SKELETAL MUSCLE WASTING OCCURS IN ADULT RATS UNDER CHRONIC TREATMENT WITH PARACETAMOL WHEN GLUTATHIONE-DEPENDENT DETOXIFICATION IS HIGHLY ACTIVATED
INTRODUCTION
The function of skeletal muscle is to ensure posture and locomotion, and this tissue is the main reservoir of body proteins. This reservoir is mobilized in case of disease, providing amino acids to tissues involved in the physiological defense necessary for recovery (1, 2). Muscle wasting is known to occur in a wide range of conditions like chronic and inflammatory diseases, renal failure, cachexia or cancer as well as physical inactivity or old age. When advanced, muscle loss potentiates morbidity and increases mortality (3-6). Consequently, preserving and maintaining muscle mass is an important challenge, especially when ensuring optimal defense in case of disease.
Diseases are often associated with acute or chronic pain and fever and the first-line treatment recommended for pain management is paracetamol (acetaminophen, APAP) (7, 8). APAP is therefore the analgesic and antipyretic drug the most commonly used in Europe/USA (9-11) and is considered as safe (7). APAP metabolism in the liver consists mainly in phase II detoxification (up to 90%) by direct sulfate or glucuronide conjugation of APAP. In phase I metabolism (up to 10%) APAP is converted by cytochrome P450 in the highly reactive compound N-acetyl-p-benzoquinone imine (NAPQI) which is readily detoxified by conjugation with glutathione (GSH) then converted to cysteine (Cys) and mercapturate conjugates (12, 13). Cys loss is therefore expected to increase with APAP consumption as its metabolism is associated with irreversible utilization of Cys. Cys is a sulfur amino acid (SAA) which can be synthesized from methionine and serine. Cys is mainly used for proteins and GSH synthesis and is also the precursor of taurine and sulfate (14). Up to 40% of APAP intake can be metabolized as sulfur containing compounds at therapeutic dosage (13).
Physiological disruptions due to APAP have been shown in growing rats or mice (15-17). In the liver, where APAP detoxification mainly occurs, reductions of hepatic protein synthesis and GSH concentration have been observed in growing mice fed a 0.6% APAP diet (w/w)(16). These data support the idea that competition occurs in the liver between the use of Cys for detoxification of chronic administration of APAP and the other uses of Cys. Splanchnic or gastrointestinal first-pass extractions of dietary Cys represent 40–60% of the dietary intake (18, 19). Hepatic Cys use for APAP detoxification would increase the already important splanchnic first-pass extraction and lead to a decrease in peripheral Cys availability for muscle protein synthesis. Liver GSH is known to be exported and is considered as a significant supplier of GSH and Cys to other tissues (20-22). Therefore, indirectly, APAP-induced depletion of hepatic GSH would worsen the decrease in peripheral Cys availability.
Effects of chronic treatment with APAP at whole body level have been shown in growing rats or mice fed APAP-containing diets for 2–3 weeks. Studies using diets supplemented with 0.8% or 1% of APAP showed that rodents failed to grow (15, 16). Furthermore, a decrease in the nutritional utilization of dietary proteins has been reported in growing rats submitted to long-term APAP treatment (17). It was hypothesized that in the presence of APAP, Cys became conditionally indispensable in young growing rats for which the Cys requirement for tissue growth was elevated.
However, it is questionable as to whether such negative effects of chronic treatment with APAP would also occur in adults who exhibit milder needs for protein metabolism than growing rodents. The aim of the study was to provide an integrative view of the relation between the quantitative irreversible loss of sulfur for APAP detoxification and APAP-induced alterations regarding protein and GSH contents in various organs and tissues in adults. We hypothesized that increasing APAP in the diet of adult rats would decrease Cys availability in peripheral tissues inducing muscle loss. In this study, in addition to muscle mass, GSH and protein contents were quantified in skeletal muscle, liver and small intestine being quantitatively important tissues in terms of GSH and protein metabolisms. In order to analyze how these expected alterations could be related to the irreversible loss of sulfur, urinary APAP metabolites were quantified. Arterial plasma Cys concentration was measured as an index of peripheral Cys bioavailability. Finally, dietary intake along with feces and urine collections allowed for N balance, a complementary assessment of protein homeostasis, to be calculated.
MATERIALS AND METHODS
Animals and experimental design
This study was conducted in 2010 in accordance with the Directive 2010/63/EU of the European Parliament and the Council on the Protection of Animals used for Scientific Purposes. The experimental protocol conformed to the 3R (replacement, reduction, refinement) rule.
Since APAP-induced metabolic alterations were expected to be dependent on dosage, two doses were chosen: 0.5% and 1% w/w of APAP in the diet. These doses were designed to be respectively around and largely above the saturation of APAP sulfation (23, 24). Therefore the contribution of the GSH-dependent detoxification pathway to total APAP detoxification was expected to be higher with the 1% APAP diet than with the 0.5% APAP diet (24, 25). This would allow to assess if APAP-induced metabolic alterations were quantitatively linked to sulfur losses for APAP detoxification purposes. Of note, as the maximum therapeutic daily dose is 4 g/d for humans and the mean dry food intake is 430 g/d in the French population (26), the maximum human dose of APAP corresponds to 0.9% of the dry food intake. Consistently, the 1% APAP diet has already been considered as corresponding to the maximum therapeutic dose for humans (15).
