THE INFLUENCE OF MODERATE-INTENSITY PHYSICAL EFFORT ON PERIPHERAL BLOOD IN ADULTS WITH DOWN SYNDROME - A PILOT STUDY
INTRODUCTION
Down syndrome is the most common pathology in the human genotype. Half a century ago, only a few people with Down syndrome reached adulthood. In 1900, the average life expectancy of these people was 9–11 years. Presently, they live to the age of 49–56 years (1, 2). Considering the fact that the average life expectancy is increasing, it is necessary to concentrate research studies on adults with Down syndrome, especially that the available literature lacks the reports addressing this age group.
Numerous experimental studies indicated that physical activity is one of the main factors influencing the changes occurring in peripheral blood in healthy subjects and also in children and adolescents with Down syndrome (3-7). Unfortunately, there is no data on the influence of physical effort on rheological and hematological blood factors in adults with Down syndrome. However, a higher level of physical activity of people with Down syndrome may significantly improve poor cardiovascular fitness and obesity (8, 9). Plonka et al. (10) also observed that long-term and intensive physical effort is an important factor influencing correct leptin production by fat cells and body mass reduction. The problem seems to be very important as people with Down syndrome present lack of physical activity, lead sedentary lifestyle, and show low awareness of the beneficial effects of physical activity on their health. That results in their poor motivation to undertake any form of exercise (2, 11). Decreased blood and plasma viscosity as well as beneficial changes in the elasticity of erythrocytes were observed in people leading a sedentary lifestyle who started regular physical activity (12-14). Additionally, the rejuvenation of erythrocytes may positively affect the process of providing tissues with oxygen (15), variability of their shape, and the tendency to aggregate. That leads to changes in the resistance to blood flow in the microcirculation vessels. The loss of elasticity of erythrocytes may limit the oxygen supply and, as a consequence, lead to hypoxia and tissue damage (16). Good knowledge of physiological and biochemical changes in response to physical effort can significantly help to create adequate rehabilitation and revalidation programmes, which would be significant for their overall condition.
The aim of the study was to evaluate the influence of a six-week physical activity implemented in the form of cycloergometer training on changes in the rheological and hematological properties of blood in the subjects with Down syndrome.
MATERIAL AND METHODS
This study was conducted in accordance with the Declaration of Helsinki (1964). The subjects’ caregivers signed a written consent form, agreeing to their wards’ participation in the study, and to their data being processed for the purposes of the project. The study was approved by the Ethics Committee of the Regional Medical Chamber in Cracow.
Subjects
The study group included 15 non-trained men aged 21–24 (22.4 years ± 0.91) with moderate (IQ 36-51) and severe (IQ 20-35) intellectual disability, suffering from Down syndrome (17).
The mean value of body weight in people undergoing examination prior to exertion equalled 67.20 kg ± 12.05, with body height of 156.53 cm ± 5.97 cm and BMI of 27.5. After the training, the body weight decreased by 1.04 kg on average, and reached the values of 66.16 kg ± 11.94 kg. The BMI also decreased and it was 27.1.
The subjects of the study group did not have any diagnosed severe or chronic cardiovascular, hematologic, respiratory, or motor disorders, nor any infections. The subjects did not take any permanent medication.
Physical effort
Subjects qualified for the study underwent physical training three times a week for a period of six weeks. The training was always conducted before midday (9:00 a.m.–12:30 p.m.) on a Monark 894 E Peak Bike (Monark Sports & Medical, USA) cycloergometer. The training included 10-minute warm-up, 20–25-minute main phase, at work intensity of 60–75% of peak heart rate (HRmax = 194.5 - (0.56 age)) (18) and 10-minute cool-down period. In order to determine lean body mass, a TANITA Body Composition Analyzer TBF-300 (Tanita Corporation, Japan) was used. Heart rate was monitored and registered by Polar Sport Tester RCX5 heart rate monitors (Polar, Finland).
Laboratory tests
The tests were carried out in the Laboratory of Motor Organ Pathology of the Academy of Physical Education in Cracow.
Twenty four hours before and twenty four hours after the beginning of the training, subjects of the study group had their venous blood samples taken from their ulnar veins into Vacuette tubes with an anticoagulant (disodium edetate).
