EFFECT OF CANNABINOID RECEPTORS 1 MODULATION ON OSTEOPOROSIS
IN A RAT MODEL OF DIFFERENT AGES
INTRODUCTION
Osteoporosis is a worldwide health problem with a high prevalence and is a skeletal disorder characterized by bone mass reduction, increased bone fracture risk and potential bone architecture alterations (1). The incidence of osteoporosis is increasing owing to increasing elderly population. Glucocorticoids are widely used for chronic treatment of a variety of diseases as bronchial asthma, collagen and skin diseases. Because of improvements in the outcome of these diseases, the long-term side effects of glucocorticoids, including osteoporosis which is the most common form of secondary osteoporosis, have become an important problem (1, 2). Activation of monomeric glucocorticoid receptor signaling participates in excessive bone remodeling, it impairs osteoblast survival and differentiation (1-4), inhibit the production of bone matrix components, increase osteoblastic and osteocytic apoptosis, enhanced secretion of the osteoclast-promoting factor receptor activator of nuclear factor kappa B ligand (RANKL) by osteogenic cells (5) and activation of osteoclastic cells (2).
Bone remodeling is a dynamic complex process that is regulated by the interplay between circulating hormones and locally produced factors that act to regulate osteoblast and osteoclast activity (6). Over recent years, there has been increasing interest in the role of the nervous system and neurotransmitters in the regulation of bone remodeling (7). Reflecting this fact, the endocannabinoid pathway has been implicated as an important regulator of bone turnover and bone mass (8-11).
Preclinical studies implicated endocannabinoids and their receptors in the regulation of bone cell activity and the pathogenesis of bone loss. Cells and intervening nerves in the skeleton express cannabinoid receptors and the machinery for the synthesis and breakdown of endocannabinoids (12-13). Cannabinoid receptor 1 (CB1) is a plasma membrane G protein-coupled receptor which regulates lipid accumulation and lipogenesis in adipose tissue (14). Idris et al. (8) had reported that osteoblasts and osteoclasts express cannabinoid receptors and mice with inactivation of CB1 have increased bone mass and are protected from ovariectomy-induced bone loss, suggesting the importance of CB1 as a regulator of bone resorption. In contrast, CB1 deficient mice were found by Tam et al. (10), to have reduced peak bone mass. Another study noticed that aged mice with CB1 gene deficiency have low bone mass and adipocyte number (15). It has been reported that CB1 signaling affects the glucocorticoid regulation of the hypothalamus function (16). Although no distinct abnormalities in bone development were observed in healthy adult mice deficient in cannabinoid type 2 receptors (CB2), pharmacological blockage of this receptor is effective in suppressing bone loss associated with increased bone turnover (17).Wang et al. demonstrated that skeletal tissue responds to supraphysiologic levels of glucocorticoid with decreased bone formation (18, 19).
Rimonabant is a potent CB1 ligand, with 1000-fold affinity for CB1 than CB2. It is a CB1 receptor antagonist in the brain and peripheral organs as bone system with therapeutic use as an antiobesity drug (20).
The present study, investigated the modulation of CB1 function can affect the deleterious effects of glucocorticoid treatment on bone remodeling in skeletally immature and mature rats.
MATERIALS AND METHODS
Experiments were performed according to the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, National Research Council, Washington, DC, National Academy Press, no. 85-23, revised 1996). All protocols were approved by our Local Committee of Animal Care and Use Committee.
All experimental osteoporosis protocols can be implemented in skeletally immature or mature rats (21). Although rats reach sexual maturity at the age of 2.5 months, their skeleton is considered mature after the age of 10 months (22).
Sixty four male Sprague Dawley rats were obtained from the Animal House Colony of Medical Experimental Research Centre, Mansoura University, Egypt. The animals were housed in plastic cages at room temperature (25 ± 2°C) under a 12 hour dark-light cycle. They were provided with tap water and balanced diet ad libitum and allowed to acclimate for two weeks to housing conditions.