Before APAP treatment, male 4-month-old Wistar rats (Janvier Labs, Saint Berthevin, France), were acclimatized for 2 weeks in individual cages in standard conditions (22 ± 1°C, 12 h light/dark cycle) with free access to water and standard laboratory powder food (A04 from SAFE Scientific Animal Food and Engineering, Villemoisson-sur-orge, France). The composition of the diet was 16% proteins, 3% fat, 60% carbohydrates, 12% water, 4% fiber, 5% vitamins and minerals. The dietary methionine content was 25.5 µmol/g and Cys 21.6 µmol/g. During the treatment period, APAP (Sigma, L’Isle d’Abeau, France) was mixed with the powdered A04 diet at 0, 0.5 and 1% on a weight basis.
Rats were randomly divided into three groups, housed in individual cages, and received 0% APAP (n = 6; body weight 465 ± 10 g), 0.5% APAP (n = 7; body weight 440 ± 8 g) or 1% APAP (n = 7; body weight 452 ± 12 g) diets. Body weights were not significantly different between groups. All groups were allowed free access to water and food during the 17-day treatment period. Similar treatment lengths had already been used in growing rats (15, 16). Body weight and food consumption were recorded 2 to 3 times a week. During the last 6 days of treatment (D12 to D17) the rats were moved into individual metabolic cages (E5MCENS906, Charles River, L’Arbresle, France) to allow for separation of feces from urine. Daily urine and feces recovered in respective collecting tubes on D15, D16 and D17 were pooled, weighed and stored at –80°C before analysis. Prior to urine collection, 5 ml of 1 M hydrochloric acid were put into the collecting tube each morning. At the end of the experiment, all animals were euthanized under pentobarbital anesthesia (50 mg/kg, intraperitoneal injection) by aortic blood sampling. An aliquot of blood was frozen in liquid nitrogen. Plasma was separated by centrifugation at 2000 × g for 10 min at 4°C and immediately frozen in liquid nitrogen. Liver and small intestine (SI) were immediately removed, saline washed, weighed, and frozen in liquid nitrogen. Skeletal muscles: gastrocnemius (GM), tibialis anterior (TA), soleus (SOL) and extensor digitorum longus (EDL) were carefully dissected from the left posterior leg, weighed, and then GM was frozen in liquid nitrogen. All the samples frozen in liquid nitrogen were subsequently kept at –80°C before analysis. The remains of each rat (i.e. carcass) were frozen and kept at –20°C until analysis. Frozen tissues were finely pulverized in liquid nitrogen using a ball mill (Dangoumeau, Prolabo, Paris, France) prior to analysis.
Nitrogen balance
Nitrogen (N) was quantified in thawed urine and freeze-dried feces collected from D15 to D17 and powdered diets using the Kjeldahl method (Kjeldatherm block digestion system and Vapodest distillation system, Gerhardt, Les Essarts le Roi, France). N balance was calculated as: ingested N - (urinary N + fecal N) and expressed as mg per 3 days (mg/3d).
Glutathione content
Total GSH (reduced plus oxidized) was quantified with a spectrophotometer using a standard enzymatic recycling procedure and 5,5’-dithio-bis-2-nitrobenzoic acid (Ellman reagent) as oxidant as previously described (27). Total GSH was expressed as µmol per organ or muscle.
Protein content
Protein concentration was quantified after NaOH digestion of powdered tissue using a bicinchoninic acid assay reagent kit, as previously described (28). Bovine serum albumin was used as standard. Total proteins were expressed as g or mg per organ or muscle. Total whole body proteins were calculated as the product of body weight and protein concentration of rat remains and expressed in g.
Plasma biochemical analyses
Aspartate transaminase (AST) and alanine transaminase (ALT) activities were quantified in plasma using an ABX Pentra 400 analyzer (Horiba, Montpellier, France) and test kits A11A01629 and A11A01627 (Horiba), respectively.
Total non-protein cyst(e)ine (Cys plus cystine plus Cys bound to proteins and to small molecules through disulfide bridges) was measured with a colorimeter in plasma treated with dithiothreitol before deproteinization (29, 30). Free cyst(e)ine (Cys plus cystine plus Cys bound to small molecules) was quantified with the same method however dithiothreitol treatment was performed after protein precipitation. Then the ratio of free cyst(e)ine to total non-protein cyst(e)ine was calculated and expressed in percent.
