COUNTERCURRENT TRANSFER OF DOPAMINE FROM VENOUS
BLOOD IN THE CAVERNOUS SINUS TO THE ARTERIAL BLOOD SUPPLYING
THE BRAIN - THE PERFUSED RABBIT HEAD AS AN EXPERIMENTAL MODEL
Olsztyn, Poland
INTRODUCTION
In the rabbit, like humans and many other animals, the cavernous sinus lies in the cranial cavity on the sphenoid bone in the Turkish saddle, just below and around the pituitary gland on both sides, but beyond the dura mater. It is several times larger than the pituitary. In the cavernous sinus the venous blood from the brain and pituitary (and in part from the nose and eye) meets the arterial blood flowing in the opposite direction to the brain and pituitary. The physiological role of the cavernous sinus is still unexplained. It has been documented (1-5) that under physiological conditions the cavernous sinus does not protect the brain from overheating as was believed for many years (selective brain cooling system) (6-8). Moreover, it was discovered that numerous brain transmitters permeate from the venous brain outflow to the arterial blood supplying the brain and pituitary in the cavernous sinus. Retrograde counter current transfer of neuropeptides (neurotransmitters) such as GnRH (9-11), oxytocin (11, 12), beta endorphin (9, 13), and dopamine (14, 15), as well as male pheromones, (16, 17), steroid hormones (9, 18, 19) and CO released from the retina in transmitting a light signal (20, 21) has been demonstrated.
In the area of the cavernous sinus of humans and other primates as well as majority of carnivores, equines and rodents, streams of arterial and venous blood flowing in opposite directions are close to one another. Morphological adaptations of the walls of the veins and arteries further reduce the distance between the two streams of blood. In humans, rabbit, dog, rat, and many other species, the vein walls in the cavernous sinus are transformed into fragments composed of connective tissue and venous endothelium directly covers the cavernous sinus segment of the internal carotid artery. In humans, the cavernous fragment of the internal carotid artery loses the lamina elastica externa and lamina elastica interna (22). Streams of venous blood and arterial blood are separated by only four vascular layers. Moreover, it has been demonstrated in rabbits, mice and rats that alterations in the internal lamina fenestration and permeability of the vessel occur as a function of age (23-25). In many species of ruminants and swine the cavernous sinus is formed by a network of veno-venous vessels intertwined with the arterio-arterial network (veno-venous and arterio-arterial rete mirabile) (26-28). The external layer of thin veins and arteries is formed by a common tunica adventitia (29) and the inner muscular layer of arterioles is reduced to 3–5 layers of myocytes (30). Venous blood is separated from arterial blood by just five or six thin vascular layers.
Fragments of the internal carotid artery and cranial nerves pass through the corresponding left and right cavernous sinus. In humans, this fragment of the internal carotid artery is marked as fragment T6 (cavernous sinus segment of the internal carotid artery) (31). On both sides of the head a total of 10 cranial nerve trunks, such as the occulomotor nerve (III), trochlear nerve (IV), maxillare and ophthalmic branches of the trigeminal nerve (V), and obducent nerve (VI), pass through the cavernous sinus (32). The cavernous segment of the internal carotid artery is covered with sympathetic carotid plexus formed by upper cervical and sphenopalatine ganglions (33-35). However, the importance of these nerves passing through the cavernous sinus has never been explained.
Retrograde countercurrent transfer of dopamine from the cavernous sinus to the blood of the carotid internal artery has never been studied in humans or rabbits and has never been connected with the genesis of diseases such as Parkinson's, Attention Deficit Hyperactivity Disorder (ADHD) and many other psychiatric disorders. However, the function of the dopaminergic system is regulated mainly by biogenic amine transporter proteins, i.e. the dopamine transporter (DAT) (36-38), and the activity of the DAT is down-regulated by dopamine and amphetamine (39, 40). This suggests that retrograde transfer of dopamine in the cavernous sinus and its back transport to the brain could affect DAT activity by metabolites of dopamine, produced in endothelial and glial cells of the brain (41).
Therefore, the objective of the current study was to test our hypothesis developed on the basis of our previous finding of countercurrent transfer in the cavernous sinus of many neurotransmitters (including CO as a gaseous neurotransmitter) and pheromones. This concept assumes that dopamine retrograde transfer in the cavernous sinus plays an important role in the development of hyperfunction or hypofunction of the dopaminergic system. We wanted to check whether in the rabbit cavernous sinus, similar to sheep, a countercurrent transfer of dopamine takes place, despite the differences in the structure of the cavernous sinus, and whether rabbits can be used as a model animal for research involving the function of the cavernous sinus as a basic element in the emergence of ADHD and Parkinson's disease.
