PEROXISOME PROLIFERATOR ACTIVATED RECEPTOR LIGANDS AFFECT PROGESTERONE AND 17β-ESTRADIOL SECRETION BY PORCINE CORPUS LUTEUM DURING EARLY PREGNANCY
2Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, Olsztyn, Poland
INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear receptor family. Three isoforms of PPARs have been described as -α, -β/δ, and -γ. As transcriptional factors PPARs regulate expression of genes that control cell differentiation and proliferation (1, 2). They can be activated by endogenous (polyunsaturated fatty acids and arachidonic acid derivatives) and exogenous (fibrates, thiazolidinediones - TZD, non-steroid anti-inflammatory drugs) ligands (3). Numerous studies have revealed that PPARs are involved in adipocyte differentiation, lipid metabolism and glucose homeostasis (4-9). They also affect inflammatory responses (10-12) and reproductive processes (13-15). It has been reported that PPARs are expressed in the ovarian follicle, luteal cells and uterine tissues in rodents, cows, dogs and pigs (3, 15-17). They are involved in the regulation of basic ovarian processes during the estrous cycle and pregnancy. PPARs control steroidogenesis, angiogenesis, tissue remodeling, cell cycle and apoptosis (14, 18, 19). The activation of PPARs by TZDs stimulates secretion of progesterone and estradiol in rat, ovine and bovine granulosa cells (11, 18) as well as porcine theca cells (20).
During pregnancy, PPARs regulate the embryo implantation and placenta development. PPARγ inactivation in mice is lethal and leads to death of the embryo at an early stage of development (21). Moreover, tissue-specific deletion of PPARγ in mouse ovary strongly disrupted the embryo implantation (22). An important role in implantation is also assigned to beta isoform of PPAR (23, 24). PPARβ-null mice showed abnormalities in placenta development (21). An administration of a specific PPARβ agonist restored implantation disorders in rats with deficiencies of cyclooxygenase-2 (COX-2) and it even increased the number of implanted embryos compared with control group (23). A deletion of alpha isoform also affects fertility in mice. PPARα-null rodents display a higher risk of maternal abortion and neonatal mortality (25). Recent data underline a significance of PPARs in pregnancy maintenance and delivery.
Our previous results showed the presence of PPARs (-α, -β, and -γ1) mRNA in porcine endometrium collected from different stages of the estrous cycle and early pregnancy (26). A marked increase in PPARγ1 mRNA level on days 13–15 of the estrous cycle and the decrease in PPARβ on days 11–12 of pregnancy suggested that PPARs are engaged, respectively, in luteolysis (corpus luteum regression) and maternal recognition of pregnancy in the pig (26). In further experiments we found that PPARs might be mediators of PGF2α (with luteolytic properties) and PGE2 (with luteotrophic properties) synthesis/secretion by porcine endometrium during the luteal phase of the estrous cycle and the time of periimplantation (27-29).
In the present study we examined the in vitro effect of PPAR ligands on progesterone (P4) and 17β-estradiol (E2) secretion by porcine corpora lutea (CL) on days 10–12 and 14–16 of the estrous cycle or pregnancy. Additionally, the expression of gene coding 3β-hydroxysteroid dehydrogenase/Δ(5)-Δ(4) isomerase (3β-HSD), an enzyme that catalyzes the synthesis of progesterone, was also determined.
MATERIALS AND METHODS
Animals
All procedures relative to the care and use of animals were approved by the Local Animal Ethics Committee of the University of Warmia and Mazury in Olsztyn (Poland) and the study was conducted in accordance with the national guidelines for animal care (approval No 72/2007).
The study was performed on crossbred pigs (100 kg, 7 month-old) from a commercial farm. Premature gilts were treated hormonally as described previously (27-29). Briefly, single intramuscular injection of 750 IU PMSG (Folligon, Intervet, Netherlands) was followed by 500 IU hCG (Chorulon, Intervet) administered 72 hours later. The animals were divided into the following experimental groups: cyclic (days 10–12 and 14–16 of the estrous cycle, n=4–6 in each group) or pregnant (days 10-12 and 14–16 of pregnancy, n=4–6 in each group). The gilts designated to the pregnant group were inseminated twice, 24 h and 36 h after the hCG treatment. Two stages of the estrous cycle represent mid- and late-luteal phases. The analysed days of the pregnancy reflect maternal recognition of pregnancy and the beginning of implantation. During slaughter the ovaries were dissected and transported to the laboratory on ice in sterile PBS with antibiotics (penicillin and streptomycin, Polfa Tarchomin, Poland).
