IMPACT OF OBESITY AND NITRIC OXIDE SYNTHASE GENE G894T
POLYMORPHISM ON ESSENTIAL HYPERTENSION
INTRODUCTION
Essential hypertension (EH) is one of the most common diseases affecting humans worldwide and a major risk factor for cardiovascular diseases, including stroke, myocardial infarction, heart failure and coronary artery disease (1). It has been recognized that number of patients with essential hypertension is increasing, however, the reason for this increase remained uncertain (2). Hypertension is thought to be a multifactorial disease caused by environmental, metabolic and genetic determinants (3). However, little is currently known on the complex interplay between genetic, environmental and metabolic factors and its implication for healthcare.
A rising prevalence of overweight and obesity is evident in men and women, and obesity has been recognized as a major public health problem (4, 5). Obesity is associated with a high prevalence of hypertension (6, 7). However, not all obese individuals are hypertensive, indicating that there is a considerable variation in responses to metabolic dysregulation. It can be hypothesized that interaction between obesity and genetic factors involved in the regulatory pathways of blood pressure plays a significant role in hypertension development in obese individuals.
Obesity is a condition of increased adipose tissue mass. Human adipose tissue expresses all components of the renin/angiotensin system (RAS), including angiotensinogen, angiotensin-converting enzyme (ACE) as well as AT1 (angiotensin type 1), and AT2 (angiotensin type 2) receptors (6, 8, 9). Moreover, there is evidence that obesity is associated with endothelial cell dysfunction (10, 11), unbalanced circulating levels of nitric oxide (NO) metabolites due to increased oxidative stress (12), and decreased NO production (13). NO is a key paracrine regulator of vascular homeostasis and NO bioavailability is determined by a balance between NO biosynthesis by endothelial NO synthase (eNOS) and its degradation (14). Reduction in NO bioavailability may predispose to hypertension (15, 16) and administration of NG-nitro-L-arginine methyl ester (L-NAME), an inhibitor of NO synthesis, induces development of hypertension in rats (17). NO contributes to vasodilation (relaxation of vascular smooth muscle cells) and vascular tone regulation. Besides dilating peripheral arteries, NO also mediates dilation in the coronary microcirculation (18). Endothelium-derived NO prevents leukocyte adhesion to endothelium, platelet aggregation and adhesion as well as limiting the oxidation of atherogenic low density lipoproteins (19, 20). NO also inhibits the vasoconstrictive and inflammatory actions of angiotensin II (Ang II) and endothelin-1 by down-regulating the syntheses of angiotensin-converting enzyme (ACE), Ang II type 1 receptors, and endothelin-1 in the endothelium (21). A functional imbalance between NO and angiotensin II (Ang II) has been implicated in the pathogenesis of hypertension and atherosclerosis (22). Angiotensin II generation depends on activity of angiotensin converting enzyme (ACE) (23). The insertion (I)/ deletion (D) polymorphism (rs1799752) in ACE gene effects circulating ACE plasma levels and activity (24). NO synthesis is controlled by endothelial nitric oxide synthase gene (NOS3) (25). Three NOS3 gene polymorphisms; 4a/4b (rs61722009), G894T (rs1799983), and -T786C (rs2070744), seem to increase a predisposition to essential hypertension, however, the results of the association studies are inconsistent (26-32) and ethnicity may influence this association (33). In addition, there is a gap in data on the role of interactions between variants in the genes encoding angiotensin converting enzyme and endothelial nitric oxide synthase and obesity in hypertension development.
The aim of the present study was to assess the impact of obesity and polymorphisms in ACE and NOS3 genes on essential hypertension development in adult Caucasians of Polish origin.
MATERIALS AND METHODS
Patients
The study protocol was approved by the Warsaw Medical University’s Ethics Committee and the Ethics Committee at the National Food and Nutrition Institute. All participants signed an informed consent form after receiving an explanation of the study’s objectives and methodology.
For the present study, 1,027 unrelated Caucasian adults of Polish nationality, mean 45.5 ± 13.6 years old, were consecutively recruited on the basis of clinical investigation from subjects who had been directed to the Outpatient Clinic at the National Food and Nutrition Institute in Warsaw, for a routine general health screening, and from subjects admitted to the Orlowski Hospital of Warsaw due to obesity and prior to bariatric surgery. Exclusion criteria from the study were as follows: acute endocrine dysfunction, chronic kidney and liver disease, and alcoholism.