MATERIALS AND METHODS
All the procedures were carried out in compliance with Polish legal regulations (Act of January 21, 2005) that, determine the terms and conditions for performing experiments on animals, and were in accordance with the protocol of the Local Ethics Commission for Animal Experiments in Olsztyn No. 88/2012.
Study design
Mature male Great Belgian rabbits (10–12 months of age, body mass ~5–6 kg,) were used for the study. The animals were housed in the experimental farm of the Department of Fur-bearing Animal Breeding and Game Management, University of Warmia and Mazury, Olsztyn. In total 41 rabbits were used for the study. Two groups of rabbits (of the same age and similar body weight) were used: group I - animals designed to study the countercurrent transfer of dopamine (n=22); and group II - used as homologous blood and control brain tissue donors (n=19). In all animals a winged perfusion set (0.5×19 mm, Terumo, Terumo Europen, N. V., 3001 Leuven, Belgium) was inserted into the marginal vein of the ear for heparin injection (500 i.u. kg-1; Polfa, Warsaw, Poland) and for introduction of anesthetics. General anesthesia was induced with ketamine (Ketamina 10%, 6 mg kg-1; Biowet, Pulawy, Poland) and xylazine hydrochloride (Sedazin, 2 mg kg-1; Biowet, Pulawy, Poland) and maintained with supplementary doses according to the symptoms observed.
The animals in group I were divided into two subgroups: subgroup IA and IB. In rabbits in subgroup IA the head was supplied with the buffer (Hanseleit-Krebs) mixed with autologous/homologous blood in a 3:1 ratio, and in subgroup IB in a 1:1 ratio. Animals of group I and animals used as blood donors (group II) were anaesthetized and bled by opening one of the common carotid arteries. On average 150 ml of blood was collected from each animal. The autologous and homologous blood was used to supply the heads of group I rabbits.
Installing a catheter for infusion of radiolabeled dopamine to the cavernous sinus
A silastic catheter (o.d., 1 mm; i.d., 0.8 mm) was inserted into the superficial facial vein, towards the nose, cutting the vein at cheek height a few centimeters forward of the angle of the mandible in order to infuse radiolabeled dopamine into the cavernous sinus without damaging it. The profound facial vein, the nasal vein (2–3 cm before the inflow into the superficial facial vein) and the labial vein were ligatured. Under these conditions, only one direction of flow of the dopamine was possible: from the superficial facial vein to the frontal vein, which enters the angular oculi vein and passes through the ophthalmic sinus as the emissary vein into the cavernous sinus (Fig. 1).
Preparation of rabbits for artificial circulation of buffer mixed with autologous and homologous blood in the head
After installing the catheter in the superficial facial vein, 200 ml of saline was introduced into the marginal vein of the ear of the rabbit. This increased blood volume and decreased the hematocrit from 42.1 ± 1.5 to 33.5 ± 1.2. Then, two catheters (o.d., 3.0 mm; i.d., 2.1 mm) were inserted into both jugular veins as far from the head as possible. Another catheter (o.d., 1.8 mm; i.d., 1.2 mm) was inserted into the left common carotid artery. The rabbit was exsanguinated by opening the cardiac section of the left common carotid artery and both jugular veins. After exsanguination the right common carotid artery was cannulated. Both common carotid arteries were connected with the peristaltic pomp. Then, maximally oxygenated and warmed (37°C) Hanseleit-Krebs buffer mixed with autologous or homologous blood in a ratio of either 3:1 (subgroup IA) or 1:1 (subgroup IB) was pumped through both common carotid arteries into the head at a stable rate (60 ml/min) at a pressure of 80–100 mm Hg (Fig. 1).
Cannulation of the vertebral artery for cerebral arterial blood collection
Immediately after rabbit exsanguination the chest was opened and a catheter (o.d., 1 mm; i.d., 0.8 mm) was inserted into the right subclavia artery a few mm towards the head in order to collect arterial blood from the basilar artery of the brain by the vertebral artery. The left subclavia artery with the left vertebral artery was ligatured.
Infusion of 3H-DA into the cavernous sinus
Tritiated dopamine (3,4-[ring-2,5,6-3H]DA; [3H-DA]; specific activity 60 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO, USA). A 10 µCi aliquot of 3H-DA (2×107 dpm) was diluted in 10 ml of saline (Solfin, Polfa, Kutno, Poland) and infused into the right cavernous sinus through the angular oculi vein at a stable rate (1 ml/min) for 10 min using an infusion pump.