Incubation of the corpus luteum explants
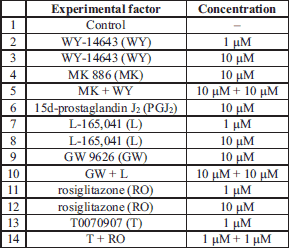
The procedure for the collection and incubation of the corpus luteum tissue was described previously (30). Isolated CLs, from cyclic gilts at the 10–12 (n=5) and 14–16 (n=4) day of estrous cycle and at the 10–12 (n=7) and 14–16 (n=5) day of pregnancy, were cut into small pieces (about 100 mg w/w) and washed twice with sterile PBS. Each tissue piece was placed in a sterile culture vial with 2 ml of medium 199 supplemented with 0.1% BSA, gentamycin (40 µg/ml) and nystatin (120 IU/ml). The pieces were pre-incubated in a water bath for 18 h in an atmosphere of 95% O2 and 5% CO2 and then treated for 6 h with the following reagents (Table 1): PPARα ligands WY-14643 (agonist; 1 and 10 µM; Cayman Chemical Company, USA) and MK 886 (antagonist; 10 µM; Enzo Life Sciences International, USA); PPARβ ligands L-165,041 (agonist; 1 and 10 µM, TOCRIS Bioscience, USA) and GW 9662 (antagonist; 10 µM; Cayman Chemical Company, USA) and PPARγ ligands 15d-prostaglandin J2 (agonist; 10 µM; Enzo Life Sciences International, USA), rosiglitazone (agonist; 1 and 10 µM; Cayman Chemical Company, USA) and T0070907 (antagonist; 1 µM, Cayman Chemical Company, USA). The PPAR ligand concentrations and incubation times were selected according to our preliminary study and previous reports as previously described (20-22). The tested compounds were added to culture media in a total volume of 20 µl dimethyl sulfoxide (DMSO, Sigma, USA). Controls (without the treatments) contained culture media or DMSO. After incubation, the CL slices were washed with PBS and snap frozen at –80°C for total RNA isolation and real-time RT-PCR quantification. Incubation media were collected for radioimmunology assay and frozen at –20°C.
Determination of P4 and E2 concentration in culture media
Concentrations of P4 in culture media collected after 6 hours incubation of corpus luteum explants with the tested factors were determined by RIA according to the protocol of Ottobre et al. (31). Media E2 was determined by RIA according to the Hotchkiss`s (32) protocol. Efficiency of P4 extraction was about 95.5% and sensitivity was 2 pg/ml. The inter- and intra- assay coefficients were less than 1.81% and 8.28%, respectively. E2 has not been extracted, sensitivity was 500 pg/ml. The inter- assay and intra- assay coefficients were less than 2.71% and 10.01%, respectively.
RNA isolation and real time RT-PCR
Total RNA was isolated with the 'Total RNA' kit (A&A Biotechnology, Poland), quantified spectrophotometrically and the integrity of the product was confirmed on 1.5% agarose gel. The sequences of primers and Taqman probe for 3β-HSD (GenBank No AF232699) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GenBank No U48832) were designed using Primer Express Software 3 (Applied Biosystems, CA, USA) and were synthesized by Applied Biosystems. The following primer and probe sequences were used: 3β-HSD forward ACCGTCATGAAGGTCAATGTGA, 3β-HSD reverse GATGAAGACCGGCACGCT, 3β-HSD probe CAGCTCCTGCTGGAGGCCTGTGTC; GAPDH forward CATCAATGGAAAGGCCATCAC, GAPDH reverse CAGCATCGCCCCATTTG and GAPDH probe CTTCCAGGAGCGAGATCCCGCC. The expressions of mRNA encoding 3β-HSD and GAPDH were determined using TaqMan®RNA-to-CTTM 1-Step Kit (Applied Biosystems). The concentrations of the PCR primers were 300 nM and 200 nM of the TaqMan fluorogenic probes labeled with FAM (6-FAM,6-carboxyfluorescein) dye. Real-time RT-PCR was carried out in an ABI PRSISM 7300 sequence detector (Applied Biosystems) using the following parameters: one cycle at 48°C for 30 min, then one cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and one cycle at 60°C for 1 min. All expression data were normalized to the amount of GAPDH mRNA and presented as arbitrary units (27, 33). GAPDH mRNA levels did not change in the presence of the tested factors.
Statistical analysis
Results were analysed by Statistica (version 8.0, StatSoft Inc, Tulsa USA). Significant differences were determined by one-way Anova for repeated measurements followed by least significant differences (LSD) post-hoc test. Statistical significances were assigned with different letters at P<0.05. The data are presented as means ± S.E.M.
RESULTS
The effect of PPAR ligands on P4 release
We observed an inhibitory effect (P<0.05) of WY-14643 (1 and 10 µM; PPARα agonist) on P4 release by the CL on days 14–16 of pregnancy (Fig. 1D). MK-886, PPARα antagonist, given alone as well as with the agonist, did not change P4 release by the CL. All tested PPARα ligands did not affect P4 release by the CL during both stages of the estrous cycle (Fig. 1A and 1B) andon days 10–12 of pregnancy (Fig. 1C).