Experimental procedures
All subjects underwent a comprehensive medical evaluation including clinical history, physical examination and anthropometric parameters. Blood pressure (BP) was measured with a standard mercury sphygmomanometer as in normal clinical practice. The sphygmomanometer cuff with appropriate cuff size was placed on the left arm of the participants while seated with his/her arm at heart level. Two readings of the systolic and diastolic blood pressure, with a 5 minute interval between them, were taken after at least 10 minutes of rest, and the average of the two readings was used for analysis. Subjects with SBP ≥ 140 mmHg and DBP ≥ 90 mmHg on examination were considered as hypertensive according to JNC-VII (1). The eligibility criteria for inclusion in the hypertension group were: an average blood pressure ≥ 140/90 mmHg, a previous diagnosis of hypertension, and/or self-reported use of antihypertensive medication. Height was measured in centimeters while the subject was standing barefoot and in a normal, straight posture. Body weight was measured in kilograms while the participants wore light clothing and no shoes using a standardized digital scale. The subjects also were asked to remove any objects from their pockets. Body mass index (BMI) was calculated as the ratio of weight (kilograms) to the square of height (meters). Obesity was classified according to World Health Organization criteria (34) and subjects with BMI ≥ 30 kg/m2 were considered obese.
Overnight peripheral fasting blood samples were taken from all subjects in commercially available vacuum tubes and analyzed on the same day. Standard assays were used to measure serum concentrations of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) and glucose. The low-density lipoprotein-cholesterol (LDL-C) levels were calculate using Friedewald formula (35). Dyslipidemia was defined based on the medical diagnosis according to the National Cholesterol Education Program-Adult Treatment Panel III (TG > 200, TC ł 240 mg/dL, and HDL-C < 40 mg/dL) (36). Patients were classified as diabetics based on the review of medical records (diagnosis of diabetes and requiring antidiabetic treatment) and as pre-diabetics when impaired fasting glucose (IFG) or impaired glucose utilization was diagnosed by a physician. All participants completed a questionnaire concerning smoking habits. Smoking status was categorized as ‘never a smoker’, ‘current smoker’, and ‘former smoker’.
Genomic DNA was extracted from peripheral blood leukocytes using the Blood Mini genomic DNA purification kit (A&A Biotechnology) according to the manufacturer’s instructions. DNA concentration and purity were determined with Quawell Q5000 micro-volume UV-Vis spectrophotometer, measuring absorbance ratios of 260 nm/280 nm. High quality DNA was considered to have an A260/A280 ratio of 1.85 – 2.10. The ACE I/D polymorphism and polymorphisms in NOS3 gene (4a/4b, G894T) and were determined by the PCR method and by agarose gel electrophoresis. The promoter NOS3 -T786C variant was assessed with Taqman Genotyping Assay (LifeTechnologies) by using the ViiA7 real-time PCR system (Applied Biosystems).
Genotyping of the angiotensin-converting enzyme I/D polymorphism
The procedures for the identification of the polymorphic region of the ACE gene described in detail by Lindpaintner et al. (37) were used. The expected insertion (I) and deletion (D) alleles were visualized after electrophoresis on a 1.5% agarose gel and ethidium bromide staining under UV light transillumination. Sense and anti-sense primers were 5’-GCCCTGCAGGTGTCT GCAGCATGT-3’ and 5’-GGATGGCTCTCCCCGCCTTGTCTC-3. To exclude preferential amplification of the D allele, each sample was retyped in a second, independent PCR amplification with a primer pair that recognizes an insertion-specific sequence (5’-TCG CCA GCC CTC CCA TGC CCA TAA-3’ and 5’-TGG GAC CAC AGC GCC CGC CAC TAC-3’) (37). The PCR product yields 335-bp only in the presence of an I allele, and no product in samples homozygous for DD.
Genotyping of the NOS3 4a/4b polymorphism
The 27-bp repeat polymorphism in intron 4 of the NOS3 gene (NOS3 4a/4b polymorphism) was analyzed by PCR amplification. PCR was performed with the primers 5’-CTATGGTAGTGCCTTGGCTGGAGG-3’ (sense) and 5’-ACCGCCCAGGGAACTCCGCT-3 (antisense) (38). The amplified products were analyzed by electrophoresis on a 3% agarose gel containing ethidium bromide and visualized under UV light. The common allele (4b) amplicon size was 210 bp, whereas the rare allele (4a) amplicon size was 183 bp.
Genotyping of the NOS3 G894T polymorphism
The G894T polymorphism (rs1799983) was assessed by polymerase chain reaction (PCR) amplification of exon 7 with the following flanking primers; sense 5-CAT GAG GCT CAG CCC CAG AAC-3 and antisense 5 -AGT CAA TCC CTT TGG TGC TCAC-3. The size of PCR product was 206 bp, followed by restriction endonuclease MboI digestion for 16 h at 37°C and separated by electrophoresis on 2.5% agarose gel and visualized after ethidium bromide staining under ultraviolet light. In the presence of a T at nucleotide 894 corresponding to Asp298, the 206-bp PCR product was cleaved into two fragments of 119 and 87 bp; the presence of a G (Glu298) removed the restriction site resulting in a fragment of 206 bp (31).