Tracking the pathway of radioactive dopamine infused into the superficial facial vein
Five milliliters of microfil (Microfil, MV-122 Yellow, Flow Tech, Inc., Carver, MA, USA) was infused into the catheter placed in the superficial facial vein of the rabbit (n=5) instead of 3H-DA. After injection the head of the rabbit was kept at 4°C for 24 hours, after which the skin was removed and the skull opened. Then, the cavernous sinus was exposed and the filling of the vein with microfil and the pathway from the superficial facial vein into the cavernous sinus was observed under a magnifying glass.
Blood sample collection and radioactivity measurement
Blood samples (n=3) were collected from both jugular veins and the vertebral artery of each rabbit after starting the artificial circulation in the head but before 3H-DA infusion in order to prepare control plasma samples. After starting the infusion of 3H-DA, samples of cerebral arterial blood were collected from the basilar artery of the brain by the vertebral artery as well as samples of venous effluent from both jugular veins. When the entire volume of autologous blood had been used, homologous blood from the donor rabbit was used to perfuse the isolated head (about a 5–6 min process). The use of homologous blood was necessary in order to maintain the circulation of blood in the head and collect blood samples for the next 5–10 min. The samples of arterial and venous blood were immediately centrifuged and the radioactivity in each plasma sample (50–100 µl of arterial blood and 500 µl of venous blood) was determined in duplicate by mixing each with dioxane scintillation cocktail and then measuring the output by liquid scintillation spectroscopy (LS 5000 TD, Beckman Instruments, USA). Each sample was measured for 5 min using a program with automatic quench compensation.
Brain tissue sample collection and radioactivity measurement
After the completion of blood sampling the head was perfused with warmed (37°C) Hanseleit-Krebs buffer for 10 min. The buffer was pumped through both common carotid arteries into the head at a stable rate (60 ml/min). Then the head was cut off, the skull was opened and the brain was removed. Tissues samples (30–50 mg on average) were collected from the midbrain (ventral tegmentum area, pons), diencephalon (hypothalamus, corpus mamillare) and cerebral cortex fragments (corpus amygdale, corpus striatum). Corresponding control tissue samples were collected from rabbits of group II (n=6). All tissue samples were weighed and then solubilized in 0.5 ml of Tissue Solubilizer (Soluene-350, Perkin Elmer Life and Analytical Sciences, Boston, USA) at 50°C overnight. The tissue samples were decolorized using 300 µl of 30% H2O2 followed by 170 µl of acetic acid. Liquid scintillation cocktail (5 ml, Ultima-Gold, ParkinElmer Life and Analytical Sciences, Boston, USA) was added to the vials and the radioactivity was measured (LS 5000 TD, Beckman Instruments, USA) for 5 min using a program with automatic quench compensation. Radioactivity was expressed in dpm per 100 mg of tissue.
3H-DA identification in samples of cerebral arterial blood
Liquid chromatography was used to determine whether the radioactivity found in the brain arterial blood collected from the vertebral artery represented unmetabolized dopamine or its metabolites. Each blood plasma sample designated for radioactivity determination was treated with cooled 10% perchloric acid to trigger protein precipitation. The sample was then centrifuged at 13,000×g for 10 min. The supernatant was transferred to another tube and treated with 3 M KOH and 3 M K2CO3 to neutralize it. The sample was then centrifuged again at 13,000×g for 10 min to separate any precipitated salt. The supernatant was then transferred to the next tube and diluted with 25 mM Tris buffer (pH 5) to a volume of 2 ml.
A chromatography column (12 mm × 60 mm) was packed with the strong cation exchanger Dowex 50WX4 Na+ (Sigma-Aldrich). Prior to adding the sample, the prepared column was rinsed with 24 ml of double distilled water and next with 24 ml of Tris buffer (25mM, pH 5.0). Then the prepared sample (2 ml, approximately 2000–3000 dpm) was applied onto the column and it was flushed successively with 3 buffers: 1. 12 ml of 25 mM Tris (pH 5); 2. 25 mM Tris and 1 M NaCl (v:v, pH 6.8); 3. 1 M NH4OH (pH 10). Fractions (1 ml each) were collected during the column flush. Afterward, 0.5 ml of each collected fraction was mixed with an appropriate scintillation cocktail (Ultima Gold, PerkinElmer Life and Analytical Sciences, Boston, USA) and radioactivity was measured. Dopamine molecules easily accept protons at acidic or neutral pH and under these conditions only dopamine, but not its metabolites, is strongly bound to the ion exchanger. Thus, a peak of radioactivity in the fractions flushed with acidic or neutral buffers indicates dopamine metabolites. Unreacted dopamine can only be flushed using an alkaline pH buffer.
