ROLE OF MAS RECEPTOR ANTAGONIST (A779) ON PRESSURE DIURESIS AND NATRIURESIS AND RENAL BLOOD FLOW IN THE ABSENCE OF ANGIOTENSIN II RECEPTORS TYPE 1 AND 2 IN FEMALE AND MALE RATS
INTRODUCTION
Clinical and epidemiologic evidences suggest the development and progression of renal and cardiovascular diseases are more slowly in women before menopause in comparison with men (1, 2). However, the mechanisms underlying this difference remain unknown, but renin angiotensin system (RAS) has been known to involve in this gender-based difference (3, 4). RAS plays an important role in regulation of arterial pressure by the kidney due to its effects on vascular tone and renal excretion (5). The activity and expression of RAS components and response to RAS receptor blockers and stimulators also have been reported to be gender-related (6, 7).
One of the main important components of RAS is angiotensin1-7 (Ang 1-7). This peptide induces its cardiovascular effect via mas receptor (MasR) activation (8, 9), and the vascular effects of angiotensin 1-7 peptidomimetic also is reported (5, 10). However, blockade of angiotensin II (AngII) receptors type 1 and 2 (AT1R and AT2R) inhibits some actions of Ang 1-7, which indicates a crosstalk between MasR and AngII receptors (9, 11-13). Moreover, increase in nitric oxide (NO), bradykinin, and prostacyclin production complicates the mechanism of actions of Ang 1-7 (14, 15). It is reported that Ang 1-7 can produce a number of physiological effects including natriuresis, diuresis, and vasodilation via activation of MasR (16). In addition, AngII receptors may also be involved in tubular function (8, 9). Intra-renal infusion of Ang 1-7 in dogs augments urinary excretion of sodium and water; indicating that Ang 1-7 increases reabsorption by AT1R-mediated mechanism. Therefore, Ang 1-7 has been reported to induce either natriuresis/diuresis or sodium and water retention via modulation of sodium transporters in the proximal tubule and loop of Henle and collecting duct water transport (8).These data therefore indicate that crosstalk mechanisms may exist between Ang 1-7 and Ang II receptors in regulation of water and sodium transport in the kidney (17, 18). Furthermore, female rats in comparison with male rats show a leftward shift in the pressure natriuresis relationship (19). It seems that Ang 1-7 and MasR have also implicated as a modulator of natriuresis (8). Pervious study showed that administration of Ang 1-7 augments sodium excretion and urinary flow rate that was eliminated by A779 (20). Also, in cultured rabbit proximal tubular cells, Ang 1-7 inhibits sodium penetration, that is associated with activation of phospholipase A2 (21). Ang 1-7 also increases sodium excretion in isolated rat proximal tubule (20). Thus, it is plausible that MasR may modulate the natriuresis relationship, contributing to gender-associated differences in blood pressure regulation (22). Accordingly, in this study, we examined the role of MasR in pressure-natriuresis in male and female rats when AT1R and AT2R were blocked.
MATERIALS AND METHODS
Animal
The experimental procedures were approved in advance by the Isfahan University of Medical Sciences Ethics Committee.
Male (n = 28, 190 ± 2.3 g) and female (n = 28, 180 ± 1.0g) Wistar rats (Animal Centre, Isfahan University of Medical Sciences) were used. The rats were individually housed at a temperature of 23–25°C with a 12-h light/dark cycle, with the dark period between 19.00–07.00.
Surgery
The rats were anaesthetised (Inactin; thiobutabarbital, 175 mg kg-1i.p. Sigma, St Louis, MO, USA.) and the trachea was isolated to insert air ventilation tube. Catheters were implanted into the jugular vein, and carotid and femoral arteries. A catheter was also inserted in the bladder to collect the urine volume. An adjustable clamp was placed around the aorta above the level of the renal arteries to control renal perfusion pressure (RPP). RPP was measured from the femoral artery. The left kidney was placed in a stable cup. The flow probe was placed and fixed around left renal artery, and renal blood flow (RBF) was monitored by transit-time ultrasound flowmetry (Transonic Systems, Ithaca, NY, USA.). Body temperature was continuously monitored through the experiment. We allowed 30–60 minutes for equilibration period.
