicz D, Warzecha Z, Dembinski A, et al. Role of hormonal axis, growth hormone - IGF-1, in the therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis. J Physiol Pharmacol 2010; 61: 599-606.
- Warzecha Z, Ceranowicz P, Dembinski A, et al. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J Physiol Pharmacol 2010; 61: 419-427.
- Gutierrez-Grobe Y, Villalobos-Blasquez I, Sanchez-Lara K, et al. High ghrelin and obestatin levels and low risk of developing fatty liver. Ann Hepatol 2010; 9: 52-57.
- Mendez-Sanchez N, Ponciano-Rodriguez G, Bermejo-Martinez L, et al. Low serum levels of ghrelin are associated with gallstone disease. World J Gastroenterol 2006; 12: 3096-3100.
- Cetin E, Kanbur M, Cetin N, Eraslan G, Atasever A. Hepatoprotective effect of ghrelin on carbon tetrachloride-induced acute liver injury in rats. Regul Pept 2011; 171: 1-5.
- Golestan Jahromi M, Nabavizadeh F, Vahedian J, Nahrevanian H, Dehpour AR, Zare-Mehrjardi A. Protective effect of ghrelin on acetaminophen-induced liver injury in rat. Peptides 2010; 31: 2114-2117.
- Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006; 130: 1707-1720.
- Konturek PC, Brzozowski T, Engel M, et al. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J Physiol Pharmacol 2009; 60: 41-47.
- De Smet B, Thijs T, Moechars D, et al. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol Motil 2009; 21: 59-70.
- Aydin S, Ozercan IH, Geckil H, et al. Ghrelin is present in teeth. J Biochem Mol Biol 2007; 40: 368-372.
- Li BB, Chen ZB, Li BC, et al. Expression of ghrelin in human salivary glands and its levels in saliva and serum in Chinese obese children and adolescents. Arch Oral Biol 2011; 56: 389-394.
- Ohta K, Laborde NJ, Kajiya M, et al. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J Dental Res 2011; 90: 1286-1292.
- Shin YK, Martin B, Kim W, et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One 2010; 5(9): e12729.
- Groschl M, Topf HG, Bohlender J, et al. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin Chem 2005; 51: 997-1006.
- Dynesen AW, Bardow A, Astrup A, Petersson B, Holst JJ, Nauntofte B. Meal-induced compositional changes in blood and saliva in persons with bulimia nervosa. Am J Clin Nutr 2008; 87: 12-22.
- Monteleone P, Tortorella A, Scognamiglio P, Serino I, Monteleone AM, Maj M. The acute salivary ghrelin response to a psychosocial stress is enhanced in symptomatic patients with bulimia nervosa: a pilot study. Neuropsychobiology 2012; 66: 230-236.
- Amano O, Mizobe K, Bando Y, Sakiyama K. Anatomy and histology of rodent and human major salivary glands -overview of the Japan salivary gland society-sponsored workshop-. Acta Histochem Cytochem 2012; 45: 241-250.
- Bodner L. Effect of parotid submandibular and sublingual saliva on wound healing in rats. Comp Biochem Physiol A Comp Physiol 1991; 100: 887-890.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Role of sensory nerves in gastroprotective effect of anandamide in rats. J Physiol Pharmacol 2011; 62: 207-217.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Heparin inhibits protective effect of ischemic preconditioning in ischemia/reperfusion-induced acute pancreatitis. J Physiol Pharmacol 2012; 63: 355-365.
- Hamilton AI, Blackwood HJJ. Cell renewal of oral mucosal epithelium of the rat. J Anat 1974; 117: 313-327.
- Warzecha Z, Dembinski A, Brzozowski T, et al. Gastroprotective effect of histamine and acid secretion on ammonia-induced gastric lesions in rats. Scand J Gastroenterol 2000; 35: 916-924.
- Sorbye H, Svanes K. The role of blood flow in gastric mucosal defence, damage and healing. Dig Dis 1994; 12: 305-317.
- Warzecha W, Dembinski A, Brzozowski T, et al. Histamine in stress ulcer prophylaxis in rats. J Physiol Pharmacol 2001; 52: 407-421.