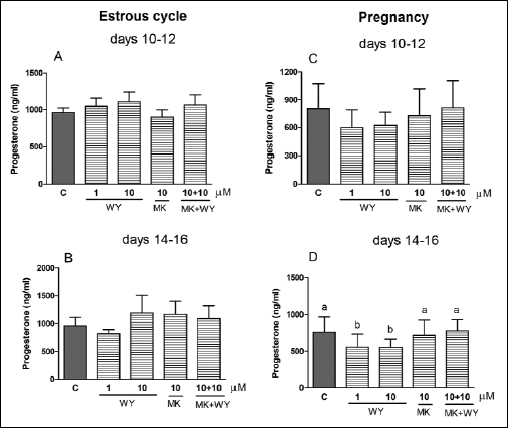 |
Fig. 1. The effect of PPARα agonist WY-14643 (WY, 1 and 10 µM) and/or its antagonist MK-886 (MK, 10 µM) on P4 release by the corpus luteum of gilts on days 10–12 (n=5) and 14–16 (n=4) of the estrous cycle (A and B) and days 10–12 (n=7) and 14–16 (n=5) of pregnancy (C and D). The tissue slices were incubated in the presence of the treatments or without (control) for 6 hours. Different letters indicate significant differences (P<0.05) between the control and the treatment within studied stage of the estrous cycle or pregnancy. |
The treatment of the CL explants with L-165,045 (1 and 10 µM, PPARβ agonist) diminished (P<0.05) P4 release by the tissue collected on days 10-12 (Fig. 2C) and days 14–16 of pregnancy (Fig. 2D). Moreover, combined addition of PPARβ agonist and the antagonist (GW 9662) also decreased (P<0.05) P4 release by the tissue during both stages of pregnancy. It should be emphasized that some doses of GW9626 may also affect the activity of PPARγ and PPARα. The PPARβ ligands did not affect P4 secretion by the CL during both stages of the estrous cycle (Fig. 2A and 2B).
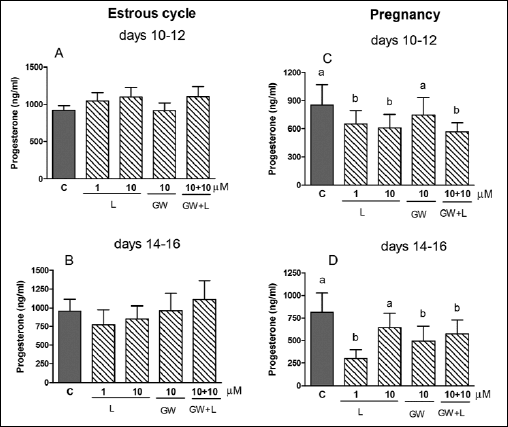 |
Fig. 2. The effect of PPARβ agonist L-165,041 (L, 1 and 10 µM) and/or its antagonist GW 9662 (GW, 10 µM) on P4 release by the corpus luteum of gilts on days 10–12 (n=5) and 14–16 (n=4) of the estrous cycle (A and B) and days 10–12 (n=7) and 14–16 (n=5) of pregnancy (C and D). The tissue slices were incubated in the presence of the treatments or without (control) for 6 hours. Different letters indicate significant differences (P<0.05) between the control and the treatment within studied stage of the estrous cycle or pregnancy. |
A natural PPARγ agonist, PGJ2, reduced (P<0.05) P4 release by the CL collected on days 14–16 (Fig. 3D). Rosiglitazone (1 µM; PPARγ agonist) inhibited (P<0.05) P4 secretion by the CL on days 10–12 of pregnancy (Fig. 3C) but the ligand was ineffective on days 14–16 of pregnancy as well as during both stages of the estrous cycle (Fig. 3A and 3B).