Quantification of urinary APAP metabolites
APAP and its metabolites were quantified in 3-day urine samples using an Agilent 1100 HPLC liquid chromatography system (Agilent Technologies, Palo Alto, CA, USA) coupled to an API 2000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA, USA), as previously described (31). Quantification was performed using calibration curves with external standards. APAP and its sulfate conjugate (APAP-S) were obtained from Sigma-Aldrich (L’Isle d’Abeau, France), APAP glucuronide (APAP-G) from Chemos GmBH (Regenstauf, Germany), N-acetylcysteine-paracetamol (APAP-NAC) and cysteine-paracetamol (APAP-Cys) from Toronto Research Chemical (Brisbane Road, Toronto, Canada). Absolute quantities of urinary APAP and its metabolites were calculated as the product of their concentrations and the 3-day urine volume for each rat. They were expressed as µmole or mmole per 3 days (µmol/3d or mmol/3d, respectively).
Statistical analysis
Results are presented as medians with standard errors of the median (S.E.) or as box plots. The Mann-Whitney U test was used to analyze differences between two groups. This non-parametric test was used since variances were not homogeneous for some of the variables (F-test, P>0.05) and the number of rats per group was 6 to 7. Statistical analyses were performed using the software R 3.0.2. P values <0.05 were considered significant. The Hodges Lehman estimator related to the Mann-Whitney U test (which estimates the difference between the medians of the two groups to be compared) was used to express all the percentages of increase or decrease in one group with respect to another (100 times the Hodges Lehman estimate divided by the median of the relative group (32)).
RESULTS
Food and APAP intakes
There were no significant differences in daily food intakes between the 0% APAP and 0.5% APAP groups throughout the treatment (Table 1). On D1-D2, food intake was 33% lower in the 1% APAP group than the 0% APAP group (P=0.003). This effect was transient since there were never any significant differences in food intakes between the 0% APAP and the 1% APAP groups from D3 to D17. During the N balance period (d15-d17), there were no significant differences in food intakes between the 3 groups. Total ingested APAP during the 17-day treatment was 0, 2.0 ± 0.1 and 3.7 ± 0.3 g in the 0% APAP, 0.5% APAP and 1% APAP groups, respectively.
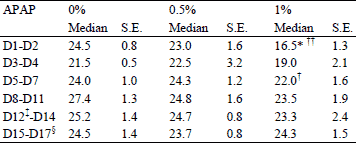
†P<0.05, †† P=0.003 vs. 0.5% APAP group, Mann-Whitney U test;
‡ the rats were housed into metabolic cages from D12 to D17 to allow for separation of feces from urine;
§ period of feces and urine collections.
Body weight, organs and skeletal muscles masses
Final body weights of the 0.5% APAP and 1% APAP groups were respectively 6% (P = 0.04) and 12% (P=0.01) lower than the 0% APAP group (Table 2). There was no significant difference in any of the organs and skeletal muscles studied between the 0% APAP and 0.5% APAP groups, neither between the 0.5% APAP and 1% APAP groups (Table 2). SI, GM, EDL masses and the sum of the four muscles studied were 13% (P=0.004), 8% (P=0.02), 13% (P=0.02), and 8% (P<0.05) lower in the 1% APAP group than the 0% APAP group.
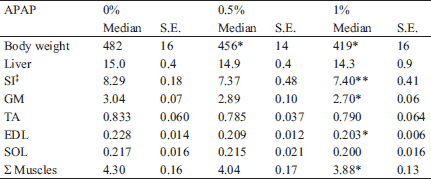
* P<0.05, ** P<0.005 vs. 0% APAP group, Mann-Whitney U test.
Body proteins and N balance
There was no significant difference in whole body proteins and N balance between the 0% APAP and 0.5% APAP groups (Fig. 1A and B). Whole body proteins and N balance were respectively 12% (P=0.04) and 37% (P=0.01) lower in the 1% APAP group than the 0% APAP group. During the 3-day N balance period rats ingested 0, 2.35 ± 0.07 and 4.83 ± 0.30 mmol of APAP in the 0% APAP, 0.5% APAP and 1% APAP groups, respectively. There was no significant difference in the amount of SAA ingested over the 3-day N balance period between the three groups (3.47 ± 0.02, 3.35 ± 0.11, 3.44 ± 0.21 mmol of SAA/3d in 0% APAP, 0.5% APAP and 1% APAP, respectively.