Genotyping of the NOS3 -T786C polymorphism
The promoter -T786C gene variant of NOS3 gene was assessed using Taqman Genotyping Assay (Life Technologies). Genotyping of polymorphism-T786C (rs2070744) was performed by TaqMan allelic discrimination real-time PCR with allele-specific Taqman probes and sequence-specific primers obtained from Life Technologies. The genotyping experiments were carried out using real-time PCR Viia7 system. The allelic discrimination plot was analyzed by Viia7 software (Applied Biosystems; Life Technologies).
About 25% of all samples were randomly selected for repeated genotyping (ACE I/D and NOS3 4a/4b, G894T, -T786C) for confirmation. Concordance between repeats was 100%.
Statistical analysis
Data was analyzed with the use of Statistica 10.0 and R 3.1. The summary statistics contain mean ± standard deviation (S.D.) for continuous variables and number of cases (percentage) for qualitative variables. The comparison of hypertensive and normotensive groups was performed with chi-square test for qualitative variables and Student t-test or Mann-Whitney test for continuous variables (depending on normality, which was verified with the use of Kolmogorov-Smirnov test). For skewed continuous variables the log-transformation was considered.
Variables that were significantly related with hypertension in univariate analyses were then included to the multivariate logistic regression model. Qualitative variables were coded as 0 – 1 dummy variables. Results from the logistic regression model are presented as odds ratios (OR) with 95% confidence interval (CI). Allele frequencies for NOS3 and ACE variants were calculated with the gene counting method. Hardy-Weinberg equilibrium (HWE) was determined by Pearson’s χ2 goodness-of-fit test. The significance of deviations of observed genotype frequencies from those predicted by the Hardy-Weinberg equation were evaluated with chi-square tests in the hypertensive and normotensive groups. A link between the NOS3 G894T polymorphism and essential hypertension in obese subjects was determined by using codominant, recessive and dominant models of inheritance. Differences in minor allele frequencies and genotype distributions among hypertensive and normotensive obese patients with corresponding odds ratios (OR) and the 95% confidence interval (CI) were analyzed by likelihood ratio tests with calculation of the p value by Chi-square (χ2) approximation to its distribution using Web-Assotest program (http://www.ekstroem.com). P-values for a model fit (Pfit) were calculated and Pfit < 0.05 indicated that given model of inheritance should be rejected. Power calculation was performed using a genetic power calculator (39). Our study could detect with power > 80% (α = 0.05) the allelic association conferring OR = 1.37. In all analyses, relations with a P-value below 0.05 were considered significant.
RESULTS
Clinical and biochemical characteristics for studied subjects are presented in Table 1. Essential hypertension was recognized in 401 participants (39%). Average body mass index (BMI) was significantly different between groups with and without hypertension (37.5 ± 8.1 kg/m2 versus 28.2 ± 7.2 kg/m2, P < 0.0001). When the BMI was transformed into a quantitative variable with cut offs at 25, 30, 35 and 40, it was found that 15.5% of hypertensive group and only 8.1% of normotensive group had a BMI in the 35 – 39.9 range. Also, a BMI above 40 kg/m2 occurred more frequently in subjects with hypertension (26.4%), compared to the normotensive group (7.7%) (Table 1).
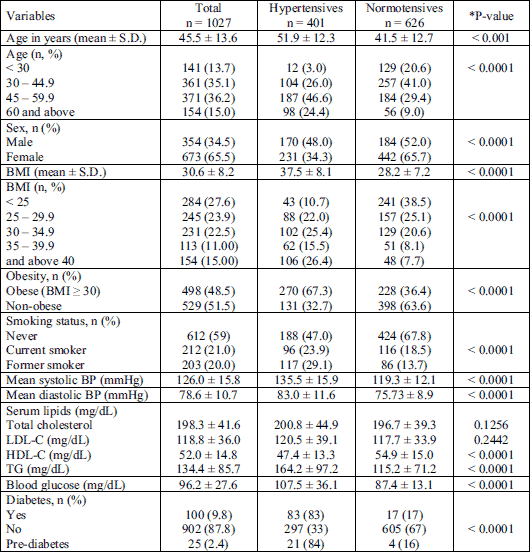
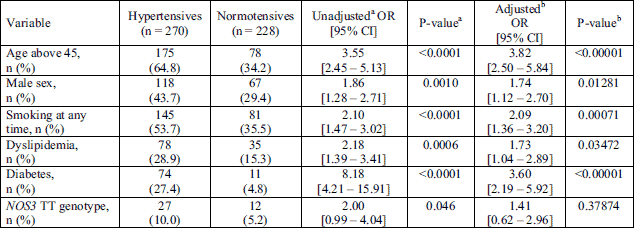
Among the hypertensive participants, almost 70% (270 of 401) were obese (BMI ≥ 30), as opposed to 36% of the obese participants in the normotensive group (P < 0.0001). Obese compared to non-obese had a 3.6-fold higher chance (OR = 3.6; 95% CI = 2.76 – 4.69) of having hypertension (Table 2). It was recognized that 53% of the hypertensive participants were current or former smokers, as opposed to 32% of normotensive participants (P < 0.0001). A higher proportion of hypertensive individuals was recognized among former smokers (29.1%) compared to current smokers (23.9%, P = 0.0118) and former smokers had higher odds of hypertension than current smokers (OR = 1.64; 95% CI = 1.15 – 2.42). Both current and former smokers had a 2.38 higher odds of having hypertension than those that never smoked (OR = 2.38; CI = 1.83 – 3.08) (Table 2). However, the presence of the assessed risk alleles was not associated with increased risk for hypertension in current or former smokers (data not shown).