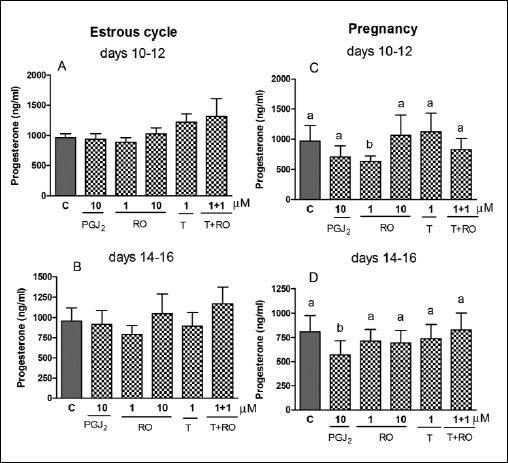 |
Fig. 3. The effect of PPARβ agonists: 15d-prostaglandin J2 (PGJ2, 10 µM), rosiglitazone (RO, 1 and 10 µM) and/or PPARβ antagonist T0070907 (T, 1 µM) on P4 release by the corpus luteum of gilts on days 10–12 (n=5) and 14–16 (n=4) of the estrous cycle (A and B) and days 10–12 (n=7) and 14–16 (n=5) of pregnancy (C and D). The tissue slices were incubated in the presence of the treatments or without (control) for 6 hours. Different letters indicate significant differences (P<0.05) between the control and the treatment within studied stage of the estrous cycle or pregnancy. |
The effect of PPAR ligands on E2 release
We observed an inhibitory effect of PPARα agonist, WY-14643 (1 and/or 10 µM) on E2 release by the explants collected from all stages of the estrous cycle and pregnancy (Fig. 4A-4D). The treatment of the CL explants with 1 and/or 10 µM of L-165,045 (PPARβ agonist) decreased E2 secretion by the tissue on days 10–12 and 14–16 of pregnancy (Fig. 5C and 5D), but the agonist did not change the steroid concentration in media during both stages of the estrous cycle (Fig. 5A and 5B). Surprisingly, the treatment of the tissue slices with GW 9662 alone and/or in combination with L-165,045 reduced E2 secretion in all tested stages of the estrous cycle and pregnancy (Fig. 5A-5D).The steroid concentration did not alter after the treatment of the CL slices with PPARγ ligands (agonists and/or antagonist) during analysed stages of the estrous cycle and pregnancy (Fig. 6A-6D).
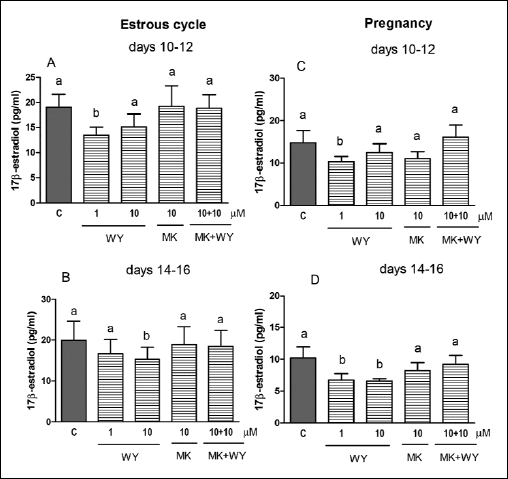 |
Fig. 4. The effect of PPARα agonist WY-14643 (WY, 1 and 10 µM) and/or its antagonist MK-886 (MK, 10 µM) on E2 release by the corpus luteum of gilts on days 10–12 (n=5) and 14–16 (n=4) of the estrous cycle (A and B) and days 10–12 (n=7) and 14–16 (n=5) of pregnancy (C and D). The tissue slices were incubated in the presence of the treatments or without (control) for 6 hours. Different letters indicate significant differences (P<0.05) between the control and the treatment within studied stage of the estrous cycle or pregnancy. |
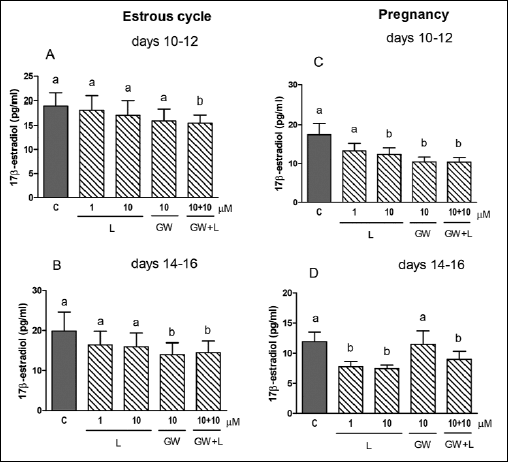 |
Fig. 5. The effect of PPARβ agonist L-165,041 (L, 1 and 10 µM) and/or its antagonist GW 9662 (GW, 10 µM) on E2 release by the corpus luteum of gilts on days 10–12 (n=5) and 14–16 (n=4) of the estrous cycle (A and B) and days 10–12 (n=7) and 14–16 (n=5) of pregnancy (C and D). The tissue slices were incubated in the presence of the treatments or without (control) for 6 hours. Different letters indicate significant differences (P<0.05) between the control and the treatment within studied stage of the estrous cycle or pregnancy. |
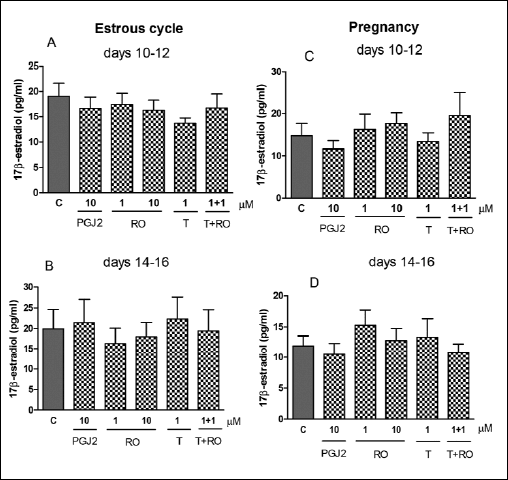 |
Fig. 6. The effect of PPARβ agonists: 15d-prostaglandin J2 (PGJ2, 10 µM), rosiglitazone (RO, 1 and 10 µM) and/or PPARβ antagonist T0070907 (T, 1 µM) on E2 release by the corpus luteum of gilts on days 10–12 (n=5) and 14–16 (n=4) of the estrous cycle (A and B) and days 10–12 (n=7) and 14–16 (n=5) of pregnancy (C and D). The tissue slices were incubated in the presence of the treatments or without (control) for 6 hours. There are no significant differences between the control and the treatment within studied stage of the estrous cycle or pregnancy. |