They were divided into two main groups each consisted of 32 rats: group 1 (G1) consisted of skeletally mature rats (12–14 month old, 300–350 g) and group 2 (G2) consisted of skeletally immature rats (3–4 month old, 250–280 g). Each main group were subdivided into four subgroups (each one consisted of 8 rats) as follows: subgroups (NC1) and (NC2), the negative control subgroups in which rats received vehicle injection, subgroups (MP1) and (MP2), were received methylprednisolone intramuscular (Pfizer Company, suspension) 0.2 mg/kg, 3 times/week for 5 weeks (23) (osteoporotic subgroups), subgroups (RIM1) and (RIM2), were received rimonabant, subanorectic dose 50 ug/kg/ intraperitoneally (cayman chemical, powder, for 5 consecutive weeks) , it was dissolved in dimethyl sulfoxide (Sigma-Chemical .CO, St. Louis, MO, USA) and then the needed concentration dissolved in 0.5% methyl cellulose (Sigma Chemical CO, St. Louis, MO, USA) and a corresponding methyl cellulose - dimethyl sulfoxide solution was given as vehicle control injection (24). Subgroups (MP + RIM1) and (MP + RIM2) were receivedwith both methylprednisolone and rimonabant in the same previous doses.
Sample collection
At the end of the experimental period, blood samples from fasting rats were withdrawn from the retro-orbital venous plexus under diethylether anesthesia. Then, centrifuged at 3000 rpm for 15 min at 4°C where the clear sera were separated stored at –20°C until analyses. The animals were then sacrificed and tibias were harvested.
Right tibia from each rat was stored in formalin buffer 10% for dual energy X-ray absorptiometry (DEXA). Bone mineral density (BMD) and bone mineral content (BMC) of each tibia were measured by DEXA using Norland XR-46, version 3.9.6/2.3.1 instrument (Norland X-R-46 version 3.9.6, Peachtree City, GA, USA) equipped with dedicated software for small animal measurements. The other tibia from each rat was stored in liquid nitrogen for gene expression of RANKL and osteoprotegerin.
Image analysis procedure
Tibia from each rat was embedded in paraffin sections and were stained with hematoxylin and eosin for image analysis technique as following, Slides were photographed using Olympus® digital camera installed on the Olympus® microscope with 1/2 X photo adaptor, using a 40X objective. The resulting images were saved as TIFF files to be analyzed with Intel® Core I3® based computer using VideoTest Morphology® software (Russia) with a specific built-in routine for length and area fraction measurement.
Cortical bone thickness was measured by a free hand line tool which is calibrated against a micrometer slide photographed under the same conditions.
The trabecular bone area was manually extracted with the aid of genius® G-Pen F509® tablet. Total area of the extracted trabecular bone was measured. Then the empty areas were subtracted according to color variation and the area was measured again. Area fraction was calculated in percents. Mean cortical bone thickness (CBT) (µm) and mean trabecular bone density (TBD) (%) were obtained by measuring 5 fields/slide from 5 slides for each rat. The reading of each animal was considered as one variable.
Serum measurements
Serum total calcium (25) and alkaline phosphatase (ALP) activity (26) were measured by colorimetric method (Chemroy, Biochemical Trade, Inc. USA) in Beckman Coulter DU-70 spectrophotometer (Beckman Coulter Inc., CA, USA). Serum osteoprotegerin (OPG), receptor activator of nuclear factor kappa B ligand (RANKL) levels were determined by enzyme linked immunosorbent assay (ELISA) technique using R&D Elisa (Sorin Biomedica, Eti-System, Denlay Instruments Ltd, England) kit as described by O'Brien et al. (27) and Teng et al. (28) respectively. Serum osteocalcin was also measured with osteocalcin (OC) rat ELISA system (Rat Bone Panel3 Millipore, MA, USA) (29).