Among the hypertensives, the prevalence of subjects aged 45 – 59.9 was significantly higher than among normotensives (46.6% versus 29.4%). On the contrary, the proportion of participants aged 30.0 – 44.9 years was significantly higher in the normotensive group when compared to the hypertensive group (41.0% versus 26.0%, respectively; P < 0.0001). The odds of hypertension were 2.5 times higher among subjects aged 45 – 59.9 years than among participants aged 30 – 44.9 years (OR = 2.51; CI = 1.85 – 3.41). About 64% of study participants aged > 60 years (98 from 154) and only 8% of subjects aged < 30 years (12 from 141) had hypertension (P < 0.0001) (Table 1). The prevalence of hypertension was 3.95 higher among subjects above 45 years than among subjects younger than 45 years (OR = 3.95; CI = 1.97 – 3.02) (Table 2).
In the total number of subjects (n = 1,027) the frequency of hypertension was significantly higher in males than in females (48.0% versus 34.3%, P < 0.0001) and males compared to females had a 77% higher chance (OR = 1.77; 95%, CI = 1.36 – 2.30) of having hypertension (Table 2). The presence of risk alleles was not associated with increased risk for hypertension in men (data not shown). The higher prevalence of hypertension among men than among women was seen in subjects younger than 30 years (20.4% versus 3.1%; P = 0.0006) in subjects aged 30 – 44.9 years: (41.7% versus 21.8 %; P < 0.0001), and in the group of 45 – 59.9 years (58.0% versus 45.9%, P = 0.0167). However, among subjects older than 60 years the prevalence of hypertension was non-significantly higher in women than in men (66% hypertensive females versus 59% hypertensive males, P = 0.407).
Hypertensive subjects were characterized by significantly higher serum glucose and triglycerides (P < 0.001), and significantly lower high-density lipoprotein cholesterol concentrations (P < 0.0001) than non-hypertensive subjects (Table 1). The prevalence of diabetes mellitus, and dyslipidemia were significantly higher in hypertensives than in normotensives (P < 0.001; Table 2). Variables that were significant in univariable models, i.e. obesity, diabetes, dyslipidemia, age, smoking status, and sex, were then combined in the multivariable logistic regression model. The results presented in Table 2 showed that all these variables were still significantly associated with hypertension, however, in the crude model diabetes was the strongest risk factor, while in the adjusted model age > 45 years appeared as the strongest one.
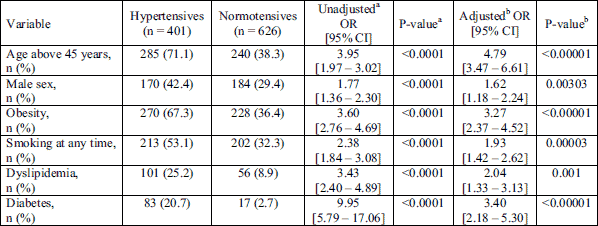
The distribution of ACE I/D (P = 0.183), NOS3 4a/4b (P = 0.683), NOS3 -T786C (P = 0.19) and NOS3 G894T (P = 0.06) genotypes in the study group were in Hardy-Weinberg equilibrium (HWE). Genotype distributions and allele frequencies of the ACE I/D, NOS3 4a/4b, G894T and -T786C variants in patients with and without hypertension were summarized in Table 3. In the total number of subjects (n = 1,027) no significant differences in genotype and allele distributions between hypertensives and normotensives were found.