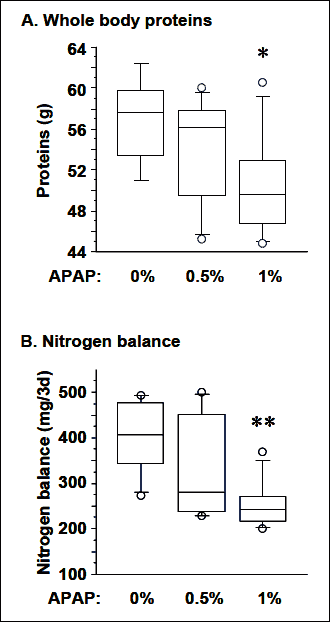 |
Fig. 1. Dose response to chronic treatment with APAP on the whole body proteins (A) and nitrogen balance (B) in adult rats. * P=0.04, ** P=0.01 vs. 0% APAP group, Mann-Whitney U test. |
Total glutathione and protein contents in organs and muscles
There was no significant difference in the blood concentration of total GSH between the 0% APAP group (711 ± 69 µM) and the 0.5% APAP group (747 ± 75 µM). Blood GSH reached 1169 ± 95 µM in the 1% APAP group, being 71% (P=0.003) and 59% (P=0.004) higher than in the 0% APAP and 0.5% APAP groups, respectively. There was no significant difference in the total GSH and protein contents in liver, SI and GM between the 0% APAP and 0.5% APAP groups (Fig. 2). Liver, SI and GM GSH contents were 63% (P=0.003), 19% (P=0.003) and 26% (P=0.010) lower in the 1% APAP group than the 0% APAP group, respectively. They were also 65% (P=0.002), 12% (P=0.01) and 12% (P=0.02) lower in the 1% APAP group than the 0.5% APAP group, respectively. There was no significant difference in the liver, SI and GM proteins between the 0% APAP and 0.5% APAP groups (Fig. 2). SI and GM protein contents were 7% (P<0.05 and P=0.03, respectively) lower in the 1% APAP group than the 0% APAP group. Liver protein content was 13% (P=0.01) lower in the 1% APAP group than the 0.5% APAP group.
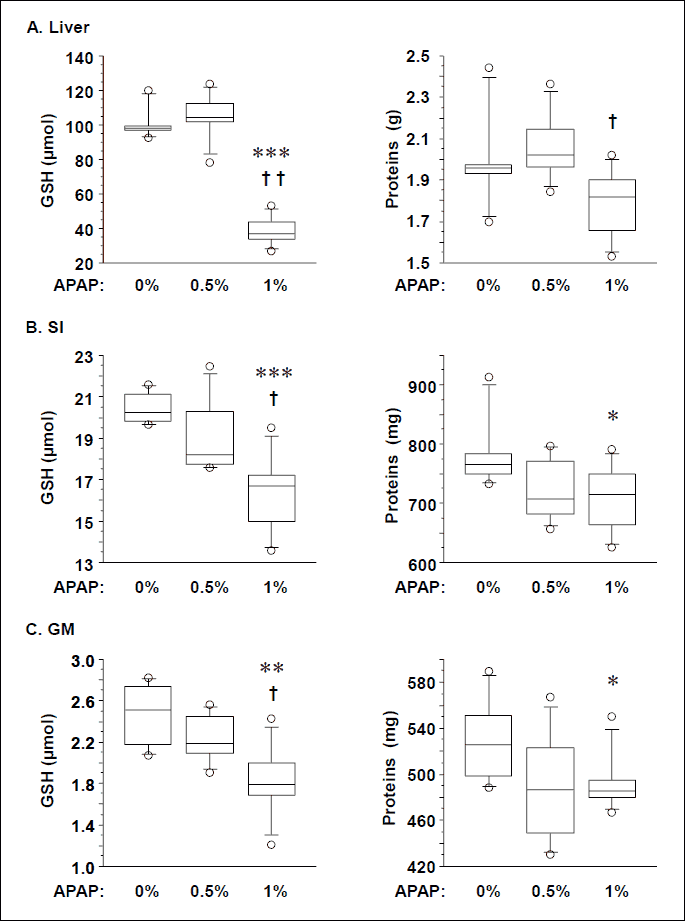
* P<0.05, ** P=0.01, *** P=0.003 vs. 0% APAP group, Mann-Whitney U test.
† P<0.02, †† P=0.002 vs. 0.5% APAP group, Mann-Whitney U test.
Urinary APAP metabolites
As expected neither APAP nor APAP metabolites were detected in the urine from the 0% APAP group. There was no significant difference in the urinary APAP-S amount between the 0.5% APAP and 1% APAP groups (Fig. 3). Urinary unmodified APAP, APAP-G, APAP-NAC and APAP-Cys were 158, 4404, 351 and 136% higher in the 1% APAP group than the 0.5% APAP group (P=0.002). Total urinary recovery of APAP was 2.27 ± 0.13 and 4.38 ± 0.45 mmol/3d in the 0.5% APAP and 1% APAP groups, respectively. These recoveries represented 98 ± 7% and 92 ± 2% of the amount ingested in the 0.5% APAP and 1% APAP groups (see above), respectively. Percentages of APAP excreted as unmodified APAP, APAP-G, APAP-S and through the GSH-dependent pathway were 3, 2, 91 and 5 in the 0.5% APAP group and 6, 41, 43 and 11% in the 1% APAP group, respectively. There was no significant difference in the total urinary sulfur-containing APAP metabolites between the two groups (2.05 ± 0.20 and 2.31 ± 0.21 mmol/3d in the 0.5% APAP and 1% APAP groups, respectively). The contribution of the GSH-dependent APAP metabolites (APAP-NAC plus APAP-Cys) to the total sulfur-containing APAP metabolites increased from 5 ± 1 in the 0.5% APAP group to 21 ± 1% in the 1% APAP group.