Real-time PCR analyses of bone osteoprotegrin and receptor activator of nuclear factor kappa B ligand
The metaphyseal portion of the tibia was cut and homogenized using a Mixer Mill MM400 (Retsch, Germany) to isolate the mRNA. Total RNA was isolated using Trizol following the manufacturer's guidelines (Invitrogen, Rockville, MD, USA). The concentration and purity of the RNA were determined by measuring the absorbance at 260 nm and 280 nm. The amount of RANKL and OPG mRNA was determined with ABI Prism 7900HT quantitative real-time PCR (Applied Biosystems, Foster City, CA). The primers were as follows: RANKL (forward, 5’-ACC AGC ATC AAA ATC CCA AG-3’, reverse, 5’-TTT GAA AGC CCC AAA GTA CG-3’) and OPG (forward, 5’-GTT CTT GCA CAG CTT CAC CA-3’, reverse, 5’-AAA CAG CCC AGT GAC CAT TC-3’). PCR amplification was carried out in a 20 µL reaction mixture (2 µL of cDNA and 200 nmol/L primers for OPG and RANKL, respectively, and 1 µL SYBR green). The temperature program was as follows: inactivation of reverse transcriptase at 95°C for 30 s, followed by 45-cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 20 s. The specificity of the PCR results was confirmed by melting curve analysis. The mRNA levels for each gene were calculated using a standard curve generated from 10-fold dilutions of a control RNA (Roche, Penzberg, Germany). The expression levels of RANKL and OPG were normalized to S18 RNA levels, and all samples were analyzed in triplicate.
Statistical analysis
The results were statistically analyzed using One-way ANOVA test to evaluate the significance between the different investigated groups using SPSS version 15 (Chicago, IL, USA). Results were presented as means and standard deviations (S.D.). P values ≤0.05 were considered statistically significant.
RESULTS
Dual x-ray absorptiometric analyses revealed that rats in the glucocorticoid-treated group had lower bone mineral density and bone mineral content than those in the vehicle-treated group after 5 weeks of glucocorticoid treatment (Tables 3 and 4). Moreover, in Tables 1 and 2 glucocorticoid treatment significantly increased alkaline phosphatase activity, decreased blood osteocalcin level, and decreased bone matrix osteoprotegerin expression together with increase in expression of osteoclast-promoting factor RANKL (Figs. 1 and 2). Image analysis examination of bone tissue from glucocorticoid-treated rats confirmed trabecular bone loss in association with enlarged fat cell accumulation in the bone marrow compartment (Table 5).
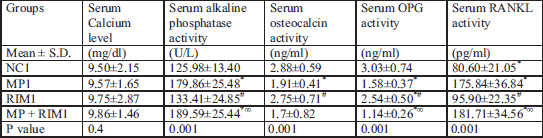
P value = ANOVA P value for comparison among old age groups; * = significance between control group and other groups; # = significance between methyl predinisolone group and rimonabant group or rimonabant + methyl predinisolone group; ∞ = significance between rimonabant group and rimonabant + methyl predinisolone group.
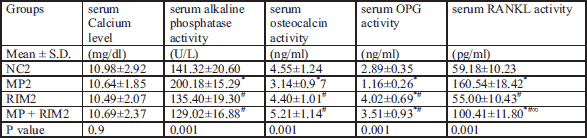
P value = ANOVA P value for comparison among young age groups; * = significance between control group and other groups; # = significance between methyl predinisolone group and rimonabant group or rimonabant + methyl predinisolone group; ∞ = significance between rimonabant group and rimonabant + methyl predinisolone group.

P value = ANOVA P value for comparison among old age groups; * = significance between control group and other groups;
# = significance between methyl predinisolone group and rimonabant group or rimonabant + methyl predinisolone group;
∞ = significance between rimonabant group and rimonabant + methyl predinisolone group.

P value = ANOVA P value for comparison among old age groups; * = significance between control group and other groups;
# = significance between methyl predinisolone group and rimonabant group or rimonabant + methyl predinisolone group;
∞ = significance between rimonabant group and rimonabant + methyl predinisolone group.