Experimental protocol
After equilibration period, male and female rats in treated groups simultaneously received MasR (A779), AT1R (losartan), and AT2R (PD123319) blockers via jugular vein by micro-infusion pumps until the end of the study. Losartan, PD123319, and A779 were purchased from Darou Pakhsh Pharma Co.(Tehran, Iran), Sigma (St. Louis MO, USA), and Bachem Bioscience Inc. (King of Prussia, PA, USA), respectively. Male and female rats in control groups had the same treatment except vehicle instead of A779. Losartan, PD123319, and A779 were administrated as 10 mg kg–1plus 10 mg kg–1 h–1 (23), 1 mg kg–1 plus 1 mg kg–1 h–1, and 50 µg kg–1plus 50 µg kg–1 h–1), respectively (24). Thirty minutes after initiating administration of the antagonists, RPP was elevated by tightening the ligatures around the celiac and mesenteric arteries, and then it was controlled at levels of about 80 or 100 mmHg via the adjustable clamp. After controlling RPP to the desired specific level of pressure, another 15 minutes was allowed for equilibration. We allowed the experiments to run for another 30 minutes as the measurement time. Urine volume was collected during this period of measurement, and MAP and RBF were measured continuously. Accordingly, we assigned eight experimental groups as follows:
Groups 1 and 2: male and female rate treated with AT1R, AT2R, and MasR blockers at controlled RPP of 80 mmHg for 30 minutes.
Groups 3 and 4: male and female rate treated with AT1R, AT2R, and MasR blockers at controlled RPP of 100 mmHg for 30 minutes.
Groups 5 and 6 were treated as groups 1 and 2 except vehicle instead of MasR blocker.
Groups 7 and 8 were treated as groups 3 and 4 except vehicle instead of MasR blocker. Blood sample was collected at the end of the experiment via catheter line. Urinary sodium concentration was measured using Convergys ISE Analyzer. The serum level of renin was measured using a rat renin ELISA kit (Glory Science Co., Ltd, USA). Finally, the animals were sacrificed humanely; the left kidney was removed immediately and weighed. Renal vascular resistance (RVR) was calculated as RPP/RBF ratio.
Statistical analysis
Data are expressed as mean ± S.E.M. Unpaired t-test was used to compare each parameter between the groups. Values of P<0.05 were considered statistically significant.
RESULTS
The role of MasR on urine parameter in both genders with two different RPP when AT1R and AT2R were blocked
There was an increase in urinary sodium excretion (UNaV, P<0.05), urine flow (UF, P<0.05) and urine weight (P<0.05) from low to high RPP in the male rats. Such observation was not seen in female rats. These data indicated that MasR is involved in pressure natriuresis and diuresis in male but not in female when AT1R and AT2R were absent. In other words, AT1R and AT2R blockade could not abolish pressure natriuresis and diuresis in male rats (Fig. 1).