Dialysis of rabbit plasma and rabbit plasma mixed with buffer containing 3H-DA
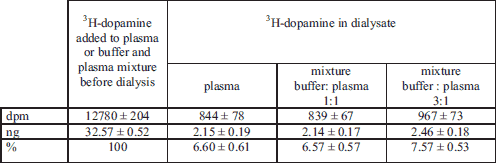
To verify whether diluting the plasma affected the amount of free dopamine, 5 ml of complete rabbit plasma or plasma diluted the same as the blood used to perfuse the rabbit head was incubated with 3H-DA (20,000 dpm) at 20°C for 1 hour. Then, the plasma was infused through minidialysators manufactured by EURU-SEP Ltd, Warsaw (42). The minidialisator contains 460 capillaries with an inner diameter of 0.15 mm and a length of 52 mm that are permeable to molecules of up to 3000 Da. On the side of the minidialisator there are two tips for the exchange of the dialyzing fluid. The Hanseleit-Krebs buffer solution used to fill the chamber (0.5 ml) was the dialysis fluid. Plasma was infused at a rate of 0.17 ml/min for 30 min at 20°C. The assay was performed with 10 replicates. After dialysis, samples of the dialysate and outflowing plasma samples were mixed with dioxane scintillation cocktail and their radioactivity was measured by liquid scintillation spectroscopy.
Statistical analysis
All statistical analyses were performed by GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA). Blood samples were analyzed using one-way ANOVA followed by Bonferroni multiple comparisons test and the T-test with Welch's correction. Tissue samples were analyzed using the T-test with Welch's correction.
RESULTS
Experiments in which microfil was introduced into the angular oculi vein (through the superficial facial vein) (Fig. 1) showed that a significant portion of the yellow microfil flowed by the nasal vein, frontal vein, and angular oculi vein into the ophthalmic sinus and hence into the cavernous sinus (through the emissary vein). A portion also went directly from the ophthalmic sinus into the jugular vein (through the ophthalmic externa vein). This study showed that microfil infused into a rabbit's angular oculi vein was present in large amounts in the cavernous sinus and confirmed that this pathway is suitable for infusing 3H-DA indirectly into the cavernous sinus without any damage to the perihypophyseal vascular complex.
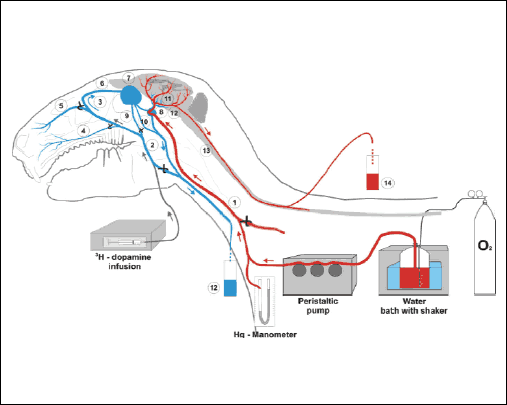 |
Fig. 1. Schematic diagram of the experiment with infusion of 3H-DA into the cavernous sinus of the rabbits head (n=14) perfused with heated and oxygenated Hanseleit-Krebs buffer solution mixed with homologous blood and collection of the cerebral arterial blood from the vertebral artery and the venous blood from both jugular veins. (1) common carotid artery and maxillary artery; (2) maxillary vein and jugular vein; (3) superficial facial vein and frontal vein, (4) labial vein; (5) nasal vein; (6) frontal vein and angular oculi vein; (7) ophthalmic sinus; (8) cavernous sinus; (9) profound facial vein; (10) ophthalmic externa vein; (11) internal carotid artery entering to the Circle of Villis and forming midle, anterior and posterior cerebral arteries; (12) basilar artery; (13) spinal cord and one of the vertebral arteries; (14) arterial blood sample; (15) venous blood sample. |
The mean concentrations of radioactive dopamine in blood collected from the jugular vein on the side of 3H-DA infusion over the entire period of arterial blood sampling in groups IA and IB are presented in Fig. 3. In the experiment on the rabbit head perfused with Hanseleit-Krebs buffer and blood in a 3:1 ratio (group IA) where 3H-DA was introduced into the angular oculi vein, the level of radioactivity in samples collected from the vertebral artery during 3H-DA infusion as well as during the next few minutes of blood sampling after the 3H-DA infusion was significantly higher than in the control samples (Fig. 2.I., P<0.001). The dynamic of dopamine transfer from the venous blood in the cavernous sinus to the artery supplying the brain every minute during the 3H-DA infusion and during the first 7 min after the infusion was complete is presented in Fig. 2.II. Significant levels of radioactivity were also found in the majority of brain tissue samples (Fig. 4, P<0.05) collected after the head perfusion was complete and additional rinsing of the head vessels with circulating pure buffer had been performed.