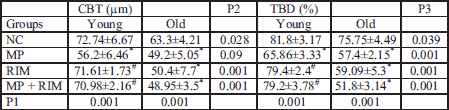
P1 = ANOVA P value for inter-comparison groups; P2 = ANOVA P value for comparison between young and old groups concerning CBT; P3 = ANOVA P value for comparison between young and old groups concerning TBD. * = significance between control group and other groups (young or old); # = significance between methyl predinisolone group and rimonabant group or rimonabant + methyl predinisolone group either for young or old.
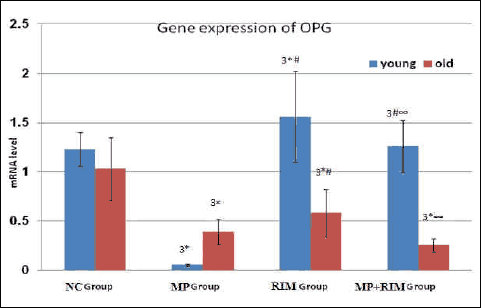 |
Fig. 1. Expression of OPG in rat bone. Values were normalized to expression of S18 RNA levels and expressed in old relative to data from young rats. Values are means ± S.D. (8 rats/group). NC Group (negative control group), MP Group (methylpredinisolone group), RIM Group (rimonabant group), MP + RIM Group (rimonabant + methylpredinisolone group), 3 = means extremely significant statistically. * = significance between control group and other groups either for young or old age groups; # = significance between MP group and RIM group or MP + RIM group either for young or old age groups; ∞ = significance between RIM group and MP + RIM group either for young or old age groups. |
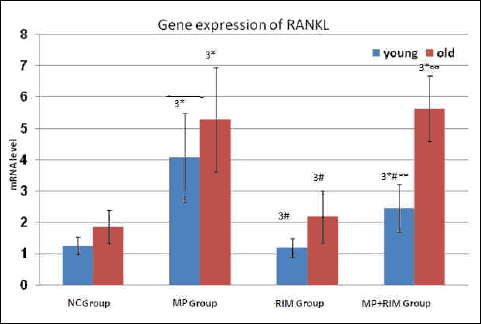 |
Fig. 2. Expression of RANKL in rat bone. Values were normalized to expression of S18 RNA levels and expressed in old relative to data from young rats. Values are means ± S.D. (8 rats/group). NC Group (negative control group), MP Group (methylpredinisolone group), RIM Group (rimonabant group), MP + RIM Group (rimonabant + methylpredinisolone group), 3 = means extremely significant statistically. * = significance between control group and other groups either for young or old age groups; # = significance between MP group and RIM group or MP + RIM group either for young or old age groups; ∞ = significance between RIM group and MP + RIM group either for young or old age groups. |
The present results revealed that treatment with rimonabant significantly attenuated the inhibitory effects of glucocorticoid on bone mineral density, bone mineral content in skeletally immature rats (young group). On the contrary it deteriorated the BMD and BMC in the old group (Tables 3 and 4). There was a significant improvement in mean CBT and mean TBD in young roup after rimonabant administration either alone or in combination with glucocorticoid (Table 5 data supported the dual X-ray analysis). On the other hand rimonabant administration alone or with glucocorticoid in the old group produced a significant decrease in CBT and TBD.
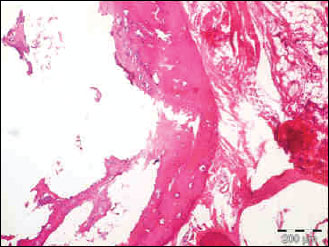 |
Fig. 3. The photomicrograph of a section in the tibia of old age rats received rimonabant+methylprednisolone, showing a significant decrease in mean cortical bone thickness and mean trabecular bone density. Hx. & E.; ×100. |
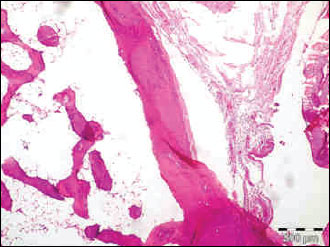 |
Fig. 4. The photomicrograph of a section in the tibia of old age rats received rimonabant, showing a significant decrease in mean cortical bone thickness and mean trabecular bone density. Hx. & E.; ×100. |
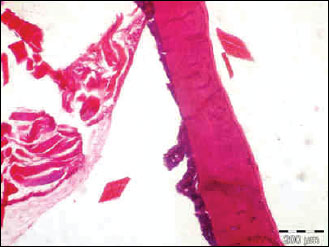 |
Fig. 5. The photomicrograph of a section in the tibia of young age rats received methyl prednisolone, showing a significant trabecular bone loss in association with enlarged fat cell accumulation in the bone marrow compartment. Hx. & E.; ×100. |
The group of young rats receiving CB1 antagonist alone showed higher bone mass than those in the corresponding control group, however, there was a marked reduction in BMD and BMC in older age than those in the corresponding control group (Table 3).