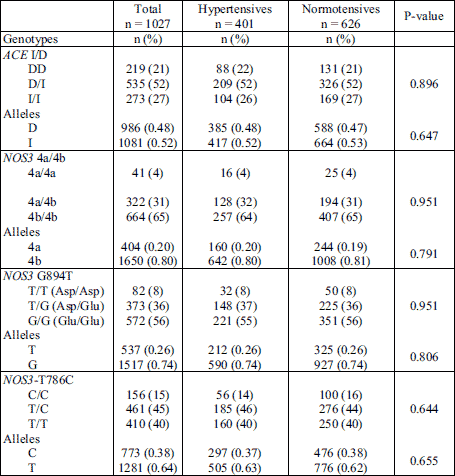
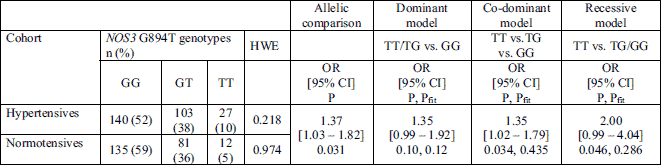
Out of a total 1,027 participants, 498 (48.5%) were obese. The presence of risk alleles, i.e. ACE D, NOS3 4a and NOS3 C-786, was not associated with increased risk for hypertension in both obese and non-obese subjects (Table 4). However, in obese individuals NOS3 T894 allele was associated with 1.37 fold higher risk of hypertension (OR = 1.37, P = 0.031). In addition, the distribution of NOS3 G894T genotypes supported the co-dominant (OR = 1.35, P = 0.034, Pfit =0.435) or recessive (OR = 2.00, P = 0.046, Pfit = 0.286), but not dominant model of inheritance (P = 0.100, Table 5). The association between T894 homozygosity and hypertension in obese subjects did not persist (P = 0.38, Table 6) when aged above 45 years, diabetes, obesity, dyslipidemia, smoking status, and male sex were taken into account.
DISCUSSION
Among various genes of the regulatory pathways of blood pressure, the endothelial NO synthase gene (NOS3) and angiotensin converting enzyme gene (ACE) appear relevant, however, inconsistent data on the association between ACE and NOS3 genetic variants and hypertension risk have been published (28, 40, 41). We found that in the whole study group the ACE I/D, NOS3 4a/4b, G894T and -T786C variants were not associated with an increased prevalence of hypertension, while the prevalence of specific genetic variants was in line with their frequencies in other European populations (42, 43). As essential hypertension is a multifactorial disease, the association between hypertension and variants in the endothelial nitric oxide synthase and angiotensin-converting enzyme genes can be modulated by multiple factors. In a number of epidemiological studies essential hypertension was found to be present in over 60% of obese individuals (44). In this report the multivariable logistic regression revealed that obese had higher chance of hypertension than non-obese. In addition, the univariate logistic regression analysis showed that among obese NOS3 GT894T polymorphism was associated with increased risk of hypertension. Similar data were found in a small group of Chinese subjects (45). Moreover, higher systolic and diastolic blood pressure was reported for TT homozygotes of G894T and CC homozygotes of -786C/T variants in NOS3 gene as compared to carriers of other NOS3 variants in young healthy individuals without history of hypertension from Czech population (46). In addition, it has been suggested that TT genotype of G894T polymorphism leads to susceptibility to proteolytic cleavage in endothelial cells and vascular tissues and results in reduced levels of functional eNOS and subsequently reduced levels of NO (47). Randomized controlled trials demonstrate that weight loss can effectively contribute to reduction in blood pressure (48, 49). It is postulated that the observed nowadays rise in BMI on population level may reduce or even reverse the observed decline in myocardial infarction incidence and coronary heart disease mortality (50), and the influence of obesity on hypertension may play a crucial role. Among various hypotheses which have been proposed to explain the development of hypertension in obese subjects, endothelial dysfunction mediated by oxidative stress and inflammation might be a key factor (6, 44). In obese subjects an excessive amount of visceral adipose tissue is associated with the inappropriate secretion of proinflammatory cytokines, chemokines, adipokines and growth factors (51). Many of them impair endothelial cell function and contribute to a variety of cardiovascular diseases (6). Enhanced levels of IL6 and TNF-α have been found in obese subjects (52) and a correlation between IL-6, body mass index, and arterial blood pressure in men with essential hypertension was reported (53). In addition, TNF-α enhances mRNA degradation of endothelial nitric oxide synthase, resulting in NO deficiency and endothelial dysfunction (54, 55). Even minor endothelial disturbances, such as decreased NO production, increased expression of cellular adhesion molecules, and adherence of leukocytes to the endothelium, could have significant implications for the initiation, development and progression of hypertension and atherosclerosis (56). The observed lack of association between the NOS3 polymorphisms and essential hypertension might be due to the fact that many subjects, both with and without the risk alleles, had increased risk of hypertension due to nicotine smoking, older age, male sex, BMI higher than 30 kg/m2, diabetes and dyslipidemia. The adjusted statistical analysis indicates that the influence of these factors on hypertension development is much stronger than the influence of 894T homozygosity in NOS3 gene. However, it cannot be ruled out that in an environment highly conducive to the development of hypertension, personal genetic profile may still influence disease development.
