ESSENTIALITY AND TOXICITY OF VANADIUM SUPPLEMENTS
IN HEALTH AND PATHOLOGY
2Department of Molecular Medicine, Medical University of Gdansk, Gdansk, Poland
INTRODUCTION
The essential role of vanadium as an inorganic enzyme cofactor in maintaining hemostasis in health and pathology has been discussed for over a century (1, 2). In several studies on animals, vanadium deficiency has been linked to several disturbances, including infertility, disturbed iron metabolism, anemia, and impaired metabolism of bone, teeth and cartilage (3-5), whereas vanadium compounds normalized blood pressure, ischemia and the metabolism of the thyroid in in vitro studies (1-3, 6-10). Importantly, several studies have focused on the insulin-tropic properties of vanadium salts in the regulation of glucose and lipid metabolism (1, 6, 11).
Our understanding of how vanadium salts regulate the glucose metabolism is described in several animal studies (2-4, 12-14). Vanadate and vanadyl salts may interact with enzymes that contain phosphate groups such as tyrosine hydroxylases, adenylate kinases, glyceraldehyde 3-phosphate dehydrogenase or ribonucleases (Fig. 1) (3, 4, 15). Reflecting the ability of vanadate/vanadyl metabolized forms in in vivo conditions to bind to transport proteins, they may be non-competitive inhibitors of K-ATPases (Fig. 1) (3, 16, 17). Consequently, the inactivation of Na-K-ATPases may enhance the transport and oxidation of glucose (2, 16, 17). Vanadium compounds also exhibited an ability to restore enzymes involved in glycogen and lipid metabolism in animal models (12, 18, 19). Interestingly, it has been shown that vanadate is a competitive inhibitor of glucose-6-phosphate phosphatase, the key enzyme in the development of insulin resistance and type 2 diabetes (3, 20). Vanadium’s oxidation state of +5 in its compounds and its structural similarity to orthophosphate may explain its role in the reduction of protein tyrosine phosphatase activity (PTPase) in adipose tissue in animal studies (Fig. 1) (1, 21).
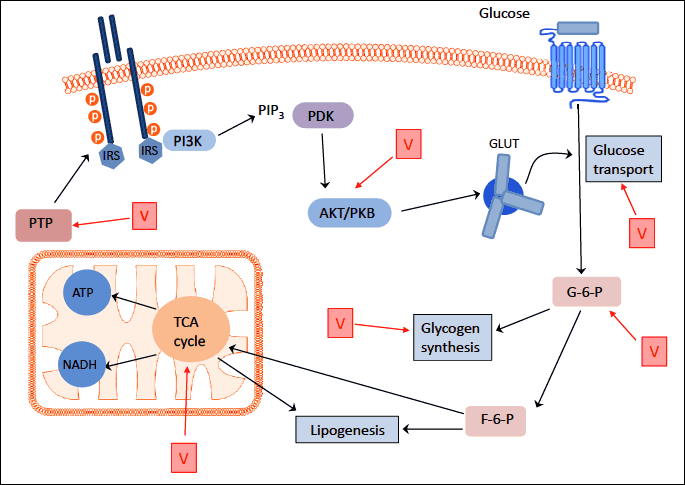
Abbreviations: V - vanadium compounds; PTP-ase - protein tyrosine phosphatase; PI3K - phosphoinositide 3-kinase; PKB - protein kinase B; PTP - tyrosine phosphatase; IRS - insulin receptor substrate; PDK -3’-phosphoinositide-dependent kinase; Glc – glucose; GLUT - glucose transporter; G-6-P - glucose-6-phosphate dehydrogenase; F-6-P - fructose 6-phosphate; TCA cycle- tricarboxylic acid cycle; NADH - nicotinamide adenine dinucleotide; ATP - adenosine triphosphate; PIP3 - phosphatidylinositol 3,4,5-triphosphate.
Vanadium compounds may also alter the metabolism of glucose by increasing its uptake and transport from the intracellular compartment to the cell surface through the insulin-dependent glucose transporter GLUT4 (11, 16, 22-24). Thus, vanadium-evoked glucose transport is similar to the action of insulin and is dependent on phosphoinositide 3-kinase (PI3K) and protein kinase B (PKB) (Fig. 1) (3, 25). However, the role of vanadium in glucose metabolism may also be insulin-independent (3, 12, 26).
At the beginning of the 20th century, vanadium was considered a panacea, due to its pharmacological and dietetic properties, useful for the treatment of syphilis, hyperlipidemia, tooth decay, anemia, malnutrition, tuberculosis and diabetes (1, 2, 5, 18, 27, 28). In recent years, vanadium compounds have again drawn the attention of researchers and their range of potential applications in medicine is constantly expanding. Accordingly, the aim of this review is to summarize the potentials of vanadium compounds in health and pathology.
VANADIUM METABOLISM
The average diet provides 10–160 µg of vanadium per day, mainly from mushrooms, seafood, black pepper, parsley, fennel seeds, grains and spinach, which contain, on average, 0.05–1.8 µg per gram. Experimental data have described dozens of inorganic and organic vanadium compounds (7, 14, 22, 28, 29). Due to the low stability of vanadate ions in the acidic environment of the stomach, average absorption for this microelement is only 1–10% (3-5, 11, 17, 18, 21). After entering the bloodstream, vanadium compounds are converted into vanadyl cations, which form complexes with transferrin and ferritin and, less frequently, with albumin, hemoglobin, or low-molecular components of plasma (citrate, lactate and phosphate) (1, 2, 5, 28, 30). In human serum, the vanadium concentration of healthy individuals is 1–2 µg/L (20). In the human body vanadium occurs in oxidation states of +4 and +5 (1). In the form of vanadyl cations (+4), it permeates cell membranes by diffusion or penetrates into cells by anion channels (1). In extracellular body fluids, vanadium occurs mainly as metavanadate (in oxidation state +5); when transported into cells it may be reduced by glutathione (GSH) to VO2+ form (18, 28, 31). Vanadium is accumulated in the kidney and to a lesser extent in the liver, bones and spleen (1, 5, 32, 33). In the general population, the mean concentration in the urine is 0.1–0.2 µg/L, and typically makes up less than 1 µg/g of creatinine.