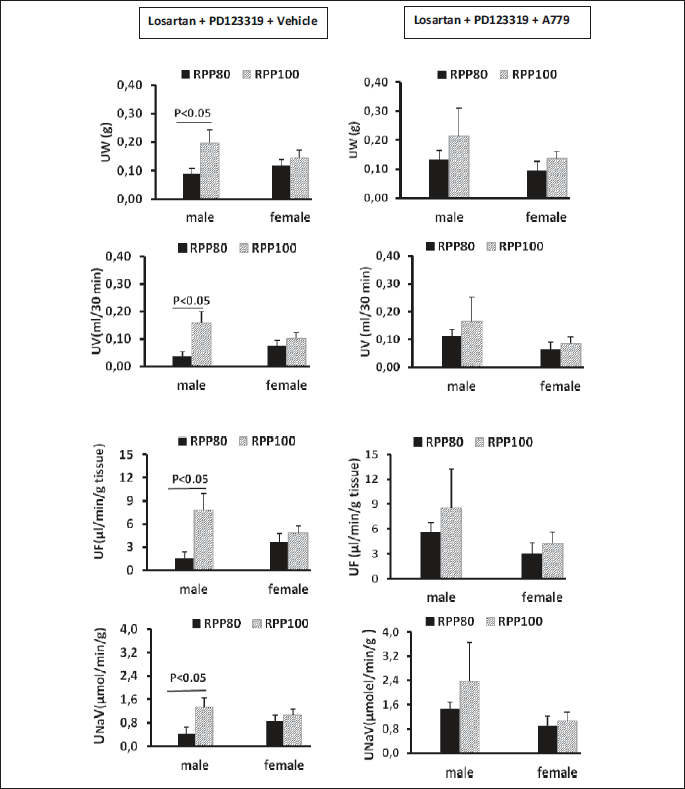
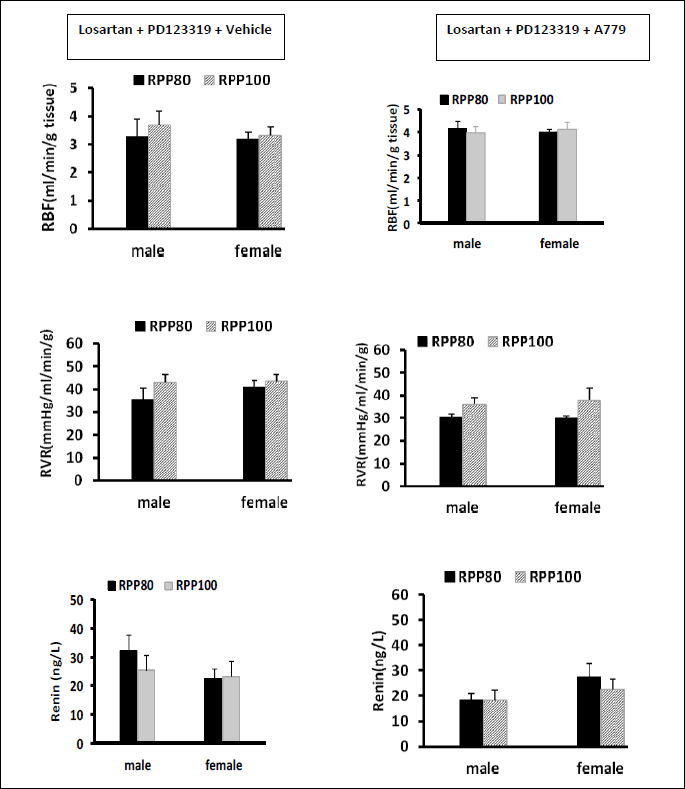
The role of MasR on RBF, RVR and serum level of renin in both genders with two different RPP when AT1R and AT2R were blocked
In both genders; RBF, RVR, and renin concentration did not differ between two levels of RPPs in both sexes. The data indicated that MasR blockade (by A779) abolished the effect of RPP on RBF, RVR and renin concentration when angiotensin II receptors are blocked (Fig. 2).
DISCUSSION
There were two major findings in this study when AT1R and AT2R were blocked. Firstly, it was shown that the impact of MasR on pressure natriuresis and diuresis is gender-related. Secondly, in both genders, RBF/wet kidney tissue weight and RVR did not alter by increased pressure in the presence or absence MasR.