Hypertension is highly prevalent in Poland, and about 42% men and 33% women aged 20 – 74 years were recognized as hypertensive in an epidemiological study (57). In the total number of subjects (n = 1 027) participating in the current study the frequency of hypertension was significantly higher in males than in females (48% versus 34%). These results also correspond to the findings from the ‘National Heart Program’ directed against high blood pressure and cardiovascular diseases in Poland (NATPOL PLUS) (58), which concluded that the awareness, detection and control of hypertension is much worse in men than in women. In the current study, a higher prevalence of hypertension in men compared to women was seen in subjects younger than 60 years, which is consistent with estrogen’s possible protection against hypertension before menopause due to its cardioprotective effect (59). Our results also indicate that the prevalence of hypertension among participants older than 60 years was slightly higher in women than in men. This finding in women corresponds to the menopause and lower estrogen levels. In addition, 64% of study participants aged > 60 years had hypertension. Our results are consistent with the fact that hypertension increases with age, and with aging the hypertension risk increases unless healthy lifestyle is followed (60). Cigarette smoking is a well-recognized risk factor for hypertension and atherosclerosis (61, 62). Data suggest that the free radical components of cigarette smoke may be responsible for the morphological and functional damage to the endothelium (63) and hypertensive smokers are more likely to develop severe forms of hypertension (64). Moreover, chronic smoking increases blood pressure by affecting arterial stiffness (65). In our study, smoking at any time increased the risk of hypertension, and, similar to previous findings (61, 62), a higher proportion of hypertensive individuals was recognized among former smokers compared to current smokers. Tobacco smoking increases blood pressure acutely (66), but in accordance with epidemiological studies and hypertension paradox, we found that former smokers were more likely to be hypertensive than current smokers. In the present study no influence of interaction between smoking and genetic variants on hypertension risk was recognized. In Indian population the influence of smoking and alcohol intake on the risk of hypertension was dependent on the NOS3 genotypes including G894T variants (67). However, differences in lifestyle and ethnic differences between Indian and Polish population should be taken into account. Diabetes, obesity related dyslipidemia and hypertension frequently occur together (68, 69). The present study shows that diabetes and dyslipidemia significantly increase the risk of hypertension. Insulin resistance associated with obesity may cause sympathetic overactivity, and by influencing the activity of the RAA system, may increase intrarenal pressures due to increased sodium and water retention (70, 71). The dysfunction of the RAA axis leads to increased intravascular fluid volume and systemic vasoconstriction (6).
Some limitations of our work should be taken into consideration. Dietary habits and physical activity were not evaluated. This work was based on a limited number of hypertensive patients as well as obese subjects younger than 30 years. We did not measure eNOS activity or NO concentrations to prove the biological effect of NOS3 G894T polymorphism.
In conclusion, the present study results indicate that in obese NOS3 894T allele may enhance hypertension risk. However, in the presence of such strong risk factors as age, diabetes and smoking, the impact of this genetic variant seems to be attenuated. Further studies are needed to reveal the usefulness of G894T polymorphism in hypertension risk assessment in obese.
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; CI, confidence interval; eNOS, endothelial nitric oxide synthase; EH, essential hypertension; NOS3, endothelial nitric oxide synthase gene; OR, odds ratio
Acknowledgements: This work was supported by the Medical University of Warsaw (grant numbers FW113/NM2/13, FW113/NM1/14); and carried out with the use of CePT infrastructure financed by the European Union- the European Regional Development Fund within the Operational Programme ‘Innovative economy’ for 2007-2013.
Conflict of interests: None declared.
REFERENCES
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206-1252.
- Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 2011; 17: 1402-1409.
- Hall JE, Granger JP, do Carmo JM, et al. Hypertension: physiology and pathophysiology. Compr Physiol 2012; 2: 2393-2442.
- Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008; 8: 200.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131: 1-8.
- Kang YS. Obesity associated hypertension: new insights into mechanism. Electrolyte Blood Press 2013; 11: 46-52.
- Canale MP, Manca di Villahermosa S, Martino G, et al. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J Endocrinol 2013; 2013: 865965.
- Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol 2009; 302: 111-117.
- Schling P, Schafer T. Human adipose tissue cells keep tight control on the angiotensin II levels in their vicinity. J Biol Chem 2002; 277: 48066-48075.
- Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci 2013; 14: 10497-10538.
- Mauricio MD, Aldasoro M, Ortega J, Vila JM. Endothelial dysfunction in morbid obesity. Curr Pharm Des 2013; 19: 5718-5729.
- Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 2001; 37: 554-560.
- Gruber HJ, Mayer C, Mangge H, Fauler G, Grandits N, Wilders-Truschnig M. Obesity reduces the bioavailability of nitric oxide in juveniles. Int J Obes (Lond) 2008; 32: 826-831.
- Wang X, Tanus-Santos JE, Reiter CD, et al. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci USA 2004; 101: 11477-11482.
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001; 357: 593.
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840-844.
- Adamcova M, Ruzickova S, Simko F. Multiplexed immunoassays for simultaneous quantification of cardiovascular biomarkers in the model of H(G)-nitro-L-arginine methylester (L-NAME) hypertensive rat. J Physiol Pharmacol 2013; 64: 211-217.
- Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation 1995; 92: 320-326.
- Hogg N, Kalyanaraman B, Joseph J, Struck A, Parthasarathy S. Inhibition of low-density lipoprotein oxidation by nitric oxide. Potential role in atherogenesis. FEBS Lett 1993; 334: 170-174.
- Kubes P, Suzuki M, Granger D. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Nat Acad Sci USA 1991; 88: 4651-4655.
- Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, and hypertension. Semin Nephrol 2004; 24: 366-378.
- Raij L. Workshop: hypertension and cardiovascular risk factors: role of the angiotensin II - nitric oxide interaction. Hypertension 2001; 37: 767-773.
- Fleming I. Signaling by the angiotensin-converting enzyme. Circ Res 2006; 98: 887-896.
- Jalil JE, Ocaranza MP, Oliveri C, et al. Neutral endopeptidase and angiotensin I converting enzyme insertion/deletion gene polymorphism in humans. J Hum Hypertens 2004; 18: 119-125.
- Marsden PA, Schappert KT, Chen HS, et al. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett 1992; 307: 287-293.
- Colomba D, Duro G, Corrao S, et al. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular damage in hypertensive subjects: an Italian case-control study. Immun Ageing 2008; 5: 4.
- Dell’Omo G, Penno G, Pucci L, et al. Lack of association between endothelial nitric oxide synthase gene polymorphisms, microalbuminuria and endothelial dysfunction in hypertensive men. J Hypertens 2007; 25: 1389-1395.
- Niu W, Qi Y. An updated meta-analysis of endothelial nitric oxide synthase gene: three well-characterized polymorphisms with hypertension. PLoS One 2011; 6: e24266.
- Rossi GP, Cesari M, Zanchetta M, et al. The T-786C endothelial nitric oxide synthase genotype is a novel risk factor for coronary artery disease in Caucasian patients of the GENICA study. J Am Coll Cardiol 2003; 41: 930-937.
- Wolff B, Grabe HJ, Schluter C, et al. Endothelial nitric oxide synthase Glu298Asp gene polymorphism, blood pressure and hypertension in a general population sample. J Hypertens 2005; 23: 1361-1366.
- Zhao Q, Su SY, Chen SF, Li B, Gu DF. Association study of the endothelial nitric oxide synthase gene polymorphisms with essential hypertension in northern Han Chinese. Chin Med J (Engl) 2006; 119: 1065-1071.
- Augeri AL, Tsongalis GJ, Van Heest JL, Maresh CM, Thompson PD, Pescatello LS. The endothelial nitric oxide synthase -786 T> C polymorphism and the exercise-induced blood pressure and nitric oxide responses among men with elevated blood pressure. Atherosclerosis 2009; 204: e28-e34.
- de Las Fuentes L, Sung YJ, Schwander KL, et al. The role of SNP-loop diuretic interactions in hypertension across ethnic groups in HyperGEN. Front Genet 2013; 4: 304.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: 1-253.
- Gadomska H, Grzechocinska B, Janecki J, Nowicka G, Powolny M, Marianowski L. Serum lipids concentration in women with benign and malignant ovarian tumours. Eur J Obstet Gynecol Reprod Biol 2005; 120: 87-90.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143-3421.
- Lindpaintner K, Pfeffer MA, Kreutz R, et al. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med 1995; 332: 706-711.
- Fatini C, Sofi F, Gori AM, et al. Endothelial nitric oxide synthase -786T>C, but not 894G>T and 4a4b, polymorphism influences plasma homocysteine concentrations in persons with normal vitamin status. Clin Chem 2005; 51: 1159-1164.
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 2003; 19: 149-150.
- Zhou YF, Yan H, Hou XP, et al. Association study of angiotensin converting enzyme gene polymorphism with elderly diabetic hypertension and lipids levels. Lipids Health Dis 2013; 12: 187.
- Martinez-Rodriguez N, Posadas-Romero C, Villarreal-Molina T, et al. Single nucleotide polymorphisms of the angiotensin-converting enzyme (ACE) gene are associated with essential hypertension and increased ACE enzyme levels in Mexican individuals. PLoS One 2013; 8: e65700.