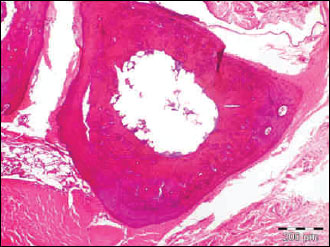 |
Fig. 6. The photomicrograph of a section in the tibia of young age rats received rimonabant showing a significant improvement in mean cortical bone thickness and mean trabecular bone density. Hx. & E.; ×100. |
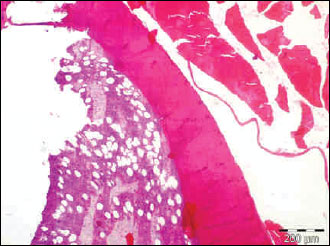 |
Fig. 7. The photomicrograph of a section in the tibia of old age rats received methyl prednisolone, showing a significant trabecular bone loss in association with enlarged fat cell accumulation in the bone marrow compartment. Hx. & E.; ×100. |
Rimonabant increased the expression of osteoprotegerin together with increase in activity of osteocalcin and inhibited alkaline phosphatase activity in comparison to glucocorticoid treated subgroup in the young rats (Table 2). On the contrary, in Table 1 rimonabant in the old group showed more deterioration in bone remodeling markers in comparison to the osteoporosis subgroup. These parameters revealed more osteoporosis with combined rimonabant and glucorticoid than glucocorticoid alone. Rimonabant alone in old rats (subgroup RIM1) showed osteoporotic indices like increase in levels of RANKL/OPG ratio, decrease in osteocalcin activity, together with increase in alkaline phosphatase activity.
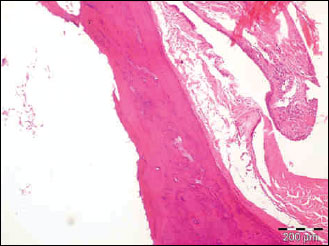 |
Fig. 8. The photomicrograph of a section in the tibia of control normal old age rats showing mean cortical bone thickness and mean trabecular bone density. Hx. & E.; ×100. |
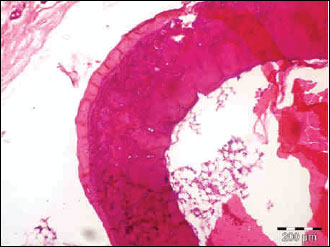 |
Fig. 9. The photomicrograph of a section in the tibia of control normal young age rats showing mean cortical bone thickness and mean trabecular bone density. Hx. & E.; ×100. |
DISCUSSION
Osteoporosis is a metabolic bone disease that is characterized by reduced bone mass and deterioration of bone microarchitecture (30, 31). By examining the present DEXA and morphometry bone results, they showed that a chronic glucocorticoid therapy was associated with low BMD and BMC with suggestion of low bone turnover and the high susceptibility of fractures, this finding agreed with other researches such as Sosa et al. (32) and Migliaccio et al. (33) who found that prednisolone administration induces apoptosis of both osteoblasts and osteocytes that lead to the suppression of bone formation and low BMD. Moreover, Surve et al. (34, 35) noticed that glucocorticoid decreases the periosteal mineralizing surface.