After the blood sample was taken, it was subjected to the following analyses:
l hematological tests (using an ABX Miros 60 analyzer), including: the number of RBC and RBC indicators: MCV, MCHC, MCH, RBC’s level of reduced glutathione - GSH evaluation (19), a poikilocytrometric examination of RBC (19), fibrinogen determination (20), hematocrit determination, and plasma viscosity measurement (using a Myrenne viscometer) according to standard methods;
- blood rheology tests, including:
- EI – the principle of determination involved shear stress being gradually applied to erythrocytes suspended in a PVP mixture and dissolved in PBS (phosphate buffered saline; a buffer solution of physiological saline with calcium chloride and pH 7.4 magnesium chloride) in a LORCA laser-assisted optical analyzer
- AMP – representing total extent of aggregation
- T½ – describing the kinetics of the aggregation process
- AI – calculated on the basis of a syllectogram, which was a curve of a relation of the intensity of scattered light to the time in which blood cell aggregates were created.
Statistical analysis
The results of the examination were compiled using Excel (Microsoft, USA) and STATISTICA PL version 6.0 (StatSoft, USA). Statistical data analysis was made on the basis of all the taken parameters. The quantitative results of the analysis were described with the use of minimal, maximal and mean value. The obtained results were subjected to a statistical analysis with the use of the Wilcoxon test. The significance of the observed changes was ascertained at P<0.05.
RESULTS
Statistically significant decrease in the RBC was noted, from the baseline value of 4.65 106/mm3 ± 0.45 to the value of 4.32 106/mm3 ± 0.49 106/mm3 after the training period.
Six weeks of cycloergometer training caused a decrease in the average MCV from 98.00 ± 4.55 µm3 to 93.00 ± 5.24 µm3 after the training period.
However, statistically significant increase in the average MCH was noted, from the level of 32.20 ± 1.82 pq to the level of 35.30 ± 4.43 pq after the training period.
An increase in the MCHC was also observed, from 33.70 ± 0.46 g/dl, to 38.30 ± 4.24 g/dl.
Statistically significant increase in the GSH level was noted, from 1.79 ± 1.04 g/l to 2.65 ± 0.93 g/l after the training period.
Six weeks of cycloergometer training also caused a statistically significant (by 48.04%) decrease in the number of macrocytes, from the level of 6 ± 0.98% to 2 ± 1.06% after the training period.
The tests indicated a post-effort decrease in the concentration of fibrinogen from the level of 2.97 ± 1.17g/l to the level of 2.43 ± 0.93 g/l. A decrease in plasma viscosity from the level of 1.62 ± 0.03 mPas to the level of 1.34 ± 0.06 mPas after the training was also observed. The level of hematocrit also decreased from the level of 44.20 ± 2.76% to the value of 39.30 ± 4.22% (Table 1).
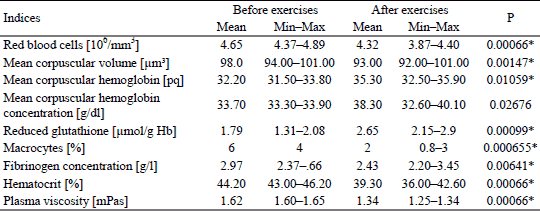
The tests also included an examination of erythrocyte deformability as it was expected to be significantly lower due to a high level of oxidative stress. However, no statistically significant changes in the deformability of erythrocytes after the training were observed. The only statistically significant change occurred at the shear stress value of 0.58, and it changed from the value of 0.038 ± 0.02 Pa to the value of 0.028 ± 0.019 Pa, but it had no influence on blood rheology.
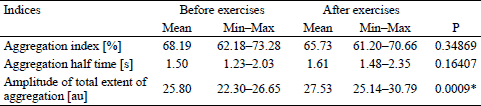
In the conducted tests, the influence of physical training on the AI in subjects with Down syndrome was observed for the first time and it changed from the value 68.19 ± 6.55 to the value 65.73 ± 6.76, but the change was not statistically significant.
Statistically insignificant increase in the average t½ was noted, from the level of 1.50 ± 3.33 s to the level of 1.61 ± 3.11 s after the training period.
Statistically significant increase in the AMP was noted, from the level of 25.80 ± 0.57 au to the level of 27.53 ± 0.85 au after the training period (Table 2).