The effect of PPAR ligands on 3β-HSD mRNA abundance
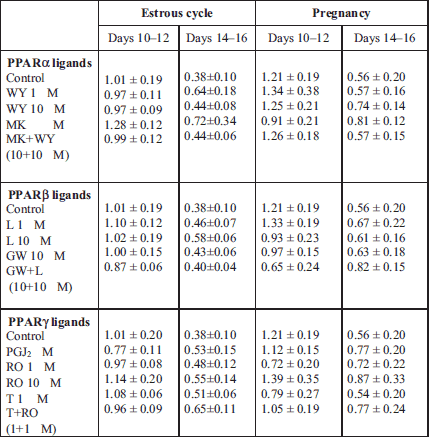
The tested compounds did not change 3β-HSD mRNA amounts in the tissue explants during both stages of the estrous cycle or pregnancy (Table 2).
DISCUSSION
In the present study we found that PPARs are involved in the regulation of P4 and E2 release by porcine corpus luteum explants cultured in vitro. Generally, PPAR agonists reduced P4 secretion by the tested tissue during analysed stages of pregnancy, whereas they were ineffective during the estrous cycle. In turn, PPAR ligands effect on E2 release was differential. While WY-14643 (PPARα agonist) diminished E2 secretion by the CL explants collected from the tested stages of the estrous cycle and pregnancy, PGJ2 and rosiglitazone (PPARγ ligands) did not induce any change in E2 level. In turn, L-165,041 (PPARβ agonist) reduced E2 release by the tissue during both stages of pregnancy but did not affect the secretion during the estrous cycle.
The role of PPARs in the regulation of reproductive processes has been previously reported, although -γ isoform has been studied most extensively compared with two other forms. It has been demonstrated that PPARγ might be an important regulator of steroidogenesis in the ovarian follicular cells of different species (34). However, available reports are inconsistent. For example, the exposure of mixed human ovarian (stroma, theca and granulosa) cells to rosiglitazone or pioglitazone (TZDs, PPARγ agonists) for 24 hours stimulated P4 accumulation in the culture media (35). In the same experiment, pioglitazone inhibited E2 production, whereas rosiglitazone (TZD, PPARγ agonist) had no effect (35). An enhanced P4 production was also noted after treatment of porcine theca (48 h) cells with TZDs (troglitazone or ciglitazone) or a natural PPARγ agonist PGJ2 (20, 36). In turn, a lack of changes in P4 and E2 production was observed after exposure (72 h) of human granulosa-lutein cells (GLCs) to rosiglitazone (37). There are also studies showing the reduction in P4 release by porcine and human granulosa cells in the presence of troglitazone (38, 39). Recent data showed that the activation of PPARs plays an important role in the regulation of steroidogenesis in polycystic ovary syndrome (PCOS). Rosiglitazone reduced media concentrations of E2 and P4 secreted by human granulosa cells (37). Progesterone production was also diminished in human choriocarcinoma JEG-3 cells as well as in MA-10 Leydig cells after treatment with PPARα activators (40, 41). The above contradictory results indicate that PPARγ mediates steroids production, however, a different effect of the receptor ligands on P4 and E2 synthesis might be dependent on the cell or ligand types, time of incubation with the treatments or different species. It should also be underlined, that phthalates, endocrine-disrupting chemicals, present in the environment and in food, cause profound and long-lasting changes of the reproductive functions. In particular, they may involve an interaction with PPARs and, in turn, affect steroid synthesis (42-46).