Sexual differences in blood pressure are associated with the angiotensin converting enzyme 2 (ACE2)/Ang 1-7/MasR axis, while the effects of ACE and AT1R blockers partly depend on ACE2 and Ang 1-7 (25). It was demonstrated that ACE2 activity and Ang 1-7 levels increased (5-25-fold) in male rats treated with an ACE inhibitor or AT1R blockers and blood pressure also enhanced by blocking Ang 1-7 synthesis (3, 26-29). Also, previous studies have shown that administration of A779 or an ACE2 inhibitor was accompanied by worsening of renal function and hypertension (25). Therefore, it could be concluded that Ang 1-7 is influenced by antihypertensive and renal protective actions of ACE inhibition and AT1R blocker (30). Drugs which influence RAS axis may inhibit Ang II production and activate Ang 1-7 formation (5). In this regard, it has been showed that aortic tissue of apoE knockout mice may generate AngII which could be attenuated by AVE0991; Ang 1-7 peptidomimetic (10). In the current study and in the presence of MasR, the effect of pressure-dependent increase in UF and UNaV was not observed in female rats. This observation could be related to genetic polymorphisms in ACE2 in women. The genetic polymorphisms in ACE2 in women attenuate the blood pressure-lowering effect of the ACE inhibitor, while polymorphisms in ACE may influence the effectiveness of ACE inhibition and AT1R blocker in men (31, 32). Thus, it seems that Ang 1-7 contributes to the antihypertensive action of ACE inhibition and AT1R blocker treatment via MasR (33-35).
The MasR is expressed in renal proximal tubular cells, afferent arterioles, cardiac myocytes, and neuronal cells; and it conveys Ang 1-7 signals via transcriptional factors (33). It has been shown that activation of the non-classical pathway (Ang 1-7/AT2/MasR) stimulates NO and prostaglandin production that causes vasodilation, and improves the renal blood flow and also enhances pressure natriuresis (30, 36). Therefore, endogenous Ang 1-7 and/ or an Ang 1-7-related peptide exert a vasodilator effect through MasR or through potentiation of bradykinin (15, 37). Ang 1-7 can bound to ACE that facilities the crosstalk between ACE and bradykinin B2 receptor, and this phenomenon leads to inhibition of the ACE C-domain (38, 39). In this regard, Ang 1-7 acts as an ACE inhibitor to potentiate the vasodilator actions of bradykinin through release of vasodilators, prostacyclins, NO, or endothelium-derived hyperpolarizing factor (15, 38, 40). The ACE polymorphisms in male is probably the reason for effectiveness of bradykinin potentiation by Ang 1-7 (32). Thus, the effect of pressure natriuresis will be seen in the presence of MasR. Also, potentiation of vasodilator effects of bradykinin by Ang 1-7 enhances natriuresis and diuresis by augmenting the elimination of water and sodium from the organism. This effect is mediated by an Ang 1-7 receptor (41).
In spite of extensive studies, role of Ang 1-7 in renal physiology is not still obvious and sometimes is contrary (42, 43). The diuretic/natriuretic actions of Ang 1-7 have been explained in in vitro and in vivo experimental models (20, 21). Moreover, some studies have shown an antidiuretic/ antinatiuretic effect made by Ang 1-7 (44, 45). These differences can be related to sodium and water situation, Ang 1-7 concentration, or experimental condition. Furthermore Ang 1-7 effects are related to some factors such as the nephron segment, the local expression of Ang 1-7, and its receptors (46-49). Garcia and Garvin explained that Ang 1-7 at physiologic and supraphysiologic doses differently acts in the kidney (50). Augmented circulating levels of Ang 1-7 in transgenic rats (TGR [A1-7] 3299) caused an antidiuretic effect, which was not related to vasopressin release (51). In pregnant rats, Joyner et al. reported that Ang 1-7 induces diuresis while in virgin females this heptapeptide causes antidiuresis (52). There are several reports consistent with our study that shows a natriuretic/diuretic action of Ang 1-7. For instance, Handa et al. explained that in anesthetized rats, administration of Ang 1-7 increases urinary flow rate and sodium excretion, while these effects were abolished by A779 (20). Also, Ang 1-7 seems to be a potent inhibitor of Na-K-ATPase activation in the renal cortex and in isolated proximal convoluted tubules (53). In renal tubular epithelial cells, Ang 1-7 inhibited trans cellular flux of sodium (21). Most studies indicate that although most Ang 1-7 effects seem to be mediated by MasR activation, this heptapeptide can also interact with AT1R, AT2R, bradykinin B2, and vasopressin V2 receptors (8, 45, 48, 54-56).