DISCUSSION
In the literature, there is a very detailed description of the changes that take place in the circulatory system in people suffering from Down syndrome (21, 22). Unfortunately, there is no data on the influence of physical effort on rheological and hematological factors.
The conducted tests revealed a statistically significant decrease in the number of the RBC at the end of the training period. Because the physical training was carried out on a cycloergometer, it can be assumed that the decrease in the number of erythrocytes, especially in subjects with Down syndrome, may have resulted from the process of faster ageing of red blood cells, particularly the defective ones, caused by the high levels of oxidative stress (23). Such condition observed in this group may result from the presence of an additional, third chromosome in the 21st pair or an excess fragment of this chromosome containing the gene encoding cytoplasmic superoxide dismutase (Cu, Zn, SOD) (24, 25). In turn, it contributes to an increase in the fragility of the erythrocyte membrane, thus reducing the blood cell deformability (6). The decrease in the membrane elasticity, as well as the reduced osmotic and mechanical resistance of erythrocytes eventually leads to membrane fragmentation, increased rupturing of older erythrocytes exposed to mechanical factors, and changes that occur in the peripheral blood during the physical stress (6). Kolodziejczyk (26) states that regular physical activity leads to a decrease in the number of high density erythrocytes. Because subjects with Down syndrome are characterised by the presence of a significant proportion of erythrocytes counted among macrocytes in the peripheral blood, which are the defective elements less resistant to oxidative stress (27, 28), it can be assumed that a statistically significant decrease in their value observed in this study is one of the reasons for the reduced number of the RBC in this group.
Since the generation of free radicals during the physical effort is also increased (7), the level of reduced glutathione in peripheral blood was examined in the study. Aerobic organisms have anti-oxidative systems, but the generation of free radicals during physical effort in the untrained subjects may considerably exceed the efficiency of these mechanisms (29). This is particularly important in subjects with Down syndrome, because, as Muchova et al. (30) observed, the subjects with Down syndrome, compared to their healthy peers, have a statistically lower level of the GSH. Additionally, a high level of oxidative stress contributes to the ageing of blood cells and causes neurological degeneration and immune disorders, as well as atherosclerosis and neoplastic changes (22, 24, 31). Six weeks of cycloergometer training caused a statistically significant increase in the GSH level. The increase of this index was also observed by Ordonez and Rosety-Rodriguez (18). In their work, the 8-person group of subjects with Down syndrome undergone 16 weeks of training. Their GSH levels were tested before and after the training. Then, the results were compared to the control group, also consisting of the subjects with Down syndrome, but not taking part in the training.
The results presented in this study show that the initial MCV value was increased when comparing to the norm (33). A high value of this index was also observed by Roizen et al. (28) and Dixon et al. (34). The reason for this, as already mentioned above, may be the presence of a significant proportion of macrocytes (27, 28) in the subjects’ blood. They are the defective elements less resistant to oxidative stress, and that is the reason why they undergo hemolysis sooner causing the post-training decrease in the MCV value. Stac (27) points out that the MCV values raise doubts as to whether the morphology results of healthy subjects can be compared to the morphology results of subjects with Down syndrome, and whether the norms used for healthy subjects can be used in this population.
People with Down syndrome have also higher MCH level compared to their healthy peers (34). However, it has not been confirmed in the results of this study. Yet, a statistically significant increase in this index after the training was observed. That can be caused by the deterioration of the defective erythrocytes (drepanocytes), macrocytes, whose membrane is less resistant to oxidative stress occurring during physical training, and an increase in the production of erythrocytes by the bone marrow.
Over the last years, it has become clear that the shape and the elasticity of erythrocytes play a significant role in the attempt to explain the reasons for the development of various pathologies (35). The impairment of the process may lead to worsening of tissue perfusion (16), which may be another reason of hypoxia in subjects with Down syndrome. Cicha (36) notices that the ability of erythrocytes to change their shape plays a significant role in microcirculation. The deformability of erythrocytes, along with their aggregation, complete blood and plasma viscosity, hematocrit, and fibrinogen concentration, constitute the factors determining the flow of blood in the vessels (14, 37). Hence, these are one of the main factors determining capillary flow and the process of providing tissues with oxygen. The loss of elasticity by erythrocytes may limit the oxygen supply, and as a consequence, lead to hypoxia and tissue damage (16).