In the view of the above results from experiments performed mainly on ovarian follicular cells, it is difficult to address the present data. There are only two papers showing the effect of PPARγ ligands on P4 secretion by the CL in different species. Zerani et al. (47) observed a stimulatory effect of PGJ2 (PPARγ agonist) on P4 release by incubated for 4 h corpora lutea explants, collected from pseudopregnant rabbits at early and mid-luteal stages. Lohrke et al. (20) showed that the bovine luteal cells (24 h of culture, mid-phase of the estrous cycle) also enhanced P4 secretion in the presence of PGJ2 and ciglitazone (TZD, PPARγ agonist). On the contrary, in our study we noted that PPAR agonists generally reduced P4 secretion by porcine CL explants during both stages of pregnancy, but surprisingly the ligands were ineffective during both stages of the estrous cycle.
The above observation suggests that porcine CL indicates a different receptivity to PPAR ligands depending on the reproductive status of animals. Furthermore, the regulation of P4 synthesis/secretion within the CL, dependent on PPARs, is differentially regulated during the luteal phase of the estrous cycle and early pregnancy. Since PPAR ligands did not affect P4 secretion during the estrous cycle but they reduced P4 release during pregnancy, we may suggest that PPARs would make the CL more sensitive for the ligands during early pregnancy. Moreover, differential action of L-165,045 (10 µM) or rosiglitazone (1 µM) on P4 secretion by porcine CL explants, observed at days 10–12 and 14–16 of pregnancy, would be also caused by diverse receptivity of the tissue to the ligands during the tested stages of pregnancy.
The secretory function of the CL (a main source of progesterone) is significant for the maintenance of pregnancy in the pig. Though, we do not know why such alterations in PPARs activity that change CL sensitivity occur during early pregnancy. It seems we need to be more cautious using PPAR ligands as a therapeutic agent because it may diminish P4 secretion during pregnancy. Our previous data screening showed that endometrial expressions of PPARα and PPARβ in pregnant sows were diminished during maternal recognition of pregnancy when compared with the remaining tested stages of pregnancy. Moreover PPARγ1 gene expression in the same tissue was low until day 16 of the pregnancy and rapidly increased after that. This may suggest that low expression of PPARs at analyzed stages of pregnancy requires less PPARs ligands. In the present experiment, abundance of PPAR agonists may disturb P4 secretion by the CL during early pregnancy in the pig. It may suggest that treatment with PPAR ligands during early pregnancy may affect physiological state of pregnancy. Other than in pseudopregnant rabbits (36), in our experiments PPAR ligands decreased P4 secretion and those mechanism can be explained by different immunological properties in early pregnant pigs. Due to the epitheliochorial placentation the changes in early pregnancy indicate a decrease in expression of inflammatory cytokines. A changed balance between anti- and pro-inflamatory cytokines may cause different reactivity of PPARs on their ligands (48). It should be also emphasized that in some cases PPAR antagonist - GW9626 affected P4 or E2 secretion in a manner similar to that of agonists. It is possible that the blockade of PPAR action might affect other pathways involved in the regulation of steroids production. It cannot be also excluded that the antagonists indicate a different specificity and/or affinity to the receptors present in porcine CL. A clarification of the above phenomenon should be investigated in a future research.
Progesterone production is a multi-step process which requires activation of several enzymes including the step limiting 3β-HSD. In the present study we noted a lack of PPAR ligands effect on the gene expression in the CLs during all analysed physiological statuses of sows. Reduced P4 release by porcine CL explants during pregnancy followed by the lack changes in 3β-HSD gene expression may indicate that PPARs participate rather in the release of steroids than in de novo synthesis by influence on gene expression. There are reports indicating that changes in steroids (P4 and E2) production after TZDs treatment were not followed by alterations in CYP11A1 or 3β-HSD mRNA abundance in porcine granulosa cells or the corresponding proteins in ovine granulosa cells (18, 38). There are studies showing that the PPAR ligands could modify 3β-HSD activity in porcine granulosa cells (38). Studies investigating the molecular mechanisms of PPARs action in the corpus luteum of pregnant sows are needed.
In summary, our results suggest that PPARs are involved in the regulation of progesterone and 17β-estradiol release by porcine corpus luteum. Porcine CL indicates a different receptivity to PPAR ligands depending on the reproductive status of animals. The regulation of P4 secretion within the CL, dependent on PPARs, is differentially regulated during the luteal phase of the estrous cycle and early pregnancy. All PPAR isoform agonists reduced P4 secretion by the tested tissue during both stages of pregnancy whereas they were ineffective during the estrous cycle. In turn, PPARs effect on E2 release was differential. Further studies are needed to explore the molecular mechanism of PPARs action in the porcine corpus luteum.
Acknowledgments: This research was supported by the State Committee for Scientific Research (Project N N311 360235 and UWM 5280206). We sincerely appreciate Michal Blitek, Janina Bukowska and Marta Gaglewska for technical assistance.