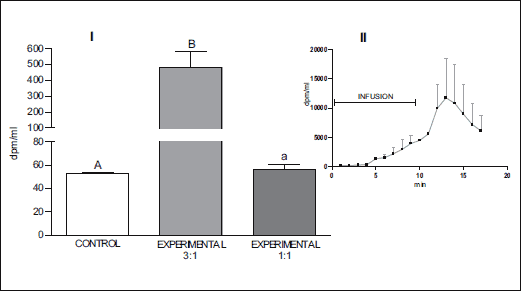 |
Fig. 2. I. Mean (± S.E.) concentration of radioactivity in the sample collected from vertebral artery during and after infusion of 3H-DA into the right cavernous sinus of the head perfused with Hanseleit-Krebs buffer solution mixed with homologous blood in a 3:1 (n=10) and 1:1 (n=4) (v:v) ratio. II. Dynamic presentation of radioactivity in the sample collected from vertebral artery during perfusion of the rabbit head with Hanseleit-Krebs buffer solution mixed with homologous blood in a 3:1 ratio. A/B, P<0.001; B/a, P<0.01; A/a, P>0.05. |
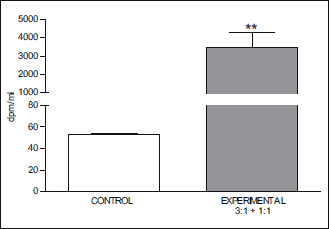 |
Fig. 3. Mean (± S.E.) concentration of radioactive dopamine in blood collected from the jugular vein on the side of 3H-DA infusion, for the entire period of arterial blood sampling, in groups IA and IB (n=14). **P<0.01. |
When the rabbit head was perfused with Henseleit-Krebs buffer and blood at a 1:1 ratio (group IB) and 3H-DA was introduced into the cavernous sinus, very low radioactivity was found in samples of perfusate collected from the vertebral arter, significantly lower than in group IA (Fig. 2.I, P<0.01). However, radioactivity was found in some brain tissue samples taken from these animals after perfusion of the 3H-DA was complete, even though the vessels of the head had been additionally perfused (rinsed) with pure buffer for 10 min. Significant radioactivity was found in the pia mater and pons (Fig. 5., P<0.05).
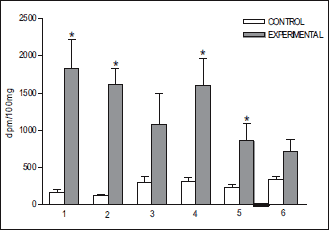 |
Fig. 4. Accumulation of radioactivity (mean ± S.E.) in some brain structures after infusion of 3H-DA into the cavernous sinus of the rabbits head (n=10) perfused with Hanseleit-Krebs buffer solution mixed with homologous blood in a 3:1 (v:v) ratio. (1) pia mater; (2) pons; (3) ventral tegmental area; (4) mammilary body; (5) hippocampus; (6) corpus striatum. *P<0.05. |
Identification of the radioactivity detected in the perfusate collected from the basilar artery (through the vertebral artery) demonstrated that 34.13 ± 2.7% of the radioactivity represented unmetabolized 3H-DA and 65.9 ± 2.7% its metabolites.
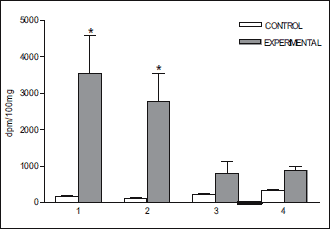 |
Fig. 5. Accumulation of radioactivity (mean ± S.E.) in some brain structures after infusion of 3H-DA into the cavernous sinus of the rabbits head (n=4) perfused with Hanseleit-Krebs buffer solution mixed with homologous blood in a 1:1 (v:v) ratio. (1) pia mater; (2) pons; (3) hippocampus; (4) corpus striatum. *P<0.05. |
Table 1 presents the results of microdialysis of complete rabbit plasma and rabbit plasma diluted with Hanseleleit-Krebs buffer solution to which a constant amount of 3H-DA had been added. The concentrations of free radiolabeled dopamine in control blood plasma dialysate and plasma diluted with buffer did not differ significantly (Table 1).
DISCUSSION
The results of the present study indicate that dopamine can be transferred from the venous blood of the cavernous sinus to the blood of the internal carotid artery in the rabbit, similar to the case with sheep (14, 15). The radioactive dopamine permeation was greater when the rabbit head was perfused with the buffer mixed with blood in a ratio of 3:1 (group IA) compared to 1:1 (group IB). In group IA high levels of radioactivity were found in samples collected from the vertebral artery (Fig. 2.I) as well as in brain tissue samples (Fig. 5). Radioactivity was already appearing in the vertebral artery 3 minutes after starting the 3H-DA infusion into the facial vein and its concentration increased dramatically over the next five minutes (Fig. 2.II).