The human body contains 100–200 µg of this element, which is the result of its absorption and excretion (1, 18, 28, 34). Data on the pharmacokinetics of vanadium in humans are scarce, indicating that its blood level is 0.42–0.08 µg/L and that it is excreted primarily in urine, where it reaches an average concentration of 22 µg/L, with a speed of excretion of about 8 µg/day (3, 5). It is also known that insulin may regulate the metabolism of vanadium, but the mechanism of this process is still unclear (5). The literature also reveals that in insulin-sensitive tissues, such as liver and adipose, vanadium metabolism may be accelerated (5, 32). This may explain why in pathological conditions (in cancer, infection or inflammation) vanadium accumulates in the liver, kidneys, lungs, spleen, adipose tissue, heart, bones and teeth (3).
THE ROLE OF VANADIUM SUPPLEMENTS IN DIABETES AND LIPID PROFILE REGULATION
The first report on the role of vanadium and its compounds in hyperglycemia appeared in 1899, when it was observed that oral supplementation with sodium orthovanadate (Na3VO4) reduced glycosuria in two of three patients with diabetes (35). Several studies pointed to the insulintropic abilities of vanadium salts in both animal and human studies (Figs. 1 and 2) (2, 7, 28, 32, 35, 36). Both inorganic and organic vanadium supplements improved glucose homeostasis in type 1 and type 2 diabetes and revealed their antioxidant properties in in vivo conditions (3, 12, 18, 19, 28, 35, 37-40). Thus, the glucose-lowering effect of 4–5 week oral metavanadate or vanadyl sulfate treatment in streptozotocin-induced diabetes (STZ-D) in rats has been associated with increased insulin sensitivity in the liver, skeletal muscle, adipose tissue and kidneys (6, 12, 31, 34). Moreover, the beneficial effect of metavanadate or vanadyl sulfate treatment on the diabetes-altered gene expression of enzymes involved in glucose and lipid metabolism, oxidative stress and signal transduction in STZ-D rats demonstrated that vanadium treatment can alleviate a significant portion of diabetic dysfunction (12, 19).
Additionally, in the same experimental model, bis(a-furancarboxylato)oxovanadium (IV) (BFOV) or bis(ethylmaltolato)oxovanadium (IV) (BEOV) reduced glucosuria, volume of urine, total cholesterol and triglyceride (TG) levels and increased concentrations of high-density lipoprotein (HDL) (Fig. 2) (41). Moreover, oral administration of BEOV (0.125–0.75 mg/ml for 12 weeks) improved bone metabolism in STZ-D rats (41). BFOV administration (0.02, 0.06 and 0.2 mmol/kg body weight) on a rat model of type 2 diabetes also caused a reduction in the release of free fatty acids (FFA) from adipocytes and their uptake by the liver, and a decrease in the synthesis of lipoproteins of very low density (VLDL) and pools of circulating triglycerides (42). In another study, the vanadyl complex bis((5-hydroxy-4-oxo-4H-pyran-2-yl)methyl 2-hydroxy-benzoatato) oxovanadium (IV) (BSOV), administered orally, effectively normalized blood glucose levels in diabetic obese (db/db) mice without affecting body weight (14). Meanwhile, in in vitro studies, sodium orthovanadate, sodium metavanadate and vanadyl sulphate accelerated the transport of glucose as well as its oxidation in adipocytes, liver tissue and skeletal muscles, increased glycogen synthesis, inhibited lipolysis, and accelerated lipogenesis in hepatocytes and adipocytes isolated from rats (Figs. 1 and 2) (6, 9, 28, 34). These observations confirmed the beneficial role of vanadium compounds in the correction of blood glycaemia and lipid metabolism in type 1 and type 2 diabetes (3, 28, 41-43).
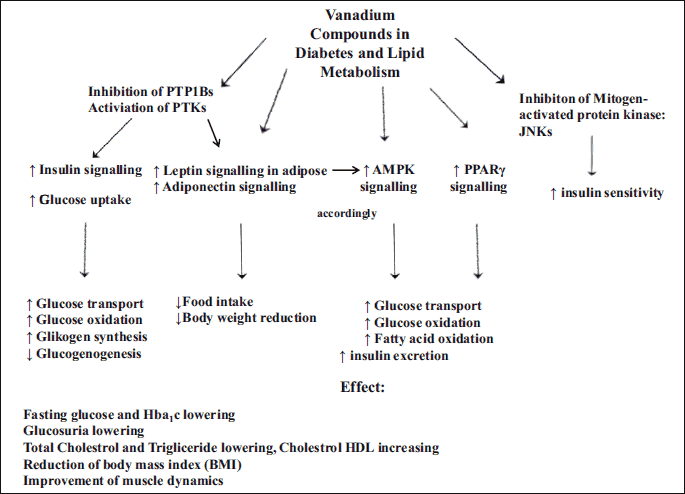
Abbreviations: PTP - protein tyrosine phosphorylase; PTP-ase - protein tyrosine phosphatase; JNKs-c - Jun N-terminal protein kinases; AMPK - AMP-activated protein kinase; PPARγ-peroxisome proliferator-activated receptor gamma.