Conflict of interests: None declared.
REFERENCES
- Mueller E, Sarraf, P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPARγ. Mol Cell 1998; 1: 465-470.
- Lazennec G, Canaple L, Saugy D, Whali W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocrinol 2000; 14: 1962-1975.
- Lambe KG, Tugwood JD. A human peroxisome-proliferator-activated receptor-gamma is activated by inducers of adipogenesis, including thiazolidinedione drugs. Eur J Biochem 1996; 239: 1-7.
- Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res 2005; 51: 85-94.
- Krey G, Braissant O, L'Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 1997; 11: 779-791.
- Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care 2004; 27: 1660-1667.
- Bogacka I, Gettys TW, De Jonge L, et al. The effect of beta-adrenergic and peroxisome proliferator-activated receptor-gamma stimulation on target genes related to lipid metabolism in human subcutaneous adipose tissue. Diabetes Care 2007; 30: 1179-1186.
- Wierzbicki M, Chabowski A, Zendzian-Piotrowska M, Gorski J. Differential effects of in vivo PPAR alpha and gamma activation on fatty acid transport proteins expression and lipid content in rat liver. J Physiol Pharmacol 2009; 60: 99-106.
- Krskova K, Filipcik P, Zilka N, et al. Angiotensinogen and angiotensin-converting enzyme mRNA decrease and AT1 receptor mRNA and protein increase in epididymal fat tissue accompany age-induced elevation of adiposity and reductions in expression of GLUT4 and peroxisome proliferator-activated receptor (PPARγ). J Physiol Pharmacol 2011; 62: 403-410.
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999; 20: 649-688.
- Komar CM, Braissant O, Wahli W, Curry TE Jr. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology 2001; 142: 4831-4838.
- Miana M, de Las Heras N, Rodriguez C, et al. Effect of eplerenone on hypertension-associated renal damage in rats: potential role of peroxisome proliferator activated receptor gamma (PPAR-g). J Physiol Pharmacol 2011; 62: 87-94.
- Lim H, Gupta RA, Ma WG, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARd. Genes Dev 1999; 13: 1561-1574.
- Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function-implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol 2005; 3: 41.
- Yang J, Chen L, Zhang X, et al. PPARs and female reproduction: evidence from genetically manipulated mice. PPAR Res 2008: 2008:723243.
- Lee SS, Pineau T, Drago J, et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 1995; 15: 3012-3022.
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996; 137: 354-366.
- Froment P, Fabre S, Dupont J, et al. Expression and functional role of peroxisome proliferator-activated receptor-gamma in ovarian folliculogenesis in the sheep. Biol Reprod 2003; 69: 1665-1674.
- Jawerbaum A, Capobianco E. Effects of PPAR activation in the placenta and the fetus: implications in maternal diabetes. Placenta 2011; 32 (Suppl. 2): S212-S217.
- Lohrke B, Viergutz T, Shahi SK, et al. Detection and functional characterisation of the transcription factor peroxisome proliferator-activated receptor gamma in lutein cells. J Endocrinol 1998; 159: 429-439.
- Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4: 585-595.
- Cui Y, Miyoshi K, Claudio E, et al. Loss of the peroxisome proliferation-activated receptor gamma (PPAR gamma) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J Biol Chem 2002; 277: 17830-17835.
- Lim H, Dey SK. PPAR delta functions as a prostacyclin receptor in blastocyst implantation. Trends Endocrinol Metab 2000; 11: 137-142.
- MacLaren LA, Guzeloglu A, Michel F, Thatcher WW. Peroxisome proliferator-activated receptor (PPAR) expression in cultured bovine endometrial cells and response to omega-3 fatty acid, growth hormone and agonist stimulation in relation to series 2 prostaglandin production. Domest Anim Endocrinol 2006; 30: 155-169.
- Yessoufou A, Hichami A, Besnard P, Moutairou K, Khan NA. Peroxisome proliferator-activated receptor alpha deficiency increases the risk of maternal abortion and neonatal mortality in murine pregnancy with or without diabetes mellitus: modulation of T cell differentiation. Endocrinology 2006; 147: 4410-4418.
- Bogacka I, Bogacki M. The quantitative expression of peroxisome proliferator activated receptors (PPARs) genes in porcine endometrium through the estrous cycle and early pregnancy. J Physiol Pharmacol 2011; 62: 559-565.
- Bogacka I, Bogacki M. Wasielak M. The effect of the embryo presence on the expression of peroxisome proliferator activated receptors (PPARs) genes in the porcine reproductive system during the periimplantation. Acta Vet Hung 2013; 61: 405-415.
- Bogacka I, Bogacki M, Kurzynska A, Chojnowska K. The involvement of peroxisome proliferator activated receptors (PPARs) in prostaglandin F2a production by porcine endometrium. Reprod Biol 2013; 13: 309-316.