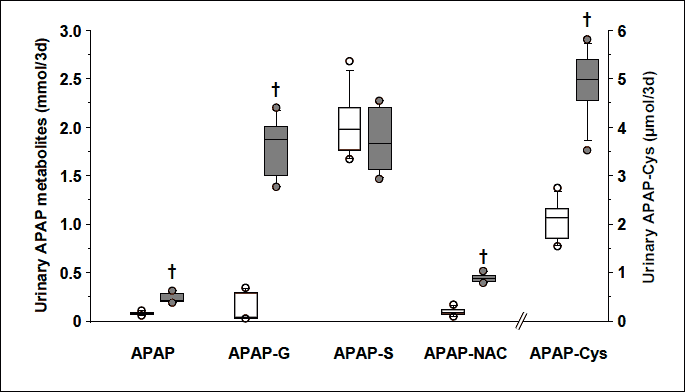
† P=0.002 vs. 0.5% APAP group, Mann-Whitney U test.
Plasma transaminase activities and cyst(e)ine concentration
There was no significant difference in the AST and ALT activities between the three groups, expect that ALT was slightly higher in the 0.5% APAP group than the 0% APAP group (+31%; P=0.02, Table 3).
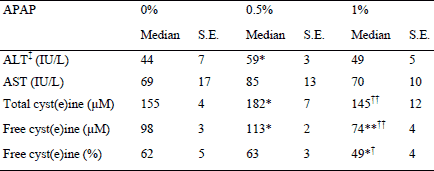
* P<0.03, ** P=0.003 vs. 0% APAP group, Mann-Whitney U test;
† P<0.04, †† P<0.01 vs. 0.5% APAP group, Mann-Whitney U test.
Total cyst(e)ine and free cyst(e)ine were 16% (P=0.01) higher in the 0.5% APAP group than the 0% APAP group as the percentage of free cyst(e)ine was unchanged (Table 3). Absolute and percent of free cyst(e)ine were respectively 33% (P=0.003) and 27% (P=0.02) lower in the 1% APAP than the 0% APAP group. Total cyst(e)ine, absolute and percent of free cyst(e)ine were 21% (P=0.007), 37% (P=0.003) and 25% (P=0.03) lower in the 1% APAP group than the 0.5% APAP group, respectively.
DISCUSSION
This study provides new data regarding the effect of APAP treatment on peripheral tissues, especially skeletal muscle. Alterations in tissue protein and GSH contents were analyzed in regards to the quantitative irreversible loss of Cys induced by chronic treatment with APAP in adult rats. The main result is that the highest studied dose, i.e. 1% APAP in the diet, which is indeed above the saturation of APAP sulfation (see below), induced muscle wasting. To our knowledge, no such effect has been previously quantified in any physiological state. In adult rats, muscle wasting was ascertained by decreases in skeletal muscle mass and protein content, and N balance in the 1% APAP group compared to the 0% APAP group. Muscle wasting occurred in the absence of hepatotoxicity as the low increase in ALT is definitively below the threshold levels usually considered for hepatotoxicity, i.e. 3 × control values of AST and ALT (33).
It is clear that muscle wasting induced by 1% APAP treatment did not result from an increase in total urinary excretion of sulfur-containing APAP metabolites, but was concomitant with the activation of the GSH-dependent detoxification pathway in the liver. It is important to stress that the origin of the urinary sulfur loss differed between the two APAP groups. APAP sulfation, which is a high affinity low-capacity reaction (34) known to be maximum at the dose of 1–2 mmol of APAP/kg of body weight (23, 24), was already the maximum in the 0.5% APAP group. Indeed, APAP-S did not differ between the two groups as the daily intake was 1.7 and 3.4 mmol of APAP/kg of body weight in the 0.5% APAP and 1% APAP groups, respectively. In the 1% APAP group, sulfation saturation induced a large increase, not only in APAP-G, but also in APAP-NAC and APAP-Cys, the two endproducts of the GSH-dependent detoxification pathway. A therapeutic dose of APAP has been shown to stimulate the turnover of hepatic GSH in vivo (35). Along the same lines, the activity of γ-glutamyl-cysteine synthetase, the limiting step in the GSH synthesis pathway, increased in liver-derived cells exposed to APAP (36). In the 1% APAP group, the use of Cy for GSH synthesis (then irreversibly lost in urine) probably increased and occurred at the expense of the synthesis of liver proteins. Such an effect has already been reported in the liver of APAP-treated mice (16). Therefore, a decrease in liver protein synthesis was probably the major mechanism by which the liver protein content decreased in the 1% APAP group. The decrease in the liver GSH content, a well-known effect of APAP (12), resulted from the fact that liver synthesis of GSH, in spite of its probable increase, was not sufficient to compensate for its total uses.
Liver GSH plays an important role in the inter-organ homeostasis of GSH and cyst(e)ine (21). About half of the SAA taken up by the liver is used for synthesis of GSH for export into plasma in rats (37). This continuous release of hepatic GSH into plasma and its extrahepatic hydrolysis (interorgan γ-glutamyl cycle) is an important mechanism for maintaining steady Cys availability in peripheral tissues (21, 38, 39). The large decrease in liver GSH observed in the 1% APAP group would have compromised its export and therefore the Cys availability for peripheral tissues. Concomitantly, the decrease in plasma free cyst(e)ine concentration, consecutive to its enhanced utilization in the liver from detoxification purposes, would have worsened peripheral Cys availability. Peripheral shortage of Cys would have limited both GSH and protein synthesis leading to the low muscle GSH and protein contents observed in muscle from the 1% APAP group.