We think that the difference in the concentration of radioactivity in the arterial blood between groups IA and IB (Fig. 2.I.) may result from the competition between endogenous dopamine and radioactive dopamine. Venous blood outflowing from the brain was mixed in the cavernous sinus with the infused radiolabeled dopamine. The different dilutions of the blood used to perfuse the rabbit head changed the ratio of the two forms of dopamine: endogenous (cold) in the blood and radiolabeled in the infusion solution. With greater dilution of the blood the endogenous dopamine competed less effectively and a greater amount 3H-DA could permeate to the arterial circulation (Fig. 2.I). We also checked whether the dilution of blood affected the ratio of free and protein-bound dopamine, and it was demonstrated that the concentration of free 3H-DA in plasma diluted with the buffer was not significantly higher than in complete plasma (Table 1).
In the experiments in which the head was supplied with buffer mixed with blood in a ratio of 1:1, radioactivity in the perfusion fluid collected from the vertebral artery was very low (Fig. 2.I), but was found in samples of brain tissue. This suggests that under these conditions radioactive dopamine permeated within the cavernous sinus to the arterial blood in small quantities and was arrested in vessels in the course of arterial blood flow throughout the internal carotid artery, arteries of the Circle of Villis (posterior communicating artery, basilar artery) and the vertebral artery.
The physiological mechanism of dopamine transfer through the wall of the internal carotid artery is not known and has never been the subject of research. Taking into account the presence of the carotid sympathetic plexus on the cavernous segment of the internal carotid artery (33-35), it can be assumed that MAO (monoamine oxidase), COMT (catechol O methyltransferase) and the norepinephrine transporter may participate in the transfer and metabolism of dopamine in this area. The activity of COMT may be also influenced by the concentration of estrogen (43), which occurs as a function of age. Moreover, many drugs contribute to the reduction of neurotransmitters biotransformation (44).
Transfer of dopamine from the venous blood of the cavernous sinus to the arterial blood supplying the brain and the pituitary gland has so far been demonstrated only in sheep (14, 15) and now is presented in rabbits for the first time. Because the structure of the cavernous sinus and the cavernous segment of the internal carotid artery in the rabbit is very similar to their counterparts in humans, in our opinion rabbits can serve as a useful model for experimental physiological studies of cavernous sinus function and retrograde dopamine transfer from the venous effluent to the arterial blood supplying the brain.
Our results indicate that, on average, 66% of the 3H-DA molecules were metabolized during transfer in the cavernous sinus through the cavernous sinus endothelial cells and the three layers of the internal carotid artery. This could mean that, as a result of the retrograde transfer of dopamine in the cavernous sinus, dopamine and its metabolites - dihydroxyphenylacetic acid, homovanilic acid and hydroxyindolacetic acid (41) - are supplied to brain capillaries. Although dopamine metabolism has been heavily investigated in the 20th century and in the last decade, to our knowledge, the influence of dopamine metabolites on the expression of DAT has not yet been elucidated. However, the results of numerous experiments support the thesis that the function of DAT is regulated by interaction with its substrates and ligands. Repeated short periods of exposure to dopamine (39) or treatment with amphetamine and dopamine (45), as well as the lesion of dopaminergic neurons (40), down-regulates the activity of DAT. Therefore we suggest that, under physiological conditions, dopamine and its metabolites chronically retrograde transferred and transported with arterial blood to the brain may down-regulate the function of DAT.
In accordance with the principle that the system of neuropeptide retrograde transfer may be a significant regulator of brain function (46), the intensity of the retrograde transfer of dopamine and its metabolites may, in our opinion, represent an important mechanism regulating the function of neural dopaminergic cell groups. This, in turn, may be crucial in explaining the genesis of dopaminergic system dysfunction including Parkinson's disease, ADHD and schizophrenia in humans.
Acknowledgements: This study was supported by the statutory funds of the Ministry of Science and Higher Education in 2013. The authors express their gratitude to Dr Janusz Strychalski for assistance in obtaining the experimental animals.
Conflict of interests: None declared.
REFERENCES
- Jessen C, Laburn HP, Knight MH, Kuhnen G, Goelst K, Mitchell D. Blood and brain temperatures of free-ranging black wildebeest in their natural environment. Am J Physiol 1994; 267: R1528-R1536.
- Mitchell D, Maloney SK, Laburn HP, Knight MH, Kuhnen G, Jessen C. Activity, blood temperature and brain temperature of free-ranging springbok. J Comp Physiol 1997; 167: 335-343.