In our study, RBF/wet kidney tissue weight, RVR, and the serum level of renin in both genders did not alter by increased pressure in the presence or absence of MasR.
Previous studies have supported contribution of the MasR to regulation of renal function by measuring the renal expression in both the tubular and vascular elements of the kidney (57). MasR mRNA expression was detected in rat cortical and proximal tubular cells, cardiac myocytes and neuronal cells (33, 58), as well as mouse afferent arterioles and tubular epithelium (59). AT1R and AT2R antagonists can involve in the Ang 1-7 effects at renal level by making oligomers including Mas-Mas sensitive to AT1R or AT2R antagonist or AT1-Mas, AT2-Mas, or AT1-AT2-Mas oligomers or cross talk (60-62). These cross talks may involve in kidney autoregulation controlling RBF, RVR, and renin concentration in the presence or absence of MasR in males and females.
Our study is partly consistent with the findings reported by Safari et al. They showed that co administration of PD123319 and A779 eliminated the effect of A779 on RBF and RVR, and they suggested that to achieve complete renal vasoconstriction effects of MasR blockade, co-activation of AT2R is required (57). Possibly the crosstalk between the AT1R, AT2R, MasR, and bradykinin receptors, which have been reported to form heterodimers, provides a situation under which expression and activity of receptors may occur differently (9, 11, 57, 63, 64). Another possibility is that the signal transduction pathways for G protein-coupled receptors, AT2R and MasR, which are weakly defined, may interact (64). Another study also showed absence of marked RBF response to Ang 1-7 infusion in male rats (65). Consistent with our findings, it has been explained that intra-renal infusion of AT1R blockers or ACE in rates produces no changes in plasma aldosterone concentration and has little effects on systemic hemodynamics, and leads to increased sodium excretion (66-68).
In the presence of MasR while AT1R and AT2R were blocked, pressure natriuresis and diuresis augmented in male rats. Such observations were not seen in female animals, which indicate the important role of MasR in pressure natriuresis and diuresis in male when both AT1R and AT2R are blocked.
Acknowledgments: This research was supported by Isfahan University of Medical Sciences.
REFERENCES
- Oparil S, Miller AP. Gender and blood pressure. J Clin Hypertens 2005; 7: 300-309.
- Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Advances in renal replacement therapy. Adv Ren Replace Ther 2003; 10: 3-14.
- Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1220-R1226.
- Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC. Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway. Hypertension 2000; 36: 330-336.
- Jawien J, Toton-Zuranska J, Gajda M, et al. Angiotensin-(1-7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol 2012; 63: 77-85.
- Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 2010; 299: R899-R906.
- Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008; 52: 666-671.
- Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. ScientificWorldJournal 2009; 9: 522-535.
- Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (London) 2011; 121: 297-303.
- Olszanecki R, Suski M, Gebska A, et al. The influence of angiotensin-(1-7) peptidomimetic (AVE 0991) and nebivolol on angiotensin i metabolism in aorta of apoE-knockout mice. J Physiol Pharmacol 2013; 64: 317-320.
- Dahl T, Hultstrom M, Iversen B, Helle F. Adenosine sensitization after angiotensin II stimulation in afferent arterioles from normal rats does not occur during two-kidney, one-clip hypertension. Acta Physiol (Oxford) 2011; 201: 289-294.
- Lyngso C, Erikstrup N, Hansen JL. Functional interactions between 7TM receptors in the renin-angiotensin system-dimerization or crosstalk? Mol Cell Endocrinol 2009; 302: 203-212.
- Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides 2011: 32: 1551-1565.
- Campbell DJ. The renin-angiotensin and the kallikrein-kinin systems. Int J Biochem Cell Biol 2003; 35: 784-791.
- Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli de Carvalho MH. Potentiation of bradykinin by angiotensin-(1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension 2001; 37: 703-709.
- Burgelova M, Kramer HJ, Teplan V, Thumova M, Cervenka L. Effects of angiotensin-(1-7) blockade on renal function in rats with enhanced intrarenal Ang II activity. Kidney Int 2005; 67: 1453-1461.
- Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 2005; 46: 937-942.
- Velkoska E, Dean R, Griggs K, Burchill L, Burrell L. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (London) 2011; 120: 335-345.
- Khraibi AA, Liang M, Berndt TJ. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 2001; 14: 893-896.
- Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7): in vivo and in vitro studies. Am J Physiol 1996; 270: F141-F147.
- Andreatta van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(l-7). Kidney Int 1993; 44: 932-936.
- Sampson AK, Moritz KM, Denton KM. Postnatal ontogeny of angiotensin receptors and ACE2 in male and female rats. Gend Med 2012: 9: 21-32.
- Zhuo J, Song K, Abdelrahman A, Mendelsohn FA. Blockade by intravenous losartan of AT1 angiotensin II receptors in rat brain, kidney and adrenals demonstrated by in vitro autoradiography. Clin Exp Pharmacol Physiol 1994; 21: 557-567.
- Santos RA, Haibara AS, Campagnole-Santos MJ, et al. Scientific contributions-characterization of a new selective antagonist for angiotensin-(1-7), D-Pro7-angiotensin-(1-7). Hypertension 2003; 41: 737-743.
- Moon JY. ACE2 and angiotensin-(1-7) in hypertensive renal disease. Electrolyte Blood Press 2011; 9: 41-44.
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000; 87: e1-e9.
- Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004; 43: 970-976.
- Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-β on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol 2002; 13: 1464-1472.
- Stegbauer J, Vonend O, Oberhauser V, Rump LC. Effects of angiotensin-(1-7) and other bioactive components of the renin-angiotensin system on vascular resistance and noradrenaline release in rat kidney. J Hypertens 2003; 21: 1391-1399.
- Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension 1998; 31: 356-361.
- Fan X, Wang Y, Sun K, et al. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of captopril in women. Clin Pharmacol Ther 2007; 82: 187-196.
- Ruggenenti P, Perna A, Zoccali C, et al. Chronic proteinuric nephropathies. II. Outcomes and response to treatment in a prospective cohort of 352 patients: differences between women and men in relation to the ACE gene polymorphism. J Am Soc Nephrol 2000; 11: 88-96.
- Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 2002; 277: 14838-14843.
- Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2005; 289: H1013-H1019.
- Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7) an evolving story in cardiovascular regulation. Hypertension 2006; 47: 515-521.
- Souza AP, Sobrinho DB, Almeida JF, et al. Angiotensin II type 1 receptor blockade restores angiotensin-(1-7)-induced coronary vasodilation in hypertrophic rat hearts. Clin Sci (London) 2013; 125: 449-459.
- Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S. Angiotensin (1-7) potentiates bradykinin-induced vasodilatation in man. J Hypertens 2001; 19: 2001-2009.
- Tom B, de Vries R, Saxena PR, Danser AJ. Bradykinin potentiation by angiotensin-(1-7) and ACE inhibitors correlates with ACE C-and N-domain blockade. Hypertension 2001; 38: 95-99.
- Prada JA, Ferreira AJ, Katovich MJ, et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 2008; 51: 1312-1317.
- Vanhoutte PM. Endothelium and control of vascular function. State of the art lecture. Hypertension 1989; 13: 658-667.
- Mattson DL, Cowley A. Kinin actions on renal papillary blood flow and sodium excretion. Hypertension 1993; 21: 961-965.