The tests also included an examination of erythrocyte deformability as it was expected to be significantly lower due to a high level of oxidative stress (31, 38-40). Since the free radicals contribute to the polymerization of cell membranes, increasing their fragility and stiffness, decreasing their deformability and speeding up hemolysis (41, 42), it was assumed that this process may be significantly limited after the six-week training. However, no statistically significant changes in the deformability of erythrocytes after the training were observed. The only statistically significant change was noted at the shear stress value of 0.58. It seems to be a significant factor. According to Ernst (12), positive changes in erythrocyte elasticity may be observed in people who lead a sedentary lifestyle and start regular physical training. Thus, efficient oxygen delivery to working muscles, in response to training, is related to rheological properties which condition the level of physical fitness.
The results presented in this study also show a decrease in plasma viscosity. Therefore, it can be assumed that the post-effort decrease in fibrinogen, observed in this study, was one of the factors contributing to the decrease in plasma viscosity. Decrease plasma viscosity and fibrinogen concentration is a positive post-training change that lowers the risk of circulatory disorders (13) which are common in subjects with Down syndrome (36-39, 43-47). Ernst (12) states that a 0.1 g/l decrease in the level of fibrinogen reduces the risk of the occurrence of heart diseases by 15%. He adds that a 0.4 g/l decrease, which was also observed in this study, limits the risk of heart diseases by 60%. The decrease in plasma viscosity through the increase of plasma volume can be very important, as it contributes to a permanent decrease in cardiac workload (26). Ernst (12, 48) reaches the similar conclusions. According to him, regular and moderate-intensity physical training leads to a decrease in plasma viscosity and hematocrit, as well as a change in the elasticity of red blood cells. A decrease in the level of hematocrit after the effort also occurs in the conducted tests.
In the conducted tests, a change in the AI index value, which was not statistically significant, was observed. That means that physical training does not increase the aggregation or hinders the blood flow in the vessels. No changes were observed in the t½ parameter in the subjects with Down syndrome after the training. However, an increase in the AMP was observed, and it can be explained by the processes of slowing down the aggregation after the training.
To sum up, on the basis of the presented innovative study, it can be assumed that physical training, implemented in the form of riding a cycloergometer in subjects with Down syndrome, can positively influence the processes of blood regeneration. Additionally, this kind of physical activity may play a significant role in the process of rehabilitation of these people as it does not contribute to the increase in their physical fitness.
Human subjects with Down syndrome are reluctant to take up any physical activity, therefore, there were limitations in the group size. What is more, they often suffer from numerous comorbidities that require medication and eliminate them from the research projects. Additionally, parents and caregivers of such people fear for their health and reluctantly consent to their participation in the research programs. Moreover, in order to achieve reliable and objective results, the study group was decided to remain as homogenous as possible, and consequently, limited in its size. Subjects of the same sex without any comorbidities were included in the study. Besides, in order to provide an equal level of physical effort, contestants preparing for the Special Olympics Games were excluded from the studies.
Initially, the group was larger (22 participants), but in the course of the project, due to various factors, the number of participants was reduced (Table 3).

Perspectives
On the basis of the presented study, it can be assumed that physical training, implemented in the form of riding a cycloergometer in the subjects with Down syndrome, positively influences the processes of blood regeneration. Such training may also be one of the factors delaying ageing processes as it influences oxygen provision to the tissue and delays the aggregation processes.
This kind of physical activity may limit the number of cardiovascular diseases in subjects with Down syndrome by decreasing the concentration of fibrinogen and plasma viscosity. Physical training also plays a significant role in the process of rehabilitation of these people. Not only can it improve their physical fitness, but it may also lead to the development in social competence. Further long-term follow-up studies are required to determine whether these red blood cell changes lead to other positive changes in the blood and allow to plan proper physical activities to improve physical efficacy and life quality in people with Down syndrome.
Abbreviations: AI, aggregation index; AMP, amplitude of total extent of aggregation; EI, elongation index; Ht, hematocrit; t½, aggregation half time; GSH, reduced glutathione; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean vorpuscular volume; RBC, red blood cell
Acknowledgements: Source of funds: a grant from The Ministry of Science and Higher Education, NN 404 008938.
Conflict of interests: None declared.