Interestingly, three- and four-week courses of oral vanadyl sulphate (100–150 mg/d) reduced fasting glucose in patients with type 2 diabetes (18, 44-49). A similar effect was also observed in patients with poorly controlled type 2 diabetes, in whom fasting glucose and HbA1c levels were reduced and continued to be reduced for 2 weeks after the end of oral supplementation (1, 50). These observations were also confirmed in a clinical trial involving 60 patients with type 2 diabetes on a 12-week oral sodium metavanadate regime (100 mg/day), during which a reduction in the level of HbA1c, LDL-cholesterol, triglycerides and body mass index (BMI) was observed (4, 10, 28).
The biochemical mechanism of vanadium proinsulin-mimetic action involved the suppression of hepatic and kidney glucose release and inhibition of enzyme activities involved in gluconeogenesis, including glucose-6-phosphatase (G-6-Pase), phosphoenolpyruvate carboxykinase (PEPCK) and pyruvate kinase (Figs. 1 and 2) (51, 52). Vanadium also exerted its insulin-sensitizing effects through the stimulation of adiponectin through a PKB-dependent transduction pathway (25).
Vanadium compounds also modulated several key regulators of lipid metabolism by improving the expression of peroxisome proliferator-activated receptor γ (PPARγ) and the activation of AMP-activated protein kinase (AMPK) (Fig. 2) (13, 14, 53, 54). Vanadium also exerted an effect on adiponectin expression and its multimerization (13, 14, 25). Thus, it may inhibit the activity of PTP1B involved in cross-talk between signal transductions and PPARγ and adiponectin regulation, as well as cooperating in insulin enhancement (Fig. 2) (53). These compounds also corrected a variety of diabetes-altered gene expressions involved in lipid metabolism, oxidative stress, muscle dynamics, protein breakdown and biosynthesis, the complement system and signal transduction (12, 19, 26). In addition, vanadium treatment caused significant suppression of the phosphorylation of c-Jun N-terminal protein kinase (JNK), which plays a key role in insulin resistance in type 2 diabetes (Fig. 2) (14).
Vanadium also exhibited its hypoglycemic effect by increasing the translocation of GLUT4 glucose transporter, as observed in myocardia after an 8-week course of oral administration of organic vanadium compound bis(maltolato)oxovanadium (IV) (BMOV) in STZ-D rats (Figs. 1 and 2) (18). Another possible mechanism of organic vanadium insulin-tropic action is a reduction of mRNA expression for neuropeptide Y (NPY) and an increase in the level of leptin in adipose tissue, or the inhibition of tyrosine phosphatase receptor type 1 activity (PTP1B), resulting in a loss of appetite and body weight reduction in animal studies (Figs. 1 and 2) (18, 39, 40, 42, 55).
CONFLICTING RISK IN HYPERTENSION AND HEART DISEASE FOLLOWING SUPPLEMENTATION WITH VANADIUM COMPOUNDS
Numerous reports indicate the cytoprotective effect of vanadium compounds on rat cardiomyocytes, both in vitro and in vivo conditions (6, 17, 33, 38-40). Studies on rats showed an enhancement in the activity of nitric oxide synthase (NOS) in heart vessels after oral administration of organic vanadium chelate bis((1-oxy-2-pyridinethiolato)oxovanadium (IV) (VO(OPT)) (Fig. 1) (38). In another study, VO(OPT), injected intraperitoneally, demonstrated a cardioprotective effect in terms of exposure to hypoxia through the activation of tyrosine kinase, which reduced mitochondrial apoptosis and the extent of myocardial infarction (Fig. 1) (39). Orthovanadate also showed a similar mechanism for the prevention of myocardial infarction induced by hypoxia (17). The cardioprotective effect of BMOV (3.3 mg/kg) in intravenous infusion has been confirmed in rats treated with premedication (40). This effect is explained by a BMOV-dependent increase in tyrosine phosphorylation followed by inhibition of tyrosine phosphatase, which protects against ischemia (Fig. 1) (40). It is speculated that vanadium may also induce relaxation of cardiac muscle and help to improve its tolerance to hypoxia (34, 51). In addition, vanadium compounds have been found to contribute to reductions in plasma triglycerides and to prevent the development of hypertension and coronary heart disease (34, 51, 56). It is believed that their cardioprotective properties result from the activation of protein kinase B and phosphatidylinositol-3 (IP3) (Fig. 1) (38). The most likely mechanism seems to be based on the insulin-tropic and antioxidant properties of vanadium salts (6). On the other hand, it has been demonstrated that vanadium compounds can modulate the vasoconstrictive properties of vascular smooth muscle through the inhibition of Na+-K+-ATPase and an increase in intracellular calcium ions which may lead to the development of high blood pressure (3). The underlying mechanism of vanadium’s vasoconstrictive action was caused by the calmodulin-dependent phosphorylation of myosin light chains (Fig. 1) (3). Thus, in view of conflicting data, the mechanism of action of vanadium compounds on cardiomyocytes remains unclear.