- Lara L, De Carvalho T, Leao-Ferreira LR, Lopes AG, Caruso-Neves C. Modulation of the (Na(+)+K+) ATPase activity by angiotensin-(1-7) in MDCK cells. Regul Pept 2005; 129: 221-226.
- Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol 1999; 10 (Suppl. 12): S258-S265.
- Simoes e Silva AC, Bello AP, Baracho NC, Khosla MC, Santos RA. Diuresis and natriuresis produced by long term administration of a selective angiotensin-(1-7) antagonist in normotensive and hypertensive rats. Regul Pept 1998; 74: 177-184.
- Vallon V, Richter K, Heyne N, Osswald H. Effect of intratubular application of angiotensin 1-7 on nephron function. Kidney Blood Press Res 1997; 20: 233-239.
- Simoes-e-Silva A, Baracho N, Passaglio K, Santos R. Renal actions of angiotensin-(1-7). Braz J Med Biol Res 1997; 30: 503-513.
- Chappell MC, Diz DI, Yunis C, Ferrario CM. Differential actions of angiotensin-(1-7) in the kidney. Kidney Int 1998; 54: S3-S6.
- Magaldi AJ, Cesar KR, de Araujo M, Simoes e Silva AC, Santos RA. Angiotensin-(1-7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflugers Archiv 2003; 447: 223-230.
- Souza Dos Santos RA, Passaglio KT, Pesquero JB, Bader M, Simoes e Silva AC. Interactions between angiotensin-(1-7), kinins, and angiotensin II in kidney and blood vessels. Hypertension 2001; 38 : 660-664.
- Garcia NH, Garvin JL. Angiotensin 1-7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol 1994; 5: 1133-1138.
- Ferreira AJ, Pinheiro SV, Castro CH, et al. Renal function in transgenic rats expressing an angiotensin-(1-7)-producing fusion protein. Regul Pept 2006; 137: 128-133.
- Joyner J, Neves LA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1073-R1080.
- Lopez Ordieres MG, Gironacci M, Rodriguez de Lores Arnaiz G, Pena C. Effect of angiotensin-(1-7) on ATPase activities in several tissues. Regul Pept 1998; 77: 135-139.
- Oudit GY, Kassiri Z, Patel MP, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 2007; 75: 29-39.
- Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther 1998; 284: 388-398.
- Santos RA, Simoes e Silva AC, Magaldi AJ, et al. Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension 1996; 27: 875-884.
- Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the Mas receptor. Acta Physiol (Oxf) 2012; 206: 150-156.
- Su Z, Zimpelmann J, Burns K. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 2006; 69: 2212-2218.
- Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1-7), ACE2 and blood pressure regulation. Contrib Nephrol 2004; 143: 77-89.
- AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem 2001; 276: 39721-39726.
- AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 2000; 407: 94-98.
- Kostenis E, Milligan G, Christopoulos A, et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 2005; 111: 1806-1813.
- Canals M, Jenkins L, Kellett E, Milligan G. Up-regulation of the angiotensin II type 1 receptor by the MAS proto-oncogene is due to constitutive activation of Gq/G11 by MAS. J Biol Chem 2006; 281: 16757-16767.
- Porrello ER, Delbridge L, Thomas WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci (Landmark). 2008; 14: 958-972.
- van der Wouden EA, Ochodnicky P, van Dokkum RP, et al. The role of angiotensin (1-7) in renal vasculature of the rat. J Hypertens 2006; 24: 1971-1978.
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251-287.
- Kimbrough H, Vaughan E, Carey RM, Ayers C. Effect of intrarenal angiotensin II blockade on renal function in conscious dogs. Circ Res 1977; 40: 174-178.
- Cervenka L, Wang CT, Navar LG. Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. Am J Physiol 1998; 274: F940-F945.
A c c e p t e d : September 12, 2012