- Fuller A, Moss DG, Skinner JD, Jessen PT, Mitchell G, Mitchell D. Brain, abdominal and arterial blood temperatures of free-ranging eland and their natural habitat. Pflugers Arch 1999; 438: 671-680.
- Fuller A, Maloney SK, Kamerman PR, Mitchell G, Mitchell D. Absence of selective brain cooling in free-ranging zebras in their natural habitat. Exp Physiol 2000; 85: 209-217.
- Fuller A, Hetem RS, Meyer LC, Maloney SK. Angularis oculi vein blood flow modulates the magnitude but not the control of selective brain cooling in sheep. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1409-R1417.
- Baker MA, Hayward JN. The influence of the nasal mucosa and the carotid rete upon hypothalamic temperature in sheep. J Physiol 1968; 198: 561-579.
- Hayward JN, Baker MA. The role of the cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol 1968; 215: 389-403.
- Hayward JN, Baker MA. A comparative study of the role of the cerebral arterial blood in the regulation of brain temperature in five mammals. Brain Res 1969; 16: 417-440.
- Krzymowski T, Skipor J, Grzegorzewski W. Cavernous sinus and carotid rete of sheep and sows as a possible place for counter current exchange of some neuropeptides and steroid hormones. Anim Reprod Sci 1992; 29: 225-240.
- Skipor J, Bao S, Grzegorzewski W, Wasowska B, Rao CV. The inhibitory effect of hCG on counter current transfer of GnRH and the presence of LH/hCG receptors in the perihypophyseal cavernous sinus-carotid rete vascular complex of ewes. Theriogenology 1999; 51: 899-910.
- Grzegorzewski WJ, Skipor J, Wasowska B, Krzymowski T. Countercurrent transfer of 125I-LHRH in the perihypophyseal cavernous sinus-carotid rete vascular complex, demonstrated on isolated pig heads perfused with autologous blood. Domest Anim Endocrinol 1997; 14: 149-160.
- Grzegorzewski W, Skipor J, Wasowska B, Krzymowski T. Counter current transfer of oxytocin from the venous blond of the perihypophyseal cavernous sinus to the arterial blood of carotid rete supplying the hypophysis and brain depends on the phase of the estrous cycle in pigs. Biol Reprod 1995; 52: 139-144.
- Skipor J, Grzegorzewski W, Wasowska B, Krzymowski T. Counter current transfer of β-endorphin in the perihypophyseal cavernous sinus-carotid rete vascular complex of sheep. Exp Clin Endocrinol Diabetes 1997; 105: 308-313.
- Skipor J, Wasowska B, Grzegorzewski W, Zezula-Szpyra A, Stefanczyk-Krzymowska S, Thiery JC. Transfer of dopamine by counter-current mechanism in the ewe changes with endocrine stage. Biogenic Amines 2001; 16: 431-445.
- Skipor J, Grzegorzewski W, Wasowska B, Krzymowski T. Luteinizing hormone and prolactin are not retrograde transferred in perihypophyseal vascular complex in ewes. Reprod Biol 2004; 4: 195-201.
- Krzymowski T, Grzegorzewski W, Stefanczyk-Krzymowska S, Skipor J, Wasowska B. Humoral pathway for transfer of the boar pheromone, androstenol, from the nasal muc osa to the brain and hypophysis of gilts. Theriogenology 1999; 52: 1225-1240.
- Stefanczyk-Krzymowska S, Krzymowski T, Grzegorzewski W, Wasowska B, Skipor J. Humoral pathway for local transfer of the priming pheromone androstenol from the nasal cavity to the brain and hypophysis in anaesthetized gilts. Exp Physiol 2000; 85: 801-809.
- Skipor J, Grzegorzewski W, Krzymowski T, Einer-Jensen N. Local transport of testosterone from the nasal mucosa to the carotid blond and the brain in the pig. Pol J Vet Sci 2000; 3: 19-22.
- Skipor J, Grzegorzewski W, Einer-Jensen N, Wasowska B. Local vascular pathway for progesterone transfer to the brain after nasal administration in gilts. Reprod Biol 2003; 3: 143-159.
- Koziorowski M, Stefanczyk-Krzymowska S, Tabecka-Lonczynska A, Gilun P, Kaminski M. Gaseous messenger carbon monoxide is released from the eye into the ophthalmic venous blood depending on the intensity of sunlight. J Biol Regul Homeost Agents 2012; 26: 111-118.
- Gilun P, Stefanczyk-Krzymowska S, Romerowicz-Misielak M, Tabecka-Lonczynska A, Przekop F, Koziorowski M. Carbon monoxide-mediated humoral pathway for the transmission of light signal to the hypothalamus. J Physiol Pharmacol 2013; 64: 761-772.