EFFECT OF VANADIUM COMPOUNDS ON CANCER DEVELOPMENT
In the mid-20th century, several studies highlighted the role of vanadium compounds as potential agents regulating the apoptosis, proliferation and transformation of cancer cells (37, 41, 57). However, vanadium compounds exhibited both pro- and antitumor properties, depending on the dose and type of vanadium compound (28, 37, 41, 57). Low concentrations of vanadium may stimulate, whereas high concentrations may inhibit, the proliferation of tumor cells (Fig. 3) (28, 41). It appears that the oxidation of vanadium salts state plays an important role in carcinogenesis (57, 58). In vitro studies have shown that the strongest antitumor effect on lymphomas, T-cell leukemia, erythroleukemia, basophilic leukemia, liver cancer cells, ovary cancer, testicular cancer, nasopharyngeal tumors, tumors of pharynx and bone and neuroblastomas is exhibited by sodium metavanadate (+4) and vanadyl sulfate (+4), while the weakest effect is observed after using BMOV (+4) (1, 3, 41, 57). Moreover, orthovanadate (+5) exhibited an indirect suppressive effect on cells in human rhabdomyosarcoma (RMS), human lung cancer, and prostate cell lines as well as on neoplastically unchanged bronchial epithelial cells (37, 58). Moreover, the biological effect of orthovanadium was multiplied by a factor of 100–1000 by peroxvanadate, which forms in the presence of H2O2 and is a potent inhibitor of tyrosine phosphatase (Fig. 3) (41). Vanadium regulated gene expression differently from carcinogenic metals, the effect of which can vary depending on the type of tumor (59). Vanadium’s anti-tumor properties may be due to its inhibitory effect on tyrosine phosphatase and activation of tyrosine phosphorylase (Figs. 1 and 3) (58). This effect may also be caused by the induction and/or stabilization of liver enzymes, especially γ-glutamyl transpeptidase (GGT) and placental glutathione S-transferase (Fig. 3) (37). Another possible mechanism of action of vanadium compounds may be the inhibition of tumor cell proliferation by blocking cell cycles in the G2/M phase as shown in studies on embryonic fibroblasts or rhabdomyosarcomas (RMS) in mice (Fig. 3) (37, 41, 57). On the other hand, vanadium compounds induce the pro-tumor intracellular production of free radicals, leading to DNA-strand breakage and chromosomal aberrations (Fig. 3) (42).
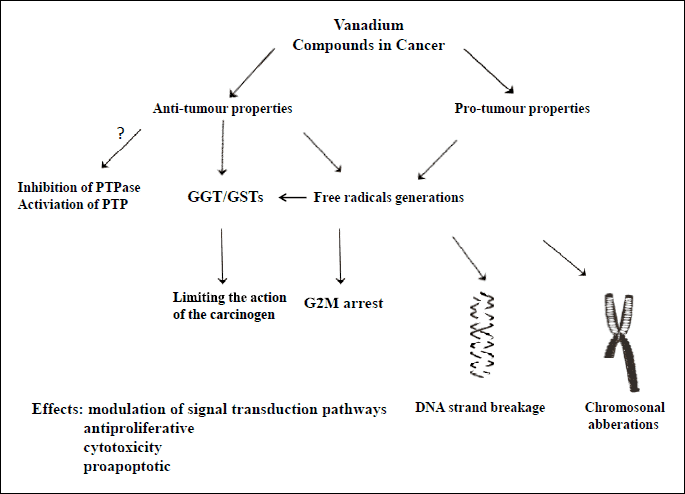
Abbreviations: PTP - protein tyrosine phosphorylase; PTP-ase - protein tyrosine phosphatase; GGT-gamma-glutamylotransferase; GST-glutathione S-transferase.
THE ROLE OF VANADIUM IN NEUROPROTECTION AND NEUROTOXICITY
There are conflicting reports on the effects of vanadium compounds on the nervous system (43, 55, 60, 61). Although studies in humans who have been exposed to vanadium showed the possibility of depression, tremors, neurasthenia and other serious movement disorders including vegetative symptoms, the neurotoxic effects of vanadium, including its ability to cause chronic neurological diseases, are not well understood (61). Accordingly, researchers have demonstrated the neurotoxic effects of vanadium compounds such as vanadyl sulfate on neurons, including cholinergic neurons in the substantia nigra of the brain in rats, suggesting a role for this compound in the development of Parkinson’s disease (55, 61). Metavanadate and orthovanadate also proved to be toxic for neuronal and astroglial cells in rats, due to a strong inhibitory effect on Na+-K+-ATPases and by strong inhibition of neuronal aconitase, respectively (Fig. 1) (60). The researchers also observed the pro-apoptotic effect of vanadyl sulphate on neurons in a mechanism dependent on ROS production and regulation of the signaling of cytochrome C and caspases 9 and 3 (60). On the other hand, studies have shown the neuroprotective properties of vanadyl sulphate in restoring acetylcholinesterase activity in hypoxia-treated hippocampal cholinergic neurons in rats with alloxan-induced diabetes in response to hypoxia in a mechanism dependent on phosphotyrosine phosphatase inhibition (Fig. 1) (43, 55). VO(OPT), has powerful neuroprotective action for brain ischemic injury. Peripheral administration of VO(OPT) is potentially relevant to therapeutic treatment of brain ischemia. VO(OPT) (at doses of 1.0, 2.5 and 5.0 mg/kg of body weight of intraperitoneral injection) preferentially inhibit expression of Fas-ligand through, thereby inhibiting apoptosis (60). The neuroprotective effect of vanadate compounds on cerebral blood flow may also be associated with protein kinase B phosphorylation (Fig. 1) (52).