Erythrocytes exhibit a much higher GSH concentration than plasma and seem to lack any cystine and GSH transport mechanism taking up only Cys (reduced form) for their needs (21). The mechanism by which blood GSH increased in the 1% APAP group could be an activation of either Cys (reduced form) uptake or intracellular GSH synthesis. Whatever the mechanism, a similar increase in blood GSH, along with a decrease in liver GSH, have been reported in rats fed a SAA-deficient diet (40). Blood GSH increase would therefore reinforce SAA deficiency for peripheral tissues.
At whole body level Cys is a conditional indispensable amino acid, i.e. it has to be present in the diet when its endogenous synthesis from methionine and serine cannot cover the metabolic demand. Cys may be indispensable for tissues exhibiting low or null activities for enzymes required for the synthesis of Cys from homocysteine, namely cystathionine synthase and cystathionase (41). To date mRNA of theses enzymes were not detected in rat or human skeletal muscle (42-44) and the activity of cystathionase appeared dramatically low in rat muscle (44). Endogenous synthesis of Cys would be null or negligible in muscle. In this study, where SAA intake was not significantly modified during APAP treatment, the increased utilization of Cys for APAP detoxification in the liver decreased the availability of Cys for peripheral tissues, as discussed above. Considering simultaneous availabilities of all the amino acids needed for protein synthesis, Cys deficiency may have limited protein synthesis in muscle, leading to an increase in the oxidation of the other amino acids, and therefore worsening of the N balance. The relevance of such a mechanism could be tested by supplementing the 1% APAP diet with Cys.
Other mechanisms could also have been involved in APAP-induced muscle wasting. Muscle homeostasis is known to depend on the equilibrium between protein synthesis and proteolysis and the equilibrium between regeneration and apoptosis. These processes are highly regulated through various signalling pathways (45, 46). Some of these pathways are sensitive to oxidative stress (47-50). Decreases in muscle GSH, the major intra-cellular anti-oxidant, and plasma free cyst(e)ine in the 1% APAP group are in favour of a pro-oxidative environment. Therefore, it is conceivable that part of the APAP-induced muscle wasting resulted from oxidative stress-driven alterations in protein turnover and/or muscle fibre renewal. The relevance of an oxidative stress-mediated mechanism for APAP-induced muscle wasting could be tested with any dietary supplementation aimed reducing oxidative stress. Indeed polyphenols, exhibiting anti-oxidants properties, have already been shown to decrease APAP-induced hepatotoxicity (51-53). In addition to anti-oxidants, new hepato-protective compounds, such as the pentadecapeptide BPC 157 (54), could also be tested.
The effect of the 1% APAP diet on body weight is consistent with the work performed in young growing rats fed with a diet containing 1% of APAP (15). The fact that a chronic treatment with APAP did not affect body weight in a more recent study (55) could be due to experimental differences with respect to age of rats, APAP dosage and length of study. Moreover, in that study APAP was administered by gavage, leading to absorption of APAP only at a specific time of the day and not spread throughout the day as in this study.
As usual, extrapolation of rat results to humans should be carried out with caution. Firstly, the 1% APAP diet is close to the maximum dose used daily in humans (4 g/d) and has already been considered as corresponding to the maximum therapeutic dose for humans (see Material and Methods). Nevertheless, interspecies comparisons of doses should take into account body surface area using adequate formulas (56, 57). According to these conversion formulas, the APAP dose consumed by the 1% APAP group was 1.5- to 1.7-fold the maximum dose used daily in humans. Secondly, recent works evaluated the influence of acute or chronic treatment with APAP on exercised skeletal muscle. In the acute study, the 24 h-postexercise-induced increase in the fractional rate of protein synthesis of exercised muscle was blunted by APAP treatment (4 g/d) in young adults (58). The authors suggested that APAP inhibited the anabolic effect of exercise. The chronic study was performed in overweight older adults subjected to resistance exercise (3 times a week) and treated with APAP (4 g/d) for 12 weeks, a significantly longer period than this study. Unexpectedly, APAP treatment potentiated the exercise-induced increase in the volume of exercised muscle (quadriceps femoris muscle) and maintained muscle protein concentration (vastus lateralis muscle) (59). This anabolic effect of APAP, mediated through prostaglandin-dependent mechanisms related to protein synthesis and protein degradation (60), was limited to exercised muscle since non-exercised muscles (semimembranosus, semitendinosus, and biceps femoris muscles) were unaffected by APAP treatment (59). The occurrence of APAP-induced anabolic effect in exercised muscle suggests that all amino acids were available for muscle. Unfortunately the dietary intake of these older adults was not reported. It has already been shown that older adults increased their protein intake when under chronic treatment with APAP (61). As already discussed in that paper, APAP-treated persons at high risk of muscle wasting are likely to be those exhibiting a low protein intake.