- Masuoka T, Hayashi N, Hori E, Kuwayama N, Ohtani O, Endo S. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol Med Chir (Tokyo) 2010; 50: 179-182.
- Wong LC, Langille BL. Developmental remodeling of the internal elastic lamina of rabbit arteries: effect of blood flow. Circ Res 1996; 78: 799-805.
- Boumaza S, Arribas SM, Osborne-Pellegrin M, et al. Fenestrations of the carotid internal elastic lamina and structural adaptation in stroke-prone spontaneously hypertensive rats. Hypertension 2001; 37: 1101-1107.
- Lee K, Forudi F, Saidel GM, Penn MS. Alterations in internal elastic lamina permeability as a function of age and anatomical site precede lesion development in apolipoprotein E-null mice. Circ Res 2005; 97: 450-456.
- Gillan LA. Blood supply to brains of ungulates with and without a rete mirabile caroticum. J Comp Neurol 1974; 153: 275-290.
- Godynicki S, Frackowiak H. Arterial branches supplying the rostral and caudal retia mirabilia in artriodactyls. Folia Morphol (Warsaw) 1979; 38: 505-510.
- Simoens P, Lauwers H, De Geest JP, De Schaepdrijver L. Functional morphology of the cranial retia mirabilia in the domestic mammals. Schweiz Arch Tierheilkd 1987; 129: 295-307.
- Khamas WA, Ghoshal NG, Bal HS. Histomorphologic structure of the carotid rete-cavernous sinus complex and its functional importance in sheep (ovis aries). Am J Vet Res 1984; 45: 156-158.
- Santamaria L, Dieguez G, Garcia-Villalon AL, Nava Hernandez E, Gomez B, Lluch S. Histomorphometry and innervations of the rete mirabile and brain vessels of Artiodactyla. In: Stroke and Microcirculation. J. Cervos-Navarra, R. Ferszt (eds.). New York, Raven Press, pp. 181-185.
- Bouthillier A, van Loveren H, Keller J. Segments of the internal carotid artery: a new classification. Neurosurgery 1996; 38: 425-433.
- Miyazaki Y, Yamamoto I, Shinozuka S, Sato O. Microsurgical anatomy of the cavernous sinus. Neurol Med Chir (Tokyo) 1994; 34: 150-163.
- Mariniello G. Microsurgical anatomy of sympathetic fibres running inside the cavernous sinus. J Neurosurg Sci 1994; 38: 1-10.
- Mariniello G, Annecchiarico H, Sardo L, Buonamassa S, de Divitiis E. Connections of sympathetic fibres inside the cavernous sinus: a microanatomical study. Clin Neurol Neurosurg 2000; 102: 1-5.
- Dalgic A, Boyaci S, Aksoy K. Anatomical study of the cavernous sinus emphasizing operative approaches. Turk Neurosurg 2010; 20: 186-204.
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol 2003; 479: 159-170.
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann NY Acad Sci 2010; 1187: 316-340.
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord 2013; 28: 715-724.
- Gulley JM, Doolen S, Zahniser NR. Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem 2002; 83: 400-411.
- Afonso-Oramas D, Cruz-Muros I, Barroso-Chinea P, et al. The dopamine transporter is differentially regulated after dopaminergic lesion. Neurobiol Dis 2010; 40: 518-530.
- Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal 2013; 11: 34. doi: 10.1186/1478-811X-11-34.
- Goraca A, Traczyk WZ. Increase in cardiodepressant factor release from the posterior pituitary lobe after angiotensin II infusion into the internal carotid artery. J Physiol Pharmacol 1997; 48: 225-237.
- Schendzielorz N, Rysa A, Reenila I, Raasmaja A, Mannisto PT. Complex estrogenie regulation of catechol-o-methyltransferase (COMT) in rats. J Physiol Pharmacol 2011; 62: 483-490.
- Czubak A, Nowakowska E, Golembiowska K, Kus K, Burda K, Metelska J. Effect of venlafaxine and nicotine on the level of neurotransmitters and their metabolites in rat brains. J Physiol Pharmacol 2010; 61: 339-346.
- Saunders C, Ferrer JV, Shi L, et al. Amphethamine-induced loss of human dopamine transporter activity: An internalization-dependent and cocaine-sensitive mechanism. Proc Nat Acad Sci USA 2000; 97: 6850-6855.
- Krzymowski T, Stefanczyk-Krzymowska S. Local retrograde and destination transfer of physiological regulators as an important regulatory system and its role. Facts and hypothesis. J Physiol Pharmacol 2012; 63: 1-14.
A c c e p t e d : August 18, 2014