VANADIUM SUPPLEMENTS IN OTHER DISORDERS
Vanadium is the subject of several studies on bone and lipid metabolism as well as viral infections, including HIV (33, 62). Due to the similarity of vanadate salts to phosphate, vanadium (BEOV) accumulates in the bones, and thus affected bone metabolism in in vitro studies in rats through a direct stimulatory effect on osteoblasts (33). Oxovanadium may also exhibit contraceptive properties through reduction of sperm motility, prompting researchers to attempt the development of two-phase contraceptives with an anti-HIV effect, due to the fact that sperm cells are one of the factors involved in the transfer of the virus into the hosting body (62). This would be an effective approach for the prevention of type 1 virus transmission through sexual intercourse, and explains why two representative oxovanadium-thiourea (OVT) non-nucleoside reverse transcriptase inhibitors (NNIs: 1(N-(2-(2-chlorophenethyl))-N0-(2-(5-bromopyridyl)-thiourea)) and 2(N-(2-(2-methoxyphenethyl))-N0-(2-(pyridyl)-thiourea))) currently used to block HIV reverse transcriptase and the replication of the virus (whether in animals or humans) are complexed with oxovanadium (62, 63) Moreover, this approach reduced drug toxicity and sperm motility (62).
THE TOXICITY OF VANADIUM COMPOUNDS
Vanadium and its salts have therapeutic properties and are recommended as dietary supplements for patients with diabetes, bodybuilders and athletes in doses of about 60 mg/day by various National Institutes of Health (27, 39, 64). Toxicological studies on rats also showed no toxic effects at therapeutic doses (5, 28). On the other hand, high concentrations of vanadium compounds demonstrate toxic properties in the inhibition of several enzymes, including oxidative phosphorylation (3). Studies on animals have revealed that the toxic effect depends on the compounds, dose, time of administration and the degree of oxidation of vanadium ions (Table 1) (57, 65-67). In humans, vanadate-related acute or chronic poisoning, affecting the respiratory and digestive system and causing heart palpitations, exhaustion, depression, trembling fingers and hands and a characteristic green tongue, has been observed (Table 1) (3). The acute systemic toxicity of vanadium leads to the inhibition of respiratory processes in cells as a result of oxidative metabolism impairment and suppression of enzyme chains, or through exhibiting potential chemical asphyxiant properties (Table 1) (68). Organic vanadium compounds are more secure in experimental animals (5, 28). The acute toxicity of vanadium may be revealed by a slight degeneration of the renal tubules, congestion in the liver and intestinal inflammation (5). Exposure to high doses of vanadium compounds may also exert a toxic effect on the cardiovascular system, impair the function of the liver and cause gastrointestinal symptoms, including diarrhea and vomiting, leading to decreased fluid and food intake, dehydration and weight reduction (Table 1) (5, 8, 28, 30, 41, 57, 69). Furthermore, in vitro studies have shown that high doses of vanadium can initiate changes in hematopoiesis, show nephrotoxic, teratogenic and hepatotoxic activity, induce lipid peroxidation and cause degenerative changes in the respiratory system (15, 28, 65). There are several publications including data concerning the function of vanadium compounds on humans, indicating that vanadium chloride, vanadyl sulfate, meta- and orthovanadate (9.2 mg/kg of vanadium for each compound) significantly reduce the number of red blood cells (RBC) in humans, impairing their ability to deform, and inducing peroxidative changes in erythrocyte membranes, resulting in hemolysis and shortened RBC survival (Table 1) (43, 55, 70). The toxicity of vanadium compounds rises with increasing ion oxidation; hence vanadium in a +5 oxidation state appears to be most toxic (3). Some studies have also proven the genotoxicity of vanadium compounds, mainly in the +4 and +5 oxidation states, resulting in an occurrence of chromosomal aberrations, DNA-strand breakage and hydroxylation of guanosine causing the generation of free radical production (Table 1) (3, 17, 30, 71, 72). However, the researchers emphasize that this phenomenon was observed in vitro, in conjunction with the use of high doses of vanadium which are not possible to achieve in vivo (65).
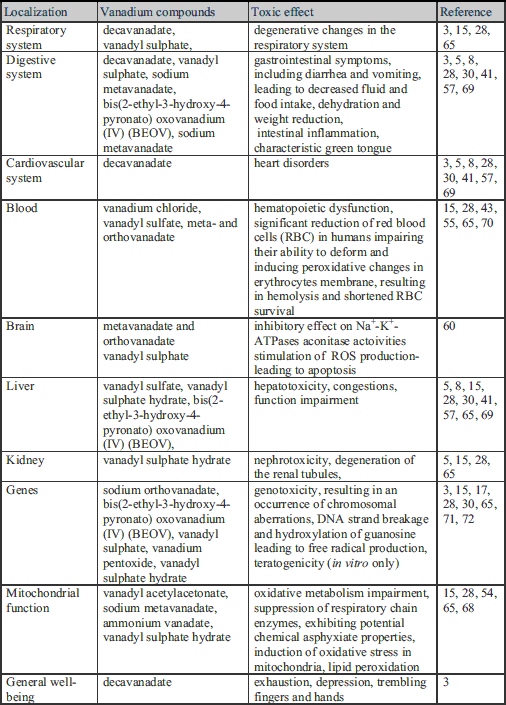
The toxic effect of vanadium is observed mainly on mitochondrial function (Fig. 1). Vanadium compounds induced oxidative stress in mitochondria and thus caused PTP opening, which led to the collapse of mitochondrial transmembrane potential (DWm) and cytochrome c (Cyt c) release as the initiation of cell apoptosis in rat livers (54). However, the toxic contribution depends on compounds of vanadium in solutions. Hence, in animal studies, decavanadate was a more potent mitochondrial depolarization agent and a more potent inhibitor of mitochondrial oxygen consumption than monomeric vanadate (67). Vanadate toxicity also occurred via two distinct pathways, one dependent on and one independent of H2O2 production, which can be blocked by catalase or glutathione (73).