REFERENCES
- Carmeli E, Kessel S, Bar-Chad S, Merrick J. A comparison between older persons with Down syndrome and a control group: clinical characteristics, functional status and sensori - motor function. Down Syndr Res Pract 2004; 9: 17-24.
- Jobling A, Cuskelly M. Young people with Down syndrome: a preliminary investigation of health knowledge and associated behaviours. J Intellect Dev Disabil 2006; 31: 210-218.
- Kivivuori SM, Rajantie J, Siimes MA. Peripheral blood cell counts in infants with Down’s syndrome. Clin Genet 1996; 49: 15-19.
- David O, Fiorucci GC, Tosi MT, et al. Hematological studies in children with Down syndrome. Pediatr Hematol Oncol 1996; 13: 271-275.
- Eberhard Y, Eterradossi J, Therminaris A. Biochemical changes and catecholamine responses in Down’s syndrome adolescents in relation to incremental maximal exercise. J Ment Defic Res 1991; 35: 140-146.
- Szygula Z. Erythrocytic system under the influence of physical exercise and training. Sports Med 1990; 10: 181-197.
- Szygula Z. Wplyw wysilku fizycznego na uklad erytrocytarny. In: Fizjologia krwi. Wybrane zagadnienia T 1. Z. Dabrowski (ed). Warszawa, PWN 2000, pp. 173-193.
- Tsimaras V, Giagazoglou P, Fotiadou E, Christoulas K, Angelopoulou N. Jog-walk training in cardiorespiratory fitness of adults with Down syndrome. Percept Mot Skills 2003; 96: 1239-1251.
- Rimmer JH, Heller T, Wang E, Valerio I. Improvements in physical fitness in adults with Down syndrome. Am J Mental Retard 2004; 109: 165-174.
- Plonka M, Toton-Morys A, Adamski P, et al. Association of the physical activity with lepton blond serum level, body mass indices and obesity in schoolgirls. J Physiol Pharmacol 2011; 62: 647-656.
- Sayers Menear K. Parents’ perceptions of health and physical activity needs of children with Down syndrome. Downs Syndr Res Pract 2007; 12: 60-68.
- Ernst E. Influence of regular physical activity on blood rheology. Eur Heart J 1987; 8 (Suppl. G): 59-62.
- Ernst E. Regular exercise reduces fibrinogen levels: a review of longitudinal studies. Br J Sports Med 1993; 27: 175–176.
- El-Sayed M. Effects of exercise and training on blood rheology. Sports Med 1998; 26: 281-292.
- Telford RD, Kovacic JC, Skinner SL, Hobbs JB, Hahn AG, Cunningham RB. Resting whole blood viscosity of elite rowers is related to performance. Eur J Appl Phys Occup Physiol 1994; 68: 470-476.
- Langenfeld JE, Machiedo GW, Lyons M, Rush BF, Dikdan G Lysz TW. Correlation between red blood cell deformability and changes in hemodynamic function. Surgery 1994; 116: 859-867.
- Marchewka A, Schmidt O. Rozwoj fizyczny i sprawnosc motoryczna osob uposledzonych umyslowo w stopniu umiarkowanym i znacznym. Fizjoterapia 2000; 8: 3-8.
- Ordonez FJ, Rosety-Rodriguez M. Correlation between glutathione peroxidase activity and anthropometrical parameters in adolescents with Down syndrome. Res Dev Disabil 2007; 28: 105-108.
- Pawelski S. Diagnostyka laboratoryjna w hematologii. Warszawa, PZWL, 1990.
- Blomback B, Blomback M. Purification of human and bovine fibrinogen. Arkiv Kemi 1956; 10: 415-419.
- Fernhall B, Pitetti KH, Rimmer JH, et al. Cardiorespiratory capacity of individuals with mental retardation including Down syndrome. Med Sci Sports Exerc 1996; 28: 366-371.
- Fernhall B, McCubbin JA, Pitetti KH, et al. Prediction of maximal heart rate in individuals with mental retardation. Med Sci Sports Exerc 2001; 33: 1655-1660.
- Zitnanova I, Korytar P, Sobotova H, et al. Markers of oxidative stress in children with Down syndrome. Clin Chem Lab Med 2006; 44: 306-310.
- Bartosz G. Druga twarz tlenu. Wolne rodniki w przyrodzie. Warszawa, PWN, 2003.