- Bogacka I, Bogacki M, Gaglewska M, Kurzynska A, Wasielak M. in vitro effect of peroxisome proliferator activated receptor (PPAR) ligands on prostaglandin E2 synthesis and secretion by porcine endometrium during the estrous cycle and early pregnancy. J Physiol Pharmacol 2013; 64: 47-54.
- Zmijewska A, Franczak A, Kotwica G. Role of interleukin-1b in the regulation of porcine corpora lutea during the late luteal phase of the cycle and during pregnancy. Acta Vet Hung 2012; 60: 395-407.
- Ottobre JS, Houmard BS, Ottobre AC. Luteal production of steroids and prostaglandins during stimulated early pregnancy in primate: differential regulation of steroids production by chronic gonadotropin. Biol Reprod 1989; 41: 393-400.
- Hotchkiss J, Atkinson LE, Knobil E. Time course of serum estrogen and luteinizing hormone (LH) concentrations during the menstrual cycle of the rhesus monkey. Endocrinology 1971; 89: 177-183.
- Bogacka I, Bogacki M, Boruszewska D, Wasielak M. Expression of peroxisome proliferator activated receptor (PPAR) genes in porcine endometrium exposed in vitro to IL-6 and INFγ. Reprod Biol 2012; 12: 157-170.
- Dupont J, Chabrolle C, Rame C, Tosca L, Coyral-Castel S. Role of the peroxisome proliferator-activated receptors, adenosine monophosphate-activated kinase, and adiponectin in the ovary. PPAR Res 2008; 2008: 176275.
- Seto-Young D, Paliou M, Schlosser J, et al. Direct thiazolidinedione action in the human ovary: insulin-independent and insulin-sensitizing effects on steroidogenesis and insulin-like growth factor binding protein-1 production. J Clin Endocrinol Metab 2005; 90: 6099-6105.
- Schoppee PD, Garmey JC, Veldhuis JD. Putative activation of the peroxisome proliferator-activated receptor gamma impairs androgen and enhances progesterone biosynthesis in primary cultures of porcine theca cells. Biol Reprod 2002; 66: 190-198.
- Chen Q, Sun X, Chen J, et al. Direct rosiglitazone action on steroidogenesis and proinflammatory factor production in human granulosa-lutein cells. Reprod Biol Endocrinol 2009; 7: 147.
- Gasic S, Bodenburg Y, Nagamani M, Green A, Urban RJ. Troglitazone inhibits progesterone production in porcine granulosa cells. Endocrinology 1998; 139: 4962-4966.
- Gasic S, Nagamani M, Green A, Urban RJ. Troglitazone is a competitive inhibitor of 3beta-hydroxysteroid dehydrogenase enzyme in the ovary. Am J Obstet Gynecol 2001; 184: 575-579.
- Matsuo H, Strauss JF. Peroxisome proliferators and retinoids affect JEG-3 choriocarcinoma cell function. Endocrinology 1994; 135: 1135-1145.
- Gazouli M, Han Z, Papadopoulos V. Identification of a peptide antagonist to the peripheral-type benzodiazepine receptor that inhibits hormone-stimulated Leydig cell steroid formation. J Pharmacol Exp Ther 2002; 303: 627-632.
- Lampen A, Zimnik S, Nau H. Teratogenic phthalate esters and metabolites activate the nuclear receptors PPARs and induce differentiation of F9 cells. Toxicol Appl Pharmacol 2003; 188: 14-23.
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 2003; 111: 139-145.
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology 2005; 210: 223-233.
- Uren-Webster TM, Lewis C, Filby AL, Paull GC, Santos EM. Mechanisms of toxicity of di(2-ethylhexyl) phthalate on the reproductive health of male zebrafish. Aquat Toxicol 2010; 99: 360-369.
- Parillo F, Maranesi M, Brecchia G, Gobbetti A, Boiti C, Zerani M. in vivo chronic and in vitro acute effects of di(2-ethylhexyl) phthalate on pseudopregnant rabbit corpora lutea: possible involvement of peroxisome proliferator-activated receptor gamma. Biol Reprod 2014; 90: 41.
- Zerani M, Maranesi M, Brecchia G, Gobbetti A, Boiti C, Parillo F. Evidence for a luteotropic role of peroxisome proliferator-activated receptor gamma: expression and in vitro effects on enzymatic and hormonal activities in corpora lutea of pseudopregnant rabbits. Biol Reprod 2013; 88: 62.
- Jalali BM, Kitewska A, Wasielak M, Bodek G, Bogacki M. Effects of seminal plasma and the presence of a conceptus on regulation of lymphocyte-cytokine network in porcine endometrium. Mol Reprod Dev 2014; 81: 270-281.
A c c e p t e d : August 18, 2014