Research conducted on individuals chronically exposed to vanadium showed no changes in blood morphology, the activity of enzymes or other biochemical parameters, which may result from the rapid transportation of vanadium and its derivatives from the blood to the tissues (27, 30, 65). Importantly, in clinical studies on patients with type 1 and type 2 diabetes, oral doses of vanadyl sulphate (100–150 mg/dl) and sodium metavanadate (125 mg/dl) (in 2–6 week courses) evoked transient gastrointestinal discomfort in the majority of patients (44-49,74).
PROSPECTS FOR DETERMINING USAGE OF VANADIUM AND ITS SALTS IN HUMANS
The best-known properties of vanadium compounds are their insulin-mimetic effects (28), while their ability to regulate lipid metabolism, including lowering triglicerides and total cholesterol and increasing HDL, creates opportunities for the use of vanadium salts in epidemics of obesity, metabolic syndrome or diabetes (75). Since the occurrence of diabetes is constantly rising, exploration of an effective treatment which would serve as a supplement to insulin therapy (which sometimes is ineffective and life-threatening, and may induce insulin resistance) is still underway (3, 28, 76). Additionally, the combination of actions of several novel organic and inorganic vanadium compounds with other newly synthetized pro-apoptotic and antioxidant derivatives may be future alternative supplements used in chemotherapy regime (37, 58, 77, 78). The data on both animal and human models seem so promising that in the near future supplements of vanadium and its salts may be widely recommended for patients. Nevertheless, we still do not know the long-term effects of vanadium treatment; therefore, further studies are required to determine its toxicity, therapeutic intervals and toxic doses in different groups of patients (28). It is also necessary to determine which human body fluid is optimal for the analysis of vanadium and its metabolite concentration in order for the results to have therapeutic relevance.
Acknowledgements: This work was supported by ST-57 and MN-116.
Conflict of interests: None declared.
REFERENCES
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Biochemical and medical importance of vanadium compounds. Acta Biochim Pol 2012; 59: 195-200.
- Shechter Y, Goldwaser I, Mironchik M, Fridkin M, Gefel D. Historic perspective and recent developments on the insulin-like actions of vanadium; toward developing vanadium- based drugs for diabetes. Coord Chem Rev 2003; 237: 3-11.
- Urban J, Antonowicz-Juchniewicz J, Andrzejak R. Wanad - zagrozenia i nadzieje. Medycyna Praktyczna 2001; 52: 125-133.
- Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M. Vanadium - an element of atypical biological significance. Toxicol Lett 2004; 150; 135-143.
- [No authors listed]. Vanadium/Vanadyl sulphate. Alternative Med Rev 2009; 14: 177-180.
- Ozturk N, Olgar Y, Ozdemir S. Trace elements in diabetic cardiomyopathy: an electrophysiological overview. World J Diabetes 2013; 4: 92-100.
- Arya GS, Hedaytullah MD, Yadav RA, Sachan K. Treating diabetes mellitus with vanadium salts - a future prospectus: Int J Pharm Sci Rev Res 2011; 8: 183-185.
- Afkhami-Ardekani M, Karimi M, Mohammadi SM, Nourani F, Soheilikhah S. Comparison of the effects of sodium metavanadate and zinc sulfate supplementation an lipid and glucose in patients with type 2 diabetes. Iran J Diabetes Obes 2009; 1: 22-29.
- Mehdi MZ, Srivastava AK. Organo-vanadium compounds are potent activators of the protein kinase B signalling pathway and protein tyrosine phosphorylation: mechanism of insulinomimesis. Arch Biochem Biophys 2005; 440: 158-164.
- Goldwasar I, Gefel D. Gershonov E, Fridkin M, Shechter Y. Insulin-like effects of vanadium: basic and clinical implications. J Inorg Biochem 2000; 80: 21-25.
- Suenaga A, Ueki H. Effect of orthovanadate on platelet aggregation induced by platelet-activating factor. Biol Pharm Bull 2004; 27: 1859-1863.
- Wei D, Li M, Ding W. Effect of vanadate on gene expression of the insulin signalling pathway in skeletal muscle of streptozotocin-induced diabetic rats. J Biol Inorg Chem 2007; 12: 1265-1273.
- Park SJ, Youn C, Hyun JW, You HJ. The anti-obesity effect of natural vanadium-containing Jeju ground water. Biol Trace Elem Res 2013; 151: 294-300.
- Huang M, Wu Y, Wang N, Wang Z, Zhao P, Yang X. Is the hypoglycemic action of vanadium compounds related to the suppression of feeding? Biol Trace Elem Res 2014; 157: 242-248.
- Aureliano M, Gandara RM. Decavanadate effects in biological systems. J Inorg Biochem 2005; 99: 979-985.
- Thompson KH, Orvig C. Vanadium in diabetes - 100 years from Phase 0 to Phase I. J Inorg Biochem 2006; 100: 1925-1935.
- Takada Y, Hashimoto M, Kasahara J. Cytoprotective effect of sodium orthovanadate on ischemia/reperfusion-induced injury in the rat heart involves Akt activation and inhibition of fodrin breakdown and apoptosis. J Pharmacol Exp Ther 2004; 311: 1249-1255.
- Marzban L, McNeill JH. Insulin-like actions of vanadium: potential as a therapeutic agent. J Trace Elem Exp Med 2003; 16: 253-267.
- Willsky GR, Chi LH, Liang Y, Gaile DP, Hu Z, Crans DC. Diabetes-altered gene expression in rat skeletal muscle corrected by oral administration of vanadyl sulphate. Physiol Genomics 2006; 26: 192-201.
- Barceloux DG. Vanadium. Clin Toxicol 1999; 37: 265-278.
- Bose S, Farah MA, Jung H, Lee JH, Kim Y. Molecular mechanism of bis(maltolato)oxovanadium (IV)-induced insulin signalling in 3T3-L1 and IM9 cells: impact of dexamethasone. J Mol Endocrinol 2007; 38: 627-649.