In conclusion, in adult rats, sulfur loss through urinary APAP metabolites was independent of the treatment dose, however, the contribution of the GSH-dependent detoxification pathway to this sulfur loss dramatically increased with the 1% APAP treatment (21 vs. 5%). The extra-utilization of Cys induced by chronic treatment with 1% APAP affected protein and GSH contents in the studied tissues. This dose induced muscle wasting. Several mechanisms could contribute to the APAP-induced muscle wasting. The major one could be unsufficient bioavailability of Cys for muscle maintenance due to APAP detoxification in the liver. Decrease of muscle GSH could also generate a pro-oxidative environment, a factor known to potentially contribute to muscle wasting. It would be worthwhile to test whether muscle wasting and the other metabolic alterations observed with the 1% APAP diet could be reversed by increasing dietary SAA. This study suggests that peripheral bioavailability of Cys and muscle GSH are potential important players in the control of muscle mass in chronic treatment with APAP, an analgesic medication of widespread use, especially in the elderly.
Abbreviations: APAP, paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide); APAP-S, APAP-sulfate; APAP-G, APAP-glucuronide; APAP-NAC, APAP-N-acetylcysteine; APAP-Cys, APAP-cysteine; Cys, cysteine; EDL, extensor digitorum longus; GM, gastrocnemius; GSH, glutathione; N, nitrogen; NAPQI: N-acetyl-p-benzoquinone imine, SAA: sulfur amino acid; SI, small intestine; SOL, soleus; TA, tibilais anterior.
Acknowledgments: The authors would like to acknowledge Philippe Lhoste and Philippe Denis from the Installation Experimentale de Nutrition for animal care, Francoise Glomot and Fabienne Rambourdin for the biochemical analyses and Melanie Petera for her help in the statistical analyses. This work was supported by Institut National de la Recherche Agronomique (INRA), France.
Conflicts of interest: None declared.
REFERENCES
- Obled C, Papet I, Breuille D. Metabolic bases of amino acid requirements in acute diseases. Curr Opin Clin Nutr Metab Care 2002; 5: 189-197.
- Wolfe RR. Optimal nutrition, exercise, and hormonal therapy promote muscle anabolism in the elderly. J Am Coll Surg 2006; 202: 176-180.
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002; 50: 889-896.
- Evans WJ, Paolisso G, Abbatecola AM, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology 2010; 11: 527-36.
- Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle 2011; 2: 1-3.
- Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010; 211: 271-278.
- Klotz U. Paracetamol (acetaminophen) - a popular and widely used nonopioid analgesic. Arzneimittelforschung 2012; 62: 355-359.
- Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing 2013; 42 (Suppl. 1): I1-I57.
- Analyse des ventes de medicaments aux officines et aux hôpitaux en France 1999-2009. Rapport d’Expertise de l’Agence Francaise de Securite Sanitaire des Produits de Sante (AFSSAPS) 2011.
- Analyse des ventes de medicaments en France en 2011. Agence National de Securite du Medicament et des Produits de Sante (ANSM). 2012.
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States - The Slone survey. JAMA 2002; 287: 337-344.
- Forrest JAH, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet 1982; 7: 93-107.
- Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin 2012; 28: 499-516.
- Courtney-Martin G, Ball RO, Pencharz PB. Sulfur amino acid metabolism and requirements. Nutr Rev 2012; 70: 170-175.
- McLean AE, Armstrong GR, Beales D. Effect of D-methionine or L-methionine and cysteine on the growth inhibitory effects of feeding 1-percent paracetamol to rats. Biochem Pharmacol 1989; 38: 347-352.
- Reicks M, Hathcock JN. Prolonged acetaminophen ingestion in mice - effects on the availability of methionine for metabolic functions. J Nutr 1989; 119: 1042-1049.
- Varela-Moreiras G, Ruiz-Roso B, Varela G. Effects of long-term administration of acetaminophen on the nutritional utilization of dietary protein. Ann Nutr Metab 1991; 35: 303-308.
- Remond D, Buffiere C, Pouyet C, et al. Cysteine fluxes across the portal-drained viscera of enterally fed minipigs: effect of an acute intestinal inflammation. Amino Acids 2011; 40: 543-552.
- Bauchart-Thevret C, Cottrell J, Stoll B, Burrin DG. First-pass splanchnic metabolism of dietary cysteine in weanling pigs. J Anim Sci 2011; 89: 4093-4099.
- Cho ES, Johnson N, Snider BC. Tissue glutathione as a cyst(e)ine reservoir during cystine depletion in growing rats. J Nutr 1984; 114: 1853-1862.
- Ookhtens M, Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis 1998; 18: 313-329.
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med 2009; 30: 42-59.
- Liu L, Klaassen CD. Different mechanism of saturation of acetaminophen sulfate conjugation in mice and rats. Toxicol Appl Pharmacol 1996; 139: 128-134.