- Brugge KL, Nichols S, Delis D, Saitoh T, Truaner D. The role of alterations in free radical metabolism in mediating cognitive impairments in Down’s syndrome. EXS 1992; 62: 190-198.
- Kolodziejczyk J. Wysilek fizyczny a hemoreologia. Med Sportiva 1999; 3: 117-121.
- Starc TJ. Erythrocyte macrocytosis in infants and children with Down syndrome. J Pediatr 1992; 121: 578-582.
- Roizen NJ, Amarose AP. Hematologic abnormalities in children with Down syndrome. Am J Med Genet 1993; 46: 510-512.
- Benzi G. Aerobic performance and oxygen free radicals. J Sports Med Phys Fitness 1993; 33: 205-222.
- Muchova J, Garaiova I, Sustrova M, et al. The redox state of glutathione in erythrocytes of individuals with Down syndrome. Bratisl Lek Listy 2007; 18: 70-74.
- Flore P, Bricout VA, van Biesen D, et al. Oxidative stress and metabolism at rest and during exercise in persons with Down syndrome. Eur J Cardiovasc Prev Rehabil 2008; 15: 35-42.
- Zambrano JC, Marquina R, Sulbaran N, Rodriguez-Malaver AJ, Reyes RA. Aerobic exercise reduced oxidative stress in saliva of person with Down syndrome. Res Sports Med 2009; 17: 195-203.
- Pawelski S, Maj S. Normy i kliniczne interpretacje badan diagnostycznych w medycynie wewnetrznej. Warszawa, PZWL, 1987.
- Dixon N, Kishnani PS, Zimmerman S. Clinical manifestations of hematologic and oncologic disorders in patients with Down syndrome. Am J Med Genet C Semin Med Genet 2006; 142: 149-157.
- Mokken FC, Kedaria M, Henny CP, Hardeman MR, Gelb AW. The clinical importance of erythrocyte deformability, a hemorheological parameter. Ann Hematol 1992; 64: 113-122.
- Cicha I. Reologia erytrocytow - podstawowe parametry i metody pomiarow. Acta Haematol Pol 1997; 28: 223-229.
- El-Sayed M, Sale C, Jones PGW, Chester M. Blood hemostasis in exercise and training. Med Sci Sports Exerc 2000; 32: 918-925.
- Carratelli M, Porcaro L, Ruscica M, De Simone E, Bertelli AA, Corsi MM. Reactive oxygen metabolites and prooxidant status in children with Down’s syndrome. Int J Clin Pharmacol Res 2001; 21: 79-84.
- Pallardo FV, Degan P, d’Ischia M, et al. Multiple evidence for an early age pro-oxidant state in Down Syndrome patients. Biogerontology 2006; 7: 211-220.
- Ordonez FJ, Rosety I, Rosety MA, et al. Aerobic training at moderate intensity reduced protein oxidation in adolescents with Down syndrome. Scand J Med Sci Sports 2012; 22: 91-94.
- Takeshi S, Nobuji M, Kazunori K. Erythrocyte rheology. Oncol Hemat 1990; 10: 9-48.
- Spodaryk K, Dabrowski Z. Blood flow in different regions of bone marrow after short-term exercise. Acta Phys Hung 1991; 77: 13-17.
- Guerra M, Llorens N, Fernhall B. Chronotropic incompetence in persons with Down syndrome. Arch Phys Med Rehabil 2003; 84: 1604-1608.
- Baynard T, Pitetti KH, Guerra M, Fernhall B. Heart rate variability at rest and during exercise in person with Down syndrome. Arch Phys Med Rehabil 2004; 85: 1285-1290.
- Fernhall B, Figueroa A, Collier S, Baynard T, Giannopoulou I, Goulopoulou S. Blunted heart rate response to upright tilt in people with Down syndrome. Arch Phys Med Rehabil 2005; 86: 813-818.
- Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther 2007; 87: 1399-1406.
- Giagkudaki F, Dimitros E, Kouidi E, Deligiannis A. Effects of exercise training on heart-rate-variability indices in individuals with down syndrome. J Sports Rehabil 2010; 19: 173-183.
- Ernst E, Daburger L, Saradeth T. The kinetics of blood rheology during and after prolonged standardized exercise. Clin Hemorheol Microcirc 1991; 11: 429-439.
A c c e p t e d : August 19, 2014