Bone resorption and formation are tightly orchestrated via the RANK/ RANKL/OPG system (36, 37). OPG is produced by osteoblast and plays a key role in the physiological regulation of osteoclastic bone resorption. It has no direct signaling capacity and acts by binding to its natural ligand which is known as RANKL. Ko et al. (38) found that this binding prevents activation of RANK receptor which is essential for osteoclast differentiation, activation and survival then inhibiting bone resorption. Furthermore, the inhibitory effect of OPG on bone resorption can also be explained as a modulator of RANKL half-life (39).
The present results proved that supraphysiologic levels of glucocorticoid lead to an imbalance in the RANKL/OPG) ratio, with increasing serum and gene expression of RANKL and decreasing serum and gene expression of OPG. These parameters revealed osteoclastogenesis and accelerated bone resorption leading to a reduction in the skeletal mass which is the hallmark of the osteoporosis. This result agreed with that of McLaughlin et al. (40) who reported that glucocorticoids promote osteoclastogenesis via increasing RANKL and decreasing the OPG expression Also, the present study showed age-related OPG elevation as a compensatory phenomenon to slow down enhanced bone resorption.
This study proved the age-related reduction in bone mineralization together with reducing expression of OPG gene and increasing the expression of RANKL gene. However, serum calcium concentration did not show age-related reductions, this was different from humans in that rats were fed a nutritionally complete diet throughout aging. In addition, Elshal et al. (41) described that serum calcium concentration changes only in a critical situation, such as undernutrition or hyperparathyroidism.
Serum OC and ALP bone markers were lower with aging in this study. OC is a biomarker of bone formation and ALP is produced by osteoblasts and is proportional to bone remodeling rates (42). These results suggested that the bone remodeling slows with growing in age in this rat model. Also, results revealed increasing susceptibility of osteoporosis and can be explained by Gimble et al. (43) who found that bone marrow stromal cells (MSCs) from elderly subjects have a reduced capacity to differentiate into osteoblasts and an increased capacity to differentiate into adipocytes, which leads to progressive accumulation of fat in the bone marrow space with increasing age.
Idris et al. (8) were first to report that genetic inactivation of the CB1 receptor results in high peak bone mass in young mice with less osteoclasts and reduced bone resorption. These findings have led to the realization that cannabinoid receptors may play a significant role in the regulation of peak bone mass. The present study agreed with Idris et al. study (8) in increasing BMD and BMC in the skeletally immature rats treated with rimonabant, but our results went on to show marked osteoporosis with CB1 blocker with increasing age due to a defect in bone formation and accumulation of marrow fat. Idris et al. (15) observed that there was a significant increase in levels of CB1 transcript between 3 and 12 months of age in freshly isolated bone marrow cells from wild-type mice, demonstrating that CB1 expression is upregulated with age in the bone marrow compartment, They also observed direct effects of CB1 on osteoblast and osteoclast differentiation in vitro.This can provide our results a possible explanation for the age-related effects of CB1 antagonist of bone loss.
Tam et al. (11) speculated that CB1 stimulates bone formation by inhibiting release of catecholamines from peripheral nerves, the present study indicates that administration of CB1 blocker caused an increase in serum osteocalcin and decrease in serum ALP activities.
The present data revealed a reduction in RANKL gene expression and an increase in 86gene expression of OPG in the skeletally immature rats. This coincides with Idris et al. (15) who suggested that the defect in osteoclast formation in CB1 deficient mice is caused by a reduction in the sensitivity of osteoclast precursors to RANKL and a reduction in the ability of CB1 deficient osteoblasts to support osteoclast formation due to reduced RANKL expression.
Many studies explained the glucocorticoid effects on BMD and BMC in the old and young (35, 38), and also, the improvement in CBT and TBD in young rats after a CB1 blocker administration either alone or in combination with glucocorticoid, while in the old group ,there was a significant decrease in CBT and TBD (13, 15, 17).
This work has important clinical implications in raising the possibility that cannabinoid receptor ligands may be of value therapeutically in enhancing peak bone mass and in preventing age-related osteoporosis, but indicates that antagonists may exert contrasting effects on the skeleton at different stages in life.