- Klaassen CD, Boles JW. Sulfation and sulfotransferases 5: the importance of 3’-phosphoadenosine 5’-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J 1997; 11: 404-418.
- Ben-Shachar R, Chen Y, Luo S, Hartman C, Reed M, Nijhout HF. The biochemistry of acetaminophen hepatotoxicity and rescue: a mathematical model. Theor Biol Med Model 2012; 9: 55. doi: 10.1186/742-4682-9-55.
- Dubuisson C, Lioret S, Touvier M, et al. Trends in food and nutritional intakes of French adults from 1999 to 2007: results from the INCA surveys. Br J Nutr 2010; 103: 1035-1048.
- Malmezat T, Breuille D, Pouyet C, Mirand PP, Obled C. Metabolism of cysteine is modified during the acute phase of sepsis in rats. J Nutr 1998; 128: 97-105.
- Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985; 150: 76-85.
- Gaitonde MK. A spectrometric method for the direct determination of cysteine in the presence of other naturally occuring amino acids. Biochem J 1967; 104: 627-633.
- Malloy MH, Rassin DK, Gaull GE. A method for measurement of free and bound plasma cyst(e)ine. Anal Biochem 1981; 113: 407-415.
- Pickering G, Schneider E, Papet I, et al. Acetaminophen metabolism after major surgery: a greater challenge with increasing age. Clin Pharmacol Ther 2011; 90: 707-711.
- Hodges JL, Lehmann EL. Estimation of location based on ranks. Ann Math Statist 1963; 34: 598-611.
- Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf 2006; 15: 241-243.
- Moldeus P. Paracetamol metabolism and toxicity in isolated hepatocytes from rat and mice. Biochem Pharmacol 1978; 27: 2859-2863.
- Lauterburg BH, Mitchell JR. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol 1987; 4: 206-211.
- Geenen S, du Preez FB, Snoep JL, et al. Glutathione metabolism modeling: a mechanism for liver drug-robustness and a new biomarker strategy. Biochim Biophys Acta 2013; 1830: 4943-4959.
- Garcia RA, Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino-acids and their metabolites in rats. J Nutr 1992; 122: 1693-1701.
- Griffith OW, Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci USA 1979; 76: 5606-5610.
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 1999; 27: 922-935.
- Richie JP, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 1994; 8: 1302-1307.
- Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem 1990; 1: 228-237.
- Chasse JF, Paly E, Paris D, et al. Genomic organization of the human cystathionine beta-synthase gene: evidence for various cDNAs. Biochem Biophys Res Commun 1995; 211: 826-832.
- Bao LM, Vlcek C, Paces V, Kraus JP. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch Biochem Biophys 1998; 350: 95-103.
- Ishii I, Akahoshi N, Yu XN, et al. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J 2004; 381: 113-123.
- Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 2014; 49: 59-68.
- Sanchez AM, Candau RB, Bernardi H. FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci 2014; 71: 1657-1671.
- Bonetto A, Penna F, Muscaritoli M, et al. Are antioxidants useful for treating skeletal muscle atrophy? Free Radic Biol Med 2009; 47: 906-916.
- Musaro A, Fulle S, Fano G. Oxidative stress and muscle homeostasis. Curr Opin Clin Nutr Metab Care 2010; 13: 236-242.
- Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim Biophys Acta 2013; 1823: 1767-1777.
- Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 2010; 11: 1509-1526.
- Kupeli E, Orhan DD, Yesilada E. Effect of Cistus laurifolius L. leaf extracts and flavonoids on acetaminophen-induced hepatotoxicity in mice. J Ethnopharmacol 2006; 103: 455-460.
- Lee CH, Kuo CY, Wang CJ, et al. A polyphenol extract of Hibiscus sabdariffa L. ameliorates acetaminophen-induced hepatic steatosis by attenuating the mitochondrial dysfunction in vivo and in vitro. Biosci Biotechnol Biochem 2012; 76: 646-651.
- Ji L, Jiang P, Lu B, Sheng Y, Wang X, Wang Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J Nutr Biochem 2013; 24: 1911-1919.
- Ilic S, Drmic D, Zarkovic K, et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol 2010; 61: 241-250.
- Carroll CC, Whitt JA, Peterson A, Gump BS, Tedeschi J, Broderick TL. Influence of acetaminophen consumption and exercise on Achilles tendon structural properties in male Wistar rats. Am J Physiol 2012; 302: R990-R995.
- Shin JW, Seol IC. Interpretation of animal dose and human equivalent dose for drug development. J Korean Orient Med 2010; 31: 1-7.
- Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research CDER, 2005. http://www.fda.gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf
- Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol 2002; 282: E551-E556.
- Trappe TA, Carroll CC, Dickinson JM, et al. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol 2011; 300: R655-R662.
- Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol 2013; 115: 909-919.
- Pujos-Guillot E, Pickering G, Lyan B, et al. Therapeutic paracetamol treatment in older persons induces dietary and metabolic adaptations related to sulfur amino acids. Age 2012; 34: 181-193.
A c c e p t e d : August 18, 2014