- West SD, Helmer KS, Chang LK, Cui Y, Greeley GH, Mercer DW. Cholecystokinin secretagogue-induced gastroprotection: role of nitric oxide and blood flow. Am J Physiol Gastrointest Liver Physiol 2003; 284: G399-G410.
- Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol 2010; 24: 873-882.
- Leung FW, Reedy TJ, Van Deventer GM, Guth PH. Reduction in index of oxygen saturation at margin of active duodenal ulcers may lead to slow healing. Dig Dis Sci 1989; 34: 417-423.
- Leung FW, Su KC, Pique JM, Thiefin G, Passaro E Jr, Guth PH. Superior mesenteric artery is more important than inferior mesenteric artery in maintaining colonic mucosal perfusion and integrity in rats. Dig Dis Sci 1992; 37: 1329-1335.
- Ahluwalia A, Li A, Cheng G, Deng X, Tarnawski AS. Reduced ghrelin in endothelial cells plays important mechanistic role in aging-related impairment of angiogenesis. J Physiol Pharmacol 2009; 60: 29-34.
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11: 633-652.
- De Koning HD, Schalkwijk J, van der Ven-Jongekrijg J, Stoffels M, van der Meer JW, Simon A. Sustained efficacy of the monoclonal anti-interleukin-1 beta antibody canakinumab in a 9-month trial in Schnitzler's syndrome. Ann Rheum Dis 2012; 72: 1634-1638.
- Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol 2011; 41: 1203-1217.
- Herlin T, Fiirgaard B, Bjerre M, et al. Efficacy of anti-IL-1 treatment in Majeed syndrome. Ann Rheum Dis 2013; 72: 410-413.
- Norman J, Franz M, Messina J, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery 1995; 117: 648-655.
- Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 2001; 86: 4284-4291.
- Xia Q, Pang W, Pan H, Zheng Y, Kang JS, Zhu SG. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul Pept 2004; 122: 173-178.
- Chang L, Du JB, Gao LR, Pang YZ, Tang CS. Effect of ghrelin on septic shock in rats. Acta Pharmacol Sin 2003; 24: 45-49.
- Dixit VD, Yang H, Sun Y, et al. Ghrelin promotes thymopoiesis during aging. J Clin Invest 2007; 117: 2778-2790.
- Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 2004; 114: 57-66.
- Tumer C, Bilgin HM, Obay BD, Diken H, Tasdemir E, Sermet A. Effect of ghrelin administration on phagocytic activity in acute cold-restraint stress exposed rats. Regul Pept 2007; 138: 113-117.
- Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res 2010; 8: 1439-1452.
- Rho O, Kim DJ, Kiguchi K, Digiovanni J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol Carcinog 2011; 50: 264-279.
- Kuemmerle JF. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol Metab Clin North Am 2012; 41: 409-423.
- Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin's role on gastrointestinal tract cancer. Surg Oncol 2010; 19: e2-e10.
- Lanfranco F, Baldi M, Cassoni P, Bosco M, Ghe C, Muccioli G. Ghrelin and prostate cancer. Vitam Horm 2008; 77: 301-324.
- Tian C, Zhang L, Hu D, Ji J. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-κB signaling pathway. Mol Cell Biochem 2013; 382: 163-172.
- Murphy G, Kamangar F, Dawsey SM, et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst 2011; 103: 1123-1129.
- Guyton AC. Secretory function of the alimentary tract. In: Textbook of Medical Physiology, A.C. Guyton (ed.) Philadelphia, W.B. Saunders Company, 1991, pp.709-725.
- Kojima M, Hosoda H, Date Y, Nakazano M, Matsuo H, KangawaK. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature 1999; 402: 656-660.
- Ho KK, O'Sullivan AJ, Hoffman DM. Metabolic actions of growth hormone in man. Endocr J 1996; 43(Suppl): S57-S63.
- Zapf J, Froesch ER. Insulin-like growth factors/somatomedins: structure, secretion, biological actions and physiological role. Horm Res 1986; 24: 121-130.
- Skrtic S, Wallenius K, Gressner AM, Jansson JO. Insulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology 1999; 140: 5729-5735.
A c c e p t e d : October 21, 2013