- Hiromura M, Sakurai H. Action mechanism of metallo-allixin complexes as antidiabetic agents. Pure Appl Chem 2008; 12: 2727-2733.
- Kawabe K, Yoshikawa Y, Adachi Y, Sakurai H. Possible mode of action for insulinomimetic activity of vanadyl(IV) compounds in adipocytes. Life Sci 2006; 78: 2860-2866.
- Li SH, McNeill JH. in vivo effects of vanadium on GLUT4 translocation in cardiac tissue of STZ-diabetic rats. Mol Cell Biochem 2001; 217: 121-129.
- Seale AP, de Jesus LA, Park M, Kim YS. Vanadium and insulin increase adiponectin production in 3T3-L1 adipocytes. Pharmacol Res 2006; 54: 30-38.
- Garcia-Vicente S, Yraola F, Marti L, et al. Oral insulin-mimetic compounds that act independently of insulin. Diabetes 2007; 56: 486-493.
- Ivancsits S, Pilger A, Diem E, Schaffer A, Rudiger HW. Vanadate induces DNA strand breaks in cultured human fibroblasts at doses relevant to occupational exposure. Mutat Res 2002; 519: 25-35.
- Srivastava AK, Mehdi MZ. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabets Med 2005; 22: 2-13.
- Zhao Y, Ye L, Liu H, et al. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J Inorg Biochem 2010; 104: 371-378.
- Thompson K.H, Lichter J, LeBel C, Scaife MC, McNeill JH, Orvig C. Vanadium treatment of type 2 diabetes - a view to the future. J Inorg Biochem 2009; 103: 554-558.
- Shafrir E, Spielman S, Nachliel I, Khamaisi M, Bar-On H, Ziv E. Vanadium salts versus diabetes: an overview. Diabetes Metab Res Rev 2001; 17: 55-66.
- Gonzalez-Villalva A, Pinon-Zarate G, De la Pena Diaz A, et al. The effect of vanadium on platelet function. Environ Toxicol Pharmacol 2011; 32: 447-456.
- Facchini DM, Yuen VG, Battell ML, McNeill JH, Grynpas MD. The effects of vanadium treatment on bone in diabetic and non-diabetic rats. Bone 2006; 38: 368-377.
- Bhuiyan MS, Fukunaga K. Cardioprotection by vanadium compounds targeting Akt-mediated signalling. J Pharmacol Sci 2009; 110: 1-13.
- Gonzalez-Sanchez C, Bermudez-Pena C, Guerrero-Romero F, Trenzado CE, Montes-Bayon M, Sanz-Medel A, Llopis J. Effect of bis(maltolato)oxovanadium (IV) (BMOV) on selenium nutritional status in diabetic streptozotocin rats. Brit J Nutr 2012; 108: 893-899.
- Sanchez-Gonzalez C, Lopez-Chaves C, Trenzado CE, et al. Changes in iron metabolism and oxidative status in STZ induced-diabetic rats treated with bis(maltolato)oxovanadium (IV) as an antidiabetic agent. ScientificWorldJournal 2014; 2014: 706074.
- Dabros W, Adamczyk A, Ciurkot K, KordowiakAM. Vanadium compounds affect growth and morphology of human rhabdomyosarcoma cell line. Pol J Pathol 2011; 4: 262-268.
- Bhuiyan S, Shioda N, Shibuya M. Iwabuchi Y, Fukunaga K. Activation of endothelial nitric oxide synthase by a vanadium compound ameliorates pressure overload-induced cardiac injury in ovariectomized rats. Hypertension 2009; 53: 57-63.
- Bhuiyan S, Shibuya M, Shioda N. Cytoprotective effect of bis(1-oxy-2-pyridinethiolato)oxovanadiun (IV) on myocardial ischemia/reperfusion injury elicits inhibition of Fas ligand and Bim expression and elevation of FLIP expression. Eur J Pharmacol 2007; 571: 180-188.
- Liem DA, Gho CC, Gho BC, et al. The tyrosine phosphatase inhibitor bis(maltolato)-oxovanadium attenuates myocardial reperfusion injury by opening ATP-sensitive potassium channels. J Pharmacol Exp Ther 2004; 309: 1256-1262.
- Desoize B. Metals and metal compounds in cancer treatment. Anticancer Res 2004; 24: 1529-1544.
- Rodriguez-Mercado JJ, Roldan-Reyes E, Altamirano-Lozano M. Genotoxic effects of vanadium (IV) in human peripheral blood cells. Toxicol Lett 2003; 144: 359-369.
- Suwalsky M, Fierro P, Villena F, et al. Human ertythocytes and neuroblastoma cells are in vitro affected by sodium orthovanadate. Biochim Biophys Acta 2012; 1818: 2260-2270.
- O’Connell B. Select vitamins and minerals in the management of diabetes. Diabetes Spectrum 2001; 14: 133-148.
- Boden G, Chen X, Ruiz J, van Rossum GD, Turco S. Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism 1996; 45: 1130-1135.
- Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1995; 95: 2501-2509.
- Halberstam M, Cohen N, Shlimovich P, Rossetti L, Shamoon H. Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes 1996; 45: 659-666.
- Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003; 26: 1277-1294.
- Goldfine AB, Simonson DC, Folli F, Patti ME, Kahn CR. Metabolic effects of sodium metavanadate in humans with insulin-dependent and noninsulin-dependent diabetes mellitus in vivo and in vitro studies. J Clin Endocr Metab 1995; 80: 3311-3320.