- Zajc Petranovic M, Skaric-Juric T, Smolej Narancic N, et al. Angiotensin-converting enzyme deletion allele is beneficial for the longevity of Europeans. Age (Dordr) 2012; 34: 583-595.
- Zintzaras E, Kitsios G, Stefanidis I. Endothelial NO synthase gene polymorphisms and hypertension: a meta-analysis. Hypertension 2006; 48: 700-710.
- DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol 2014; 10: 364-376.
- Jia CQ, Zhao ZT, Wang LH, et al. [Relationship between mutation of exon G894 T of endothelial nitric oxide synthase gene and overweight to essential hypertension] [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi 2003; 37: 365-367.
- Jira M, Zavodna E, Honzikova N, et al. Association of eNOS gene polymorphisms T-786C and G894T with blood pressure variability in man. Physiol Res 2011; 60: 193-197.
- Dias RG, Gowdak MM, Pereira AC. Genetics and cardiovascular system: influence of human genetic variants on vascular function. Genes Nutr 2011; 6: 55-62.
- Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003; 42: 878-884.
- Seimon RV, Espinoza D, Ivers L, et al. Changes in body weight and blood pressure: paradoxical outcome events in overweight and obese subjects with cardiovascular disease. Int J Obes (Lond) 2014; 38: 1165-1171.
- Hardoon SL, Morris RW, Whincup PH, et al. Rising adiposity curbing decline in the incidence of myocardial infarction: 20-year follow-up of British men and women in the Whitehall II cohort. Eur Heart J 2012; 33: 478-485.
- Sikaris KA. The clinical biochemistry of obesity. Clin Biochem Rev 2004; 25: 165-181.
- Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest 2007; 30: 210-214.
- Gibas-Dorna M, Nowak D, Piatek J, Pupek-Musialik D, Krauss H, Kopczynski P. Plasma ghrelin and interleukin-6 levels correlate with body mass index and arterial blood pressure in males with essential hypertension. J Physiol Pharmacol 2015; 66: 367-372.
- Lai PF, Mohamed F, Monge JC, Stewart DJ. Downregulation of eNOS mRNA expression by TNFalpha: identification and functional characterization of RNA-protein interactions in the 3’UTR. Cardiovasc Res 2003; 59: 160-168.
- Zemankova L, Varejckova M, Dolezalova E, et al. Atorvastatin-induced endothelial nitric oxide synthase expression in endothelial cells is mediated by endoglin. J Physiol Pharmacol 2015; 66: 403-413.
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003; 170: 191-203.
- Piwonska A, Piotrowski W, Broda G. Knowledge about arterial hypertension in the Polish population: the WOBASZ study. Kardiol Pol 2012; 70: 140-146.
- Zdrojewski T, Szpakowski P, Bandosz P, et al. Arterial hypertension in Poland in 2002. J Hum Hypertens 2004; 18: 557-562.
- Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep 2006; 8: 368-376.
- Susic D, Frohlich ED. The aging hypertensive heart: a brief update. Nat Clin Pract Cardiovasc Med 2008; 5: 104-110.
- John U, Meyer C, Hanke M, Volzke H, Schumann A. Smoking status, obesity and hypertension in a general population sample: a cross-sectional study. QJM 2006; 99: 407-415.
- Lee DH, Ha MH, Kim JR, Jacobs DR, Jr. Effects of smoking cessation on changes in blood pressure and incidence of hypertension: a 4-year follow-up study. Hypertension 2001; 37: 194-198.
- Michael Pittilo R. Cigarette smoking, endothelial injury and cardiovascular disease. Int J Exp Pathol 2000; 81: 219-230.
- Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des 2010; 16: 2518-2525.
- Rhee MY, Na SH, Kim YK, Lee MM, Kim HY. Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am J Hypertens 2007; 20: 637-641.
- Omvik P. How smoking affects blood pressure. Blood Press 1996; 5: 71-77.
- Shankarishan P, Borah PK, Ahmed G, Mahanta J. Endothelial nitric oxide synthase gene polymorphisms and the risk of hypertension in an Indian population. Biomed Res Int 2014; 2014: 793040.
- Grossman E, Messerli FH. Hypertension and diabetes. Adv Cardiol 2008; 45: 82-106.
- Malecki R, Fiodorenko-Dumas Z, Jakobsche-Policht U, Malodobra M, Adamiec R. Altered monocyte calcium-sensing receptor expression in patients with type 2 diabetes mellitus and atherosclerosis. J Physiol Pharmacol 2013; 64: 521-527.
- Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens 2001; 19: 523-528.
- Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes 2004; 53: 2735-2740.
A c c e p t e d : August 12, 2015