Conclusion
It has been concluded that CB1 receptor play age related different roles in bone turnover processes. So, the beneficial effects of CB1 blocker as rimonabant in modulating the majority of bone markers and improving bone mineral density and bone mineral content in order to preserve bone tissue against secondary osteoporosis induced by glucocorticoid therapy in young. It was suggested that CB1 receptor antagonist aggravates and may mediate age related osteoporosis, so, it should be prohibited in old age.
Abbreviations: CB1, cannabinoid receptor 1; BMD, bone mineral density; BMC, bone mineral content; OPG, osteoprotegrin; RANKL, receptor activator of nuclear factor kappa B ligand; CBT, mean cortical bone thickness; TBD, mean trabecular bone density;
Acknowledgements: Experimental Medical Research Center in the Faculty of Medicine is acknowledged for significant contribution to the experimental care and biochemical study. We also acknowledge Dr Azza Abdel Aziz, Assoc. Prof. of pathology in the Faculty of Medicine for helping in performing pathology and The National Research Centre, Dokki, Giza , Egypt, for performing DEXA and real time PCR.
Conflict of interest: None declared.
REFERENCES
- Rauch A, Seitz S, Baschant U, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 2010; 11: 517-531.
- Kim HJ, Zhao H, Kitaura H, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest 2006; 116: 2152-2160.
- Lu NZ, Collins JB, Grissom SF, Cidlowski JA. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol Cell Biol 2007; 27: 7143-7160.
- Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology 2010; 151: 2641-2649.
- Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum 2008; 58: 1674-1686.
- Khosla S. The OPG/RANKL/RANK system. Endocrinology 2001; 42: 5050-5055.
- Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcif Tissue Int 2007; 80: 337-347.
- Idris AI, van't Hof RJ, Greig IR, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med 2005; 11: 774-779.
- Ofek O, Karsak M, Leclerc N, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 2006; 103: 696-701.
- Tam J, Ofek O, Fride E, et al. Involvement of neuronal cannabinoid receptor, CB1, in regulation of bone mass and bone remodeling. Mol Pharmacol 2006; 70: 786-792.
- Tam J, Trembovler V, Di Marzo MV, et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J 2008; 22: 285-294.
- Bab I, Ofek O, Tam J, Rehnelt J, Zimmer A. Endocannabinoids and the regulation of bone metabolism. J Neuroendocrinol 2008; 20 (Suppl. 1): 69-74.
- Idris AI. The promise and dilemma of cannabinoid therapy: lessons from animal studies of bone disease. IBMS Bonekey 2011; 8: 84-95.
- Buettner C, Muse ED, Cheng A, et al. Leptin controls adipose tissue lipogenesis via central STAT3-independent mechanisms. Nat Med 2008; 14: 667-675.
- Idris AI, Sophocleous A, Lando-Bassonga E, et al. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab 2009; 10: 139-147.
- Malcher-Lopes R, Di S, Marcheselli VS, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 2006; 26: 6643-6650.
- Idris AI. The promise and dilemma of cannabinoid therapy: lessons from animal studies of bone disease. Bonekey Rep 2012; 1: 224.
- Wang FS, Ko JY, Yeh DW, Ke HC, Wu HL. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation and bone mass loss. Endocrinology 2008; 149: 1793-1801.
- Wang FS, Ko JY, Weng LH, Yeh DW, Ke HJ, Wu SL. Inhibition of glycogen synthase kinase-3beta attenuates glucocorticoid-induced bone loss. Life Sci 2009; 85: 685-692.
- Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV. Rimonabant : an antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep 2009; 61: 217-224.
- Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 2001; 1: 193-207.
- Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med 2008; 58: 424-430.
- Kashani IR, Moradi F, Pasbakhsh P, Sobhani A, Nikzad H, Sobhani A. Prevention of methylprednisolone acetate-induced osteoporosis with calcium administration in rat model. Acta Med Iranica 2009; 47: 251-257.
- Tallett AJ, Blundell JE, Rodgers JR. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology (Berl) 2007; 195: 27-39.