- Ingram JL, Antao-Menezes A, Turpin EA, et al. Genomic analysis of human lung fibroblasts exposed to vanadium pentoxide to identify candidate genes for occupational bronchitis. Respir Res 2007; 8: 1-13.
- Fraqueza G, Ohlin CA, Casey WH, Aureliano M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J Inorg Biochem 2012; 107: 82-89.
- Hasegawa Y, Hamada J, Morioka M, et al. Neuroprotective effect of postischemic administration of sodium orthovanadate in rats with transient middle cerebral artery occlusion. J Cerebr Blood Flow Metab 2003; 23: 1040-1051.
- Wu Y, Huang M, Zhao P, Yang X. Vanadyl acetylacetonate upregulates PPARγ and adiponectin expression in differentiated rat adipocytes. J Biol Inorg Chem 2013; 18: 623-631.
- Zhao P, Yang X. Vanadium compounds modulate PPARg activity primarily by increasing PPARγ protein levels in mouse insulinoma NIT-1 cells. Metallomics 2013; 5: 836-43.
- Suwalsky M, Fierro P, Villena F, et al. Effects of sodium metavanadate on in vitro neuroblastoma and red blood cells. Arch Biochem Biophys 2013; 535: 248-256.
- Shehzad S. The potential effect of vanadium compounds on glucose-6-phosphatase. Bioscience Horizons 2013; 6: 1-11.
- Evangelou MA. Vanadium in cancer treatment. Crit Rev Oncol Hematol 2002; 42: 249-265.
- Holko P, Ligeza J, Kisielewska J, Kordowiak AM, Klein A. The effect of vanadyl sulphate (VOSO4) on autocrine growth of human epithelial cancer cell lines. Pol J Pathol 2008; 59: 3-8.
- Clancy HA, Sun H, Passantino L, et al. Gene expression changes in human lung cells exposed to arsenic, chromium, nickel or vanadium indicate the first steps in cancer. Metallomics 2012; 4: 784-793.
- Shioda N, Ishigami T, Han F, et al. Activation of phosphatidyloinositol 3-kinase/protein kinase B pathway by a vanadyl compound mediates its neuroprotective effect in mouse brain ischemia. Neuroscience 2007; 148: 221-229.
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, et al. Vanadium induces dopaminergic neurotoxicity via protein kinase C delta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol 2009; 240: 273-285.
- D’Cruz OJ, Dong Y, Uckun FM. Potent dual anti-HIV and spermicidal activities of novel oxovanadium(V) complexes with thiourea non-nucleoside inhibitors of HIV-1 reverse transcriptase. Biochem Biophys Res Commun 2003; 302: 253-264.
- Mohammad A. Various synthetic organic compounds with spermicidal activity. J Int Med Res 2014; 2: 1-14.
- www.nih.gov
- Villani P, Cordelli E, Leopardi P, et al. Evaluation of genotoxicity of oral exposure to tetravalent vanadium in vivo. Toxicol Lett 2007; 170: 11-18.
- Tang H, Sun Y, Xiu Q, Lu H, Han H. Cyclooxygenase-2 induction requires activation of nuclear factor of activated T-cells in Beas-2B cells after vanadium exposure and plays an anti-apoptotic role. Arch Biochem Biophys 2007; 468: 92-99.
- Soares SS, Gutierrez-Merino C, Aureliano M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J Inorg Biochem 2007; 101: 789-796.
- Boulassel B, Sadeg N, Roussel O, Perrin M, Belhadj-Tahar H. Fatal poisoning by vanadium. Forensic Sci Int 2011; 206: 79-81.
- Smith DM, Pickering RM, Lewith GT. A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus. Int J Med 2008; 101: 351-358.
- Hogan GR. Comparative erythropoietic effects of three vanadium compounds. Sci Total Environ 2000; 256: 185-189.
- Thompson KH, McNeil JH. Effect of vanadyl sulfate feeding on susceptibility to peroxidative change in diabetic rats. Res Commun Chem Pathol Pharmacol 1993; 80: 187-200.
- Montiel-Davalos A, Gonzalez-Villava A, Rodriguez-Lara V, Montano LF, Fortoul TI, Lopez-Marure R. Vanadium pentoxide induces activation and death of endothelial cells. J Appl Toxicol 2012; 32: 26-33.
- Capella MA, Capella LS, Valente RC. Vanadate-induced cell death is dissociated from H2O2 generation. Cell Biol Toxicol 2007; 23: 413-420.
- Cusi K, Cukier S, Defronzo RA, Torres M, Puchulu FM, Redondo JC. Vanadyl sulfate improves hepatic and muscle insulin sensitivity in type 2 diabetes. J Clin Endocriol Metab 2001; 86: 1410-1417.
- Leon IE, Butenko N, Di Virgilio AL, et al. Vanadium and cancer treatment: antitumoral mechanisms of three oxidovanadium (IV) complexes on a human osteosarcoma cell line. J Inorg Biochem 2013; 127: 1-12.
- Wender-Ozegowska E, Zawiejska A, Michalkowska-Wender G, Iciek R, Wender M, Brazert J. Metabolic syndrom in type 1 diabetes mellitus. Does it have any impact on the course of pregnancy? J Physiol Pharmacol 2011; 62: 567-573.
- Toton E, Ignatowicz E, Bernard MK, Kujawski J, Rybczynska M. Evaluation of apoptotic activity of new condensed pyrazole derivatives. J Physiol Pharmacol 2013; 64: 115-123.
- Reiter RJ, Tan DX, Korkmaz A, Fuentes-Broto L. Drug-mediated ototoxicity and tinnitus: alleviation with melatonin. J Physiol Pharmacol 2011; 62: 151-157.
A c c e p t e d : August 19, 2014