- Faulkner WR. Selected Methods for the Small Clinical Chemistry Laboratory. Washington, American Association for Clinical Chemistry, 1982, pp. 126.
- Vasiliades J. Reaction of alkaline sodium picrate with creatinine. Kinetics and mechanism of formation of the mono- creatinine picrate acid complex. Clin Chem 1976; 22: 1664-1671.
- O'Brien EA, Williams JH, Marshall MJ. Osteoprotegerin is produced when prostaglandin synthesis is inhibited causing osteoclasts to detach from the surface of mouse parietal bone and attach to the endocranial membrane. Bone 2001; 28: 208-214.
- Teng YT, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest 2000; 106: R59-R67.
- Netto CC, Vieira VC, Marinheiro LP, Agellon S, Weiler H, Marostica MR. Are skeletally mature female rats a suitable model to study osteoporosis? Arq Bras Endocrinol Metab 2012; 56: 259-264.
- Ferrari S. Bone remodeling: new therapeutic approaches. Rev Med Suisse 2009; 10: 1325-1328.
- Tsirella E, Mavrakanas T, Rager O, et al. Low dose pioglitazone does not affect bone formation and resorption markers or bone mineral density in streptozocin-induced diabetic rats. J Physiol Pharmacol 2012; 63: 201-204.
- Sosa M, Jodar E, Saavedra P, et al. Postmenopausal Canarian women receiving oral glucocorticoids have an increased prevalence of vertebral fractures and low values of bone mineral density measured by quantitative computer tomography and dual X-ray absorptiometry, without significant changes in parathyroid hormone. Eur J Intern Med 2008;19: 51-56.
- Migliaccio S, Brama M, Fornari R, Greco EA, Spera G, Malavolta N. Glucocorticoid-induced osteoporosis: an osteoblastic disease. Aging Clin Exp Res 2007; 19 (Suppl. 3): 5-10.
- Surve VV, AnderssonN, Lehto-Axtelius D, Hakanson R. Comparison of osteopenia after gastrectomy, ovariectomy and prednisolone treatment in the young female rat. Acta Orthop Scand 2001; 72: 525-532.
- Tomaszewska E, Dobrowolski P, Wydrych J. Postnatal administration of 2-oxoglutaric acid improves articular and growth plate cartilages and bone tissue morphology in pigs prenatally treated with dexamethasone. J Physiol Pharmacol 2012; 63: 547-554.
- Deif MM, Fawzy EM, Gad H. Effect of melatonin on some bone turnover markers in ovarectomized rats. Bull Alex Fac Med 2007; 43: 399.
- Fichna M, Zurawek M, Fichna P, Gryczynska M, Nowak J, Ruchala M. Increased serum osteoprotegerin in patients with primary adrenal insufficiency receiving conventional hydrocortisone substitution. J Physiol Pharmacol 2012; 63: 677-682.
- Ko JY, Wu RW, Kuo SJ, et al. Cannabinoid receptor 1 mediates glucocorticoid-induced bone loss in rats by perturbing bone mineral acquisition and marrow adipogenesis. Arthritis Rheum 2012; 64: 1204-1214.
- Nakamichi Y, Udagawa N. The role of OPG in regulation of bone remodeling. Clin Calcium 2006; 16: 1463-1468.
- McLaughlin F, Mackintosh J, Hayes BP, et al. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone 2002; 30: 924-930.
- Elshal MF, Almalki AL, Hussein HK, Khan JA. The synergistic antiosteoporotic effect of Lepidium sativum and alendronate in glucocorticoid-induced osteoporosis in Wistar rats. Afr J Tradit Complement Altern Med 2013; 10: 267-273.
- Perez-Castrillon JL, Olmos JM, Nan DN, et al. Polymorphisms of the WNT10B gene, bone mineral density, and fractures in postmenopausal women. Calcif Tissue Int 2009; 85: 113-118.
- Gimble JM., Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem 2006; 98: 251-266.
A c c e p t e d